Abstract
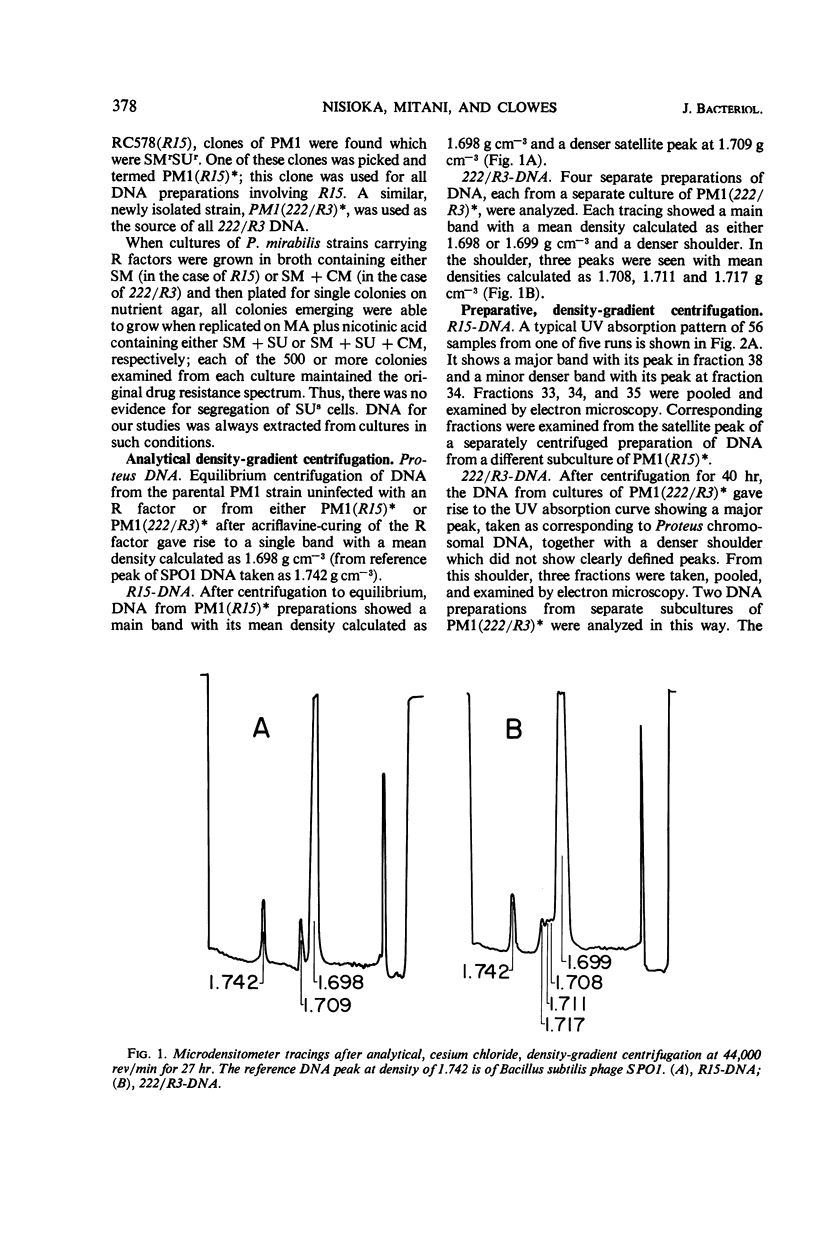
