Abstract
Conformational changes have been shown in the proteins of the red blood cell membrane, induced by lead poisoning. Evidence that the change in spatial arrangement of proteins is believed to be responsible for inhibition of the sodium (Na+)/potassium (K+) ATPase of the RBC membrane is presented.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLARKSON T. W., KENCH J. E. Uptake of lead by human erythrocytes in vitro. Biochem J. 1958 Jul;69(3):432–439. doi: 10.1042/bj0690432. [DOI] [PMC free article] [PubMed] [Google Scholar]
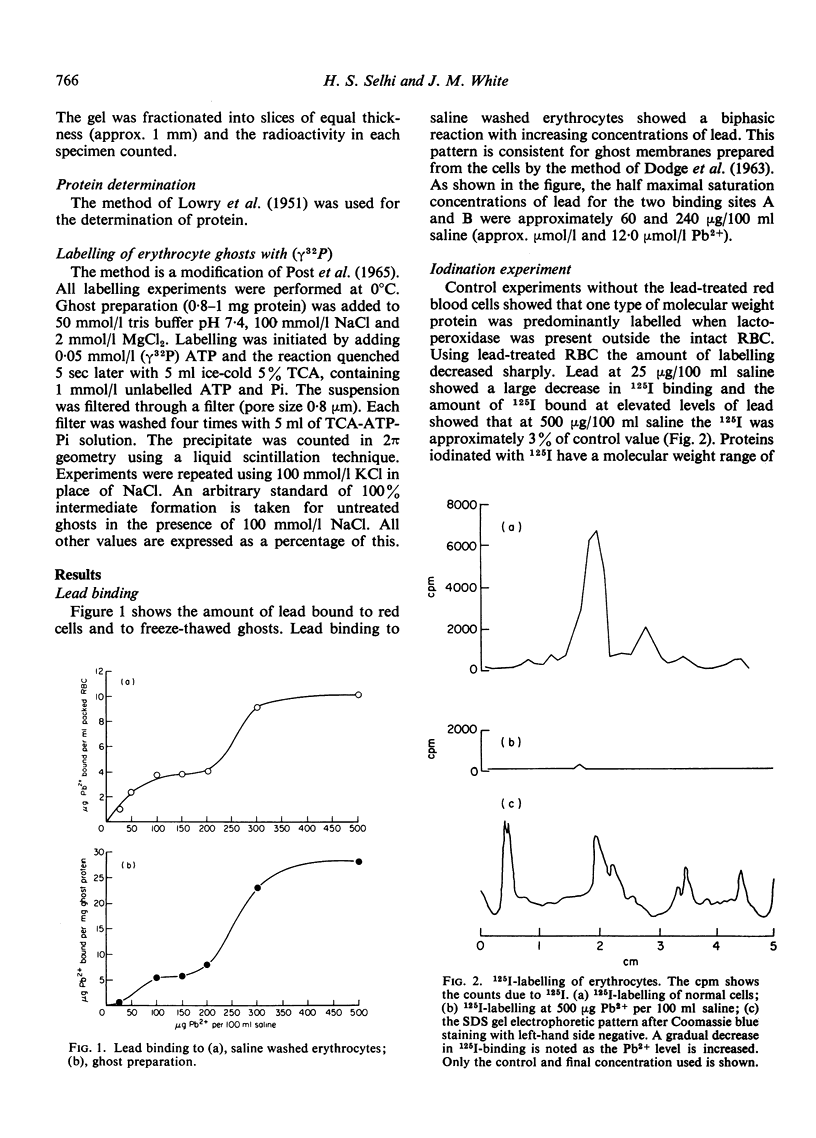
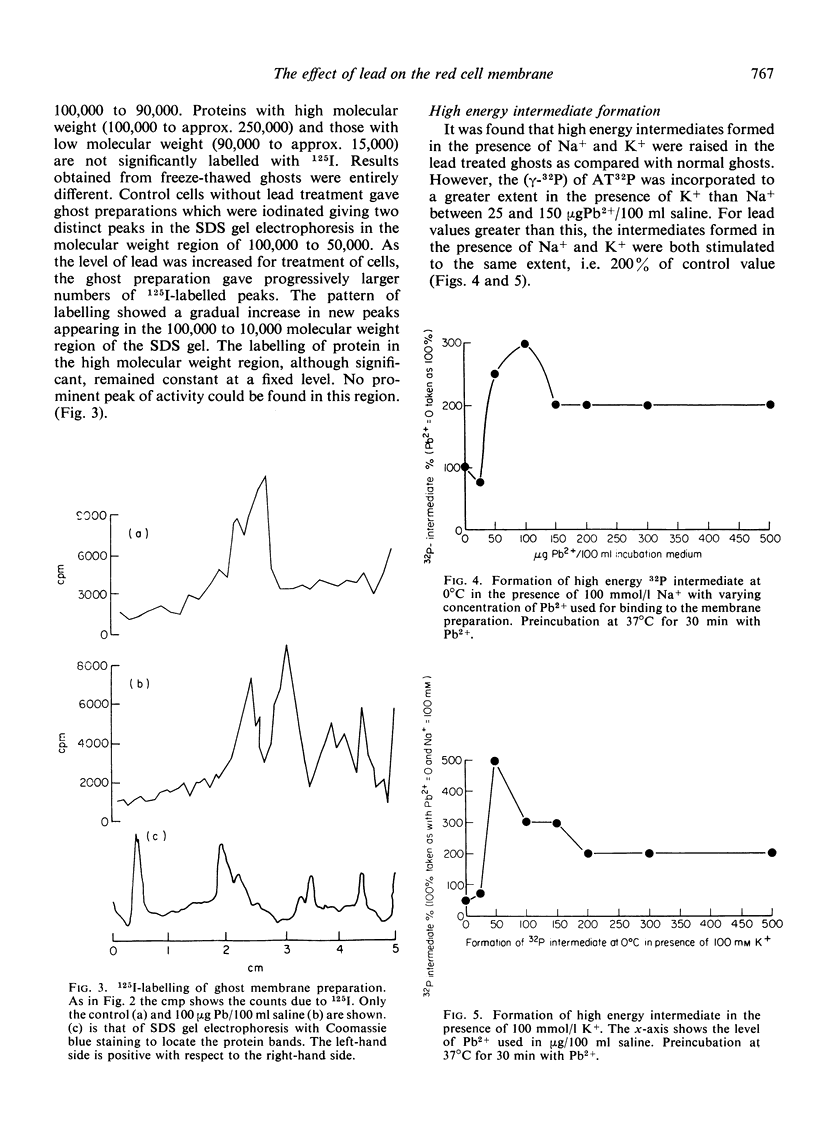
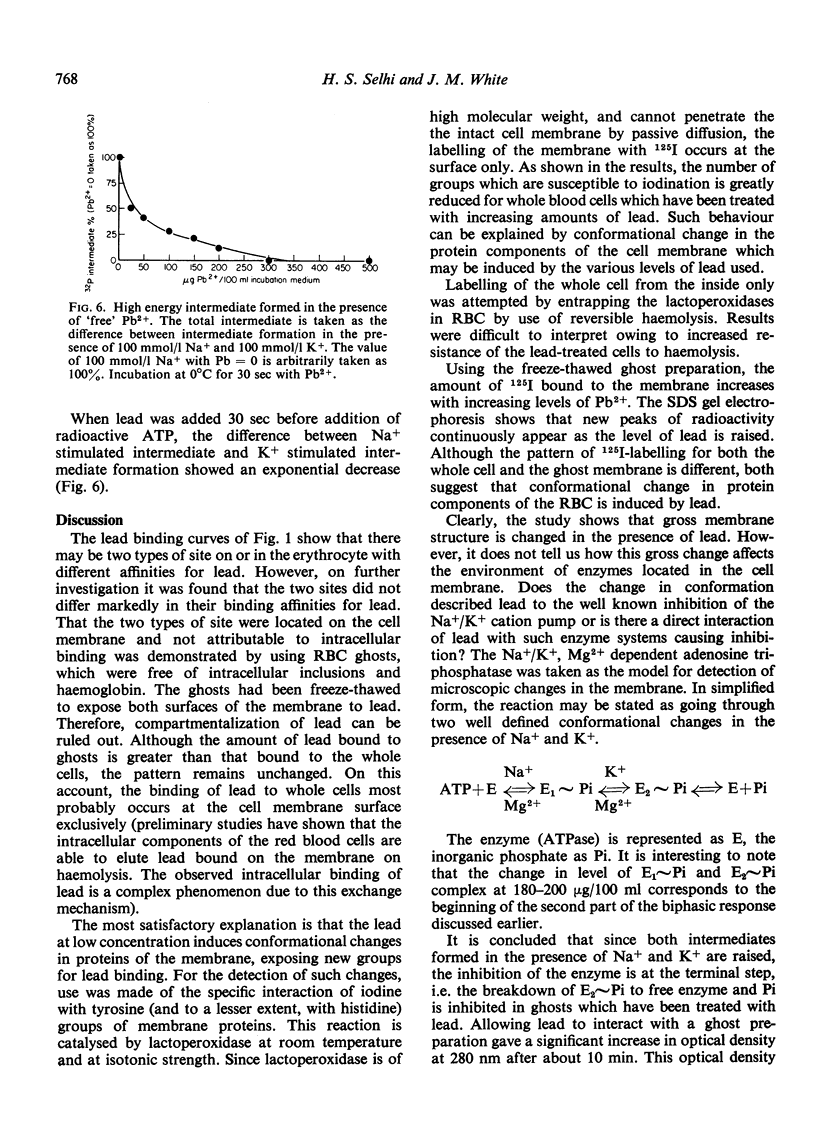
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gardner J. D., Conlon T. P. The effects of sodium and potassium on ouabain binding by human erythrocytes. J Gen Physiol. 1972 Nov;60(5):609–629. doi: 10.1085/jgp.60.