Abstract
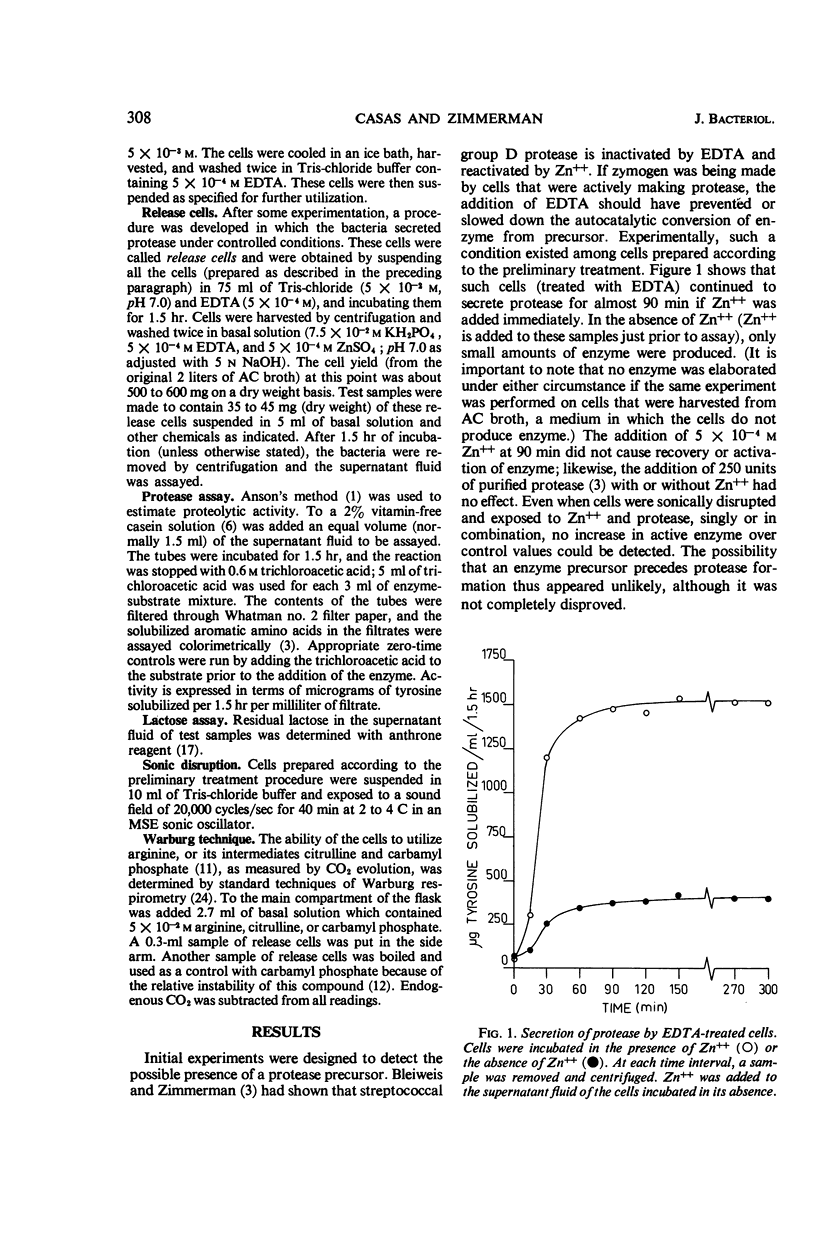
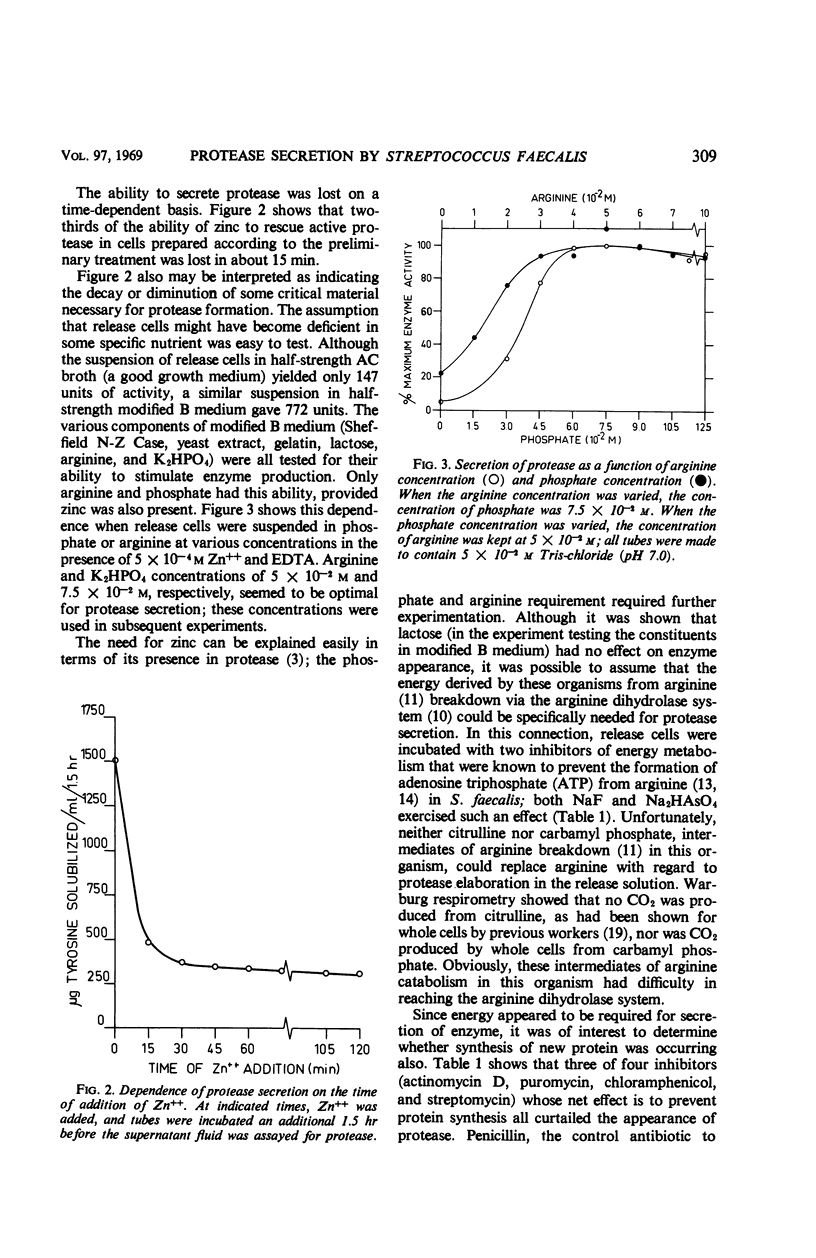
Washed cells of Streptococcus faecalis var. liquefaciens, when harvested from media that supported protease biosynthesis, continued to release this extracellular enzyme in phosphate buffer. The addition of ethylenediaminetetraacetic acid (EDTA) halted the secretion. If zinc ions were added to the EDTA-treated cells before 45 to 60 min had elapsed, a fraction of the anticipated enzyme activity was observed. After 60 min, arginine and phosphate, in addition to zinc, were necessary for the demonstration of proteolytic activity. The enzyme that was released was newly formed, because chloramphenicol, puromycin, or actinomycin D prevented its appearance. Energy for this synthetic reaction was obtained, apparently, from arginine; lactose could not be substituted for arginine. This last point is interpreted to mean that extracellular protease biosynthesis occurs in a localized area or cellular compartment into which the adenosine triphosphate derived from the fermentation of lactose cannot diffuse. No evidence was found for a protease zymogen, although this possibility is not completely precluded.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLEIWEIS A. S., ZIMMERMAN L. N. PROPERTIES OF PROTEINASE FROM STREPTOCOCCUS FAECALIS VAR. LIQUEFACIENS. J Bacteriol. 1964 Sep;88:653–659. doi: 10.1128/jb.88.3.653-659.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaton C. D. An electron microscope study of the mesosomes of a penicillinase-producing staphylococcus. J Gen Microbiol. 1968 Jan;50(1):37–42. doi: 10.1099/00221287-50-1-37. [DOI] [PubMed] [Google Scholar]