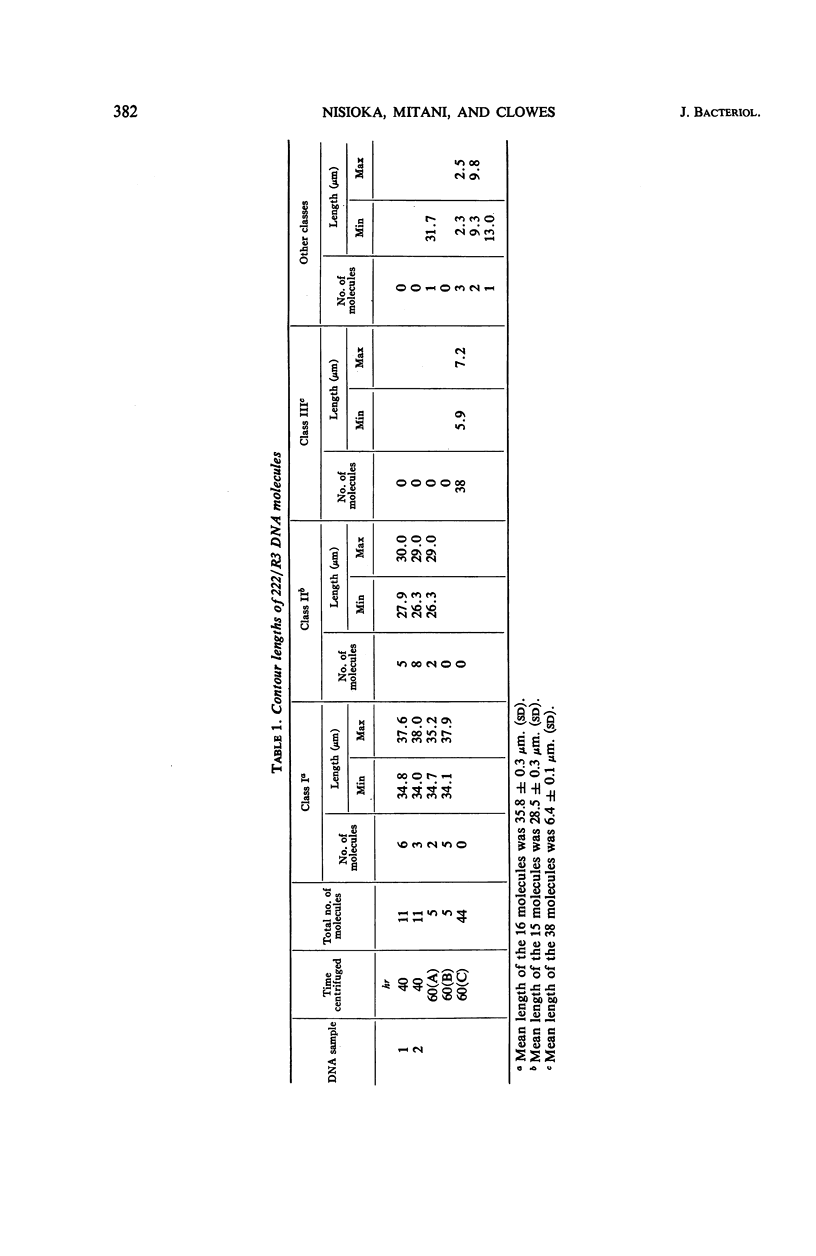
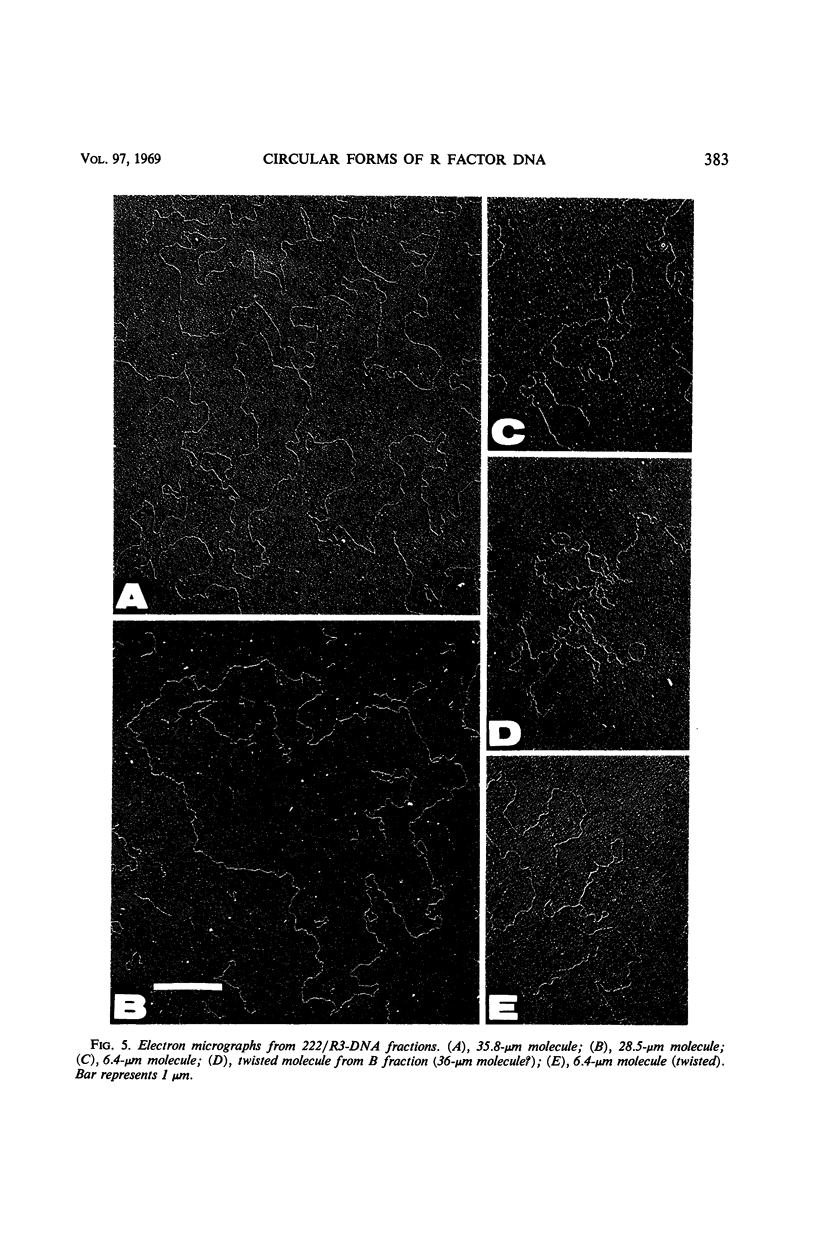