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan J., Hernberg S., Metsälä P., Vihko V. Enhanced potassium loss in blood cells from men exposed to lead. Arch Environ Health. 1967 Feb;14(2):309–312. doi: 10.1080/00039896.1967.10664737. [DOI] [PubMed] [Google Scholar]
- Hasan J., Vihko V., Hernberg S. Deficient red cell membrane /Na++K+/-ATPase in lead poisoning. Arch Environ Health. 1967 Feb;14(2):313–318. doi: 10.1080/00039896.1967.10664738. [DOI] [PubMed] [Google Scholar]
- Hernberg S., Vihko V., Hasan J. Red cell membrane ATPase in workers exposed to inorganic lead. Arch Environ Health. 1967 Feb;14(2):319–324. doi: 10.1080/00039896.1967.10664739. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Phillips D. R., Morrison M. Exposed protein on the intact human erythrocyte. Biochemistry. 1971 May 11;10(10):1766–1771. doi: 10.1021/bi00786a006. [DOI] [PubMed] [Google Scholar]
- Post R. L., Kume S., Tobin T., Orcutt B., Sen A. K. Flexibility of an active center in sodium-plus-potassium adenosine triphosphatase. J Gen Physiol. 1969 Jul 1;54(1):306–326. doi: 10.1085/jgp.54.1.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secchi G. C., Alessio L. Ricerche sul meccanismo d'inibizione della Na+-K+-ATPasi eritrocitaria ad opera del piombo. Med Lav. 1969 Nov;60(11):670–673. [PubMed] [Google Scholar]
- Steck T. L., Fairbanks G., Wallach D. F. Disposition of the major proteins in the isolated erythrocyte membrane. Proteolytic dissection. Biochemistry. 1971 Jun 22;10(13):2617–2624. doi: 10.1021/bi00789a031. [DOI] [PubMed] [Google Scholar]
- Tobin T., Akera T., Baskin S. I., Brody T. M. Calcium ion and sodium- and potassium-dependent adenosine triphosphatase: its mechanism of inhibition and identification of the E 1 -P intermediate. Mol Pharmacol. 1973 May;9(3):336–349. [PubMed] [Google Scholar]