- Coles N. W., Gross R. Liberation of surface-located penicillinase from Staphylococcus aureus. Biochem J. 1967 Mar;102(3):742–747. doi: 10.1042/bj1020742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUTTER F. H., ZIMMERMAN L. N. A proteolytic enzyme of Streptococcus zymogenes. J Bacteriol. 1955 Jun;69(6):728–732. doi: 10.1128/jb.69.6.728-732.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAROLD F. M., HAROLD R. L., ABRAMS A. A MUTANT OF STREPTOCOCCUS FAECALIS DEFECTIVE IN PHOSPHATE UPTAKE. J Biol Chem. 1965 Jul;240:3145–3153. [PubMed] [Google Scholar]
- Hammel J. M., Zimmerman L. N. The dependence of proteinase biosynthesis on the cell wall in Streptococcus faecalis var. liquefaciens. Biochim Biophys Acta. 1966 Dec 21;129(3):613–617. doi: 10.1016/0005-2787(66)90076-1. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Interaction of arsenate with phosphate-transport systems in wild- type and mutant Streptococcus faecalis. J Bacteriol. 1966 Jun;91(6):2257–2262. doi: 10.1128/jb.91.6.2257-2262.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills G. M. Ammonia production by pathogenic bacteria. Biochem J. 1940 Jul;34(7):1057–1069. doi: 10.1042/bj0341057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. E., Lipmann F. CHEMICAL AND ENZYMATIC SYNTHESIS OF CARBAMYL PHOSPHATE. Proc Natl Acad Sci U S A. 1960 Sep;46(9):1194–1205. doi: 10.1073/pnas.46.9.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNIVETT V. A. The effect of arsenate on bacterial citrulline breakdown. Biochem J. 1954 Apr;56(4):606–610. doi: 10.1042/bj0560606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU T. Y., ELLIOTT S. D. ACTIVATION OF STREPTOCOCCAL PROTEINASE AND ITS ZYMOGEN BY BACTERIAL CELL WALLS. Nature. 1965 Apr 3;206:33–34. doi: 10.1038/206033a0. [DOI] [PubMed] [Google Scholar]
- Morris D. L. Quantitative Determination of Carbohydrates With Dreywood's Anthrone Reagent. Science. 1948 Mar 5;107(2775):254–255. doi: 10.1126/science.107.2775.254. [DOI] [PubMed] [Google Scholar]
- Niven C. F., Sherman J. M. Nutrition of the Enterococci. J Bacteriol. 1944 Apr;47(4):335–342. doi: 10.1128/jb.47.4.335-342.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGINSKY E. L., GEHRIG R. F. The arginine dihydrolase system of Streptococcus faecalis. I. Identification of citrulline as an intermediate. J Biol Chem. 1952 Oct;198(2):791–797. [PubMed] [Google Scholar]
- Richmond M. H. New type of restriction to the expression of a structural gene in bacteria. Nature. 1967 Dec 23;216(5121):1191–1192. doi: 10.1038/2161191a0. [DOI] [PubMed] [Google Scholar]
- Shugart L. R., Beck R. W. Occurrence and Distribution of Proteinase of Streptococcus faecalis var. liquefaciens. J Bacteriol. 1966 Aug;92(2):338–341. doi: 10.1128/jb.92.2.338-341.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRENTINI W., CHESBRO W. Localization of the "arginine dihyldrolase system" in Streptococcus faecium. Biochim Biophys Acta. 1963 Mar 12;67:511–513. doi: 10.1016/0006-3002(63)91858-4. [DOI] [PubMed] [Google Scholar]


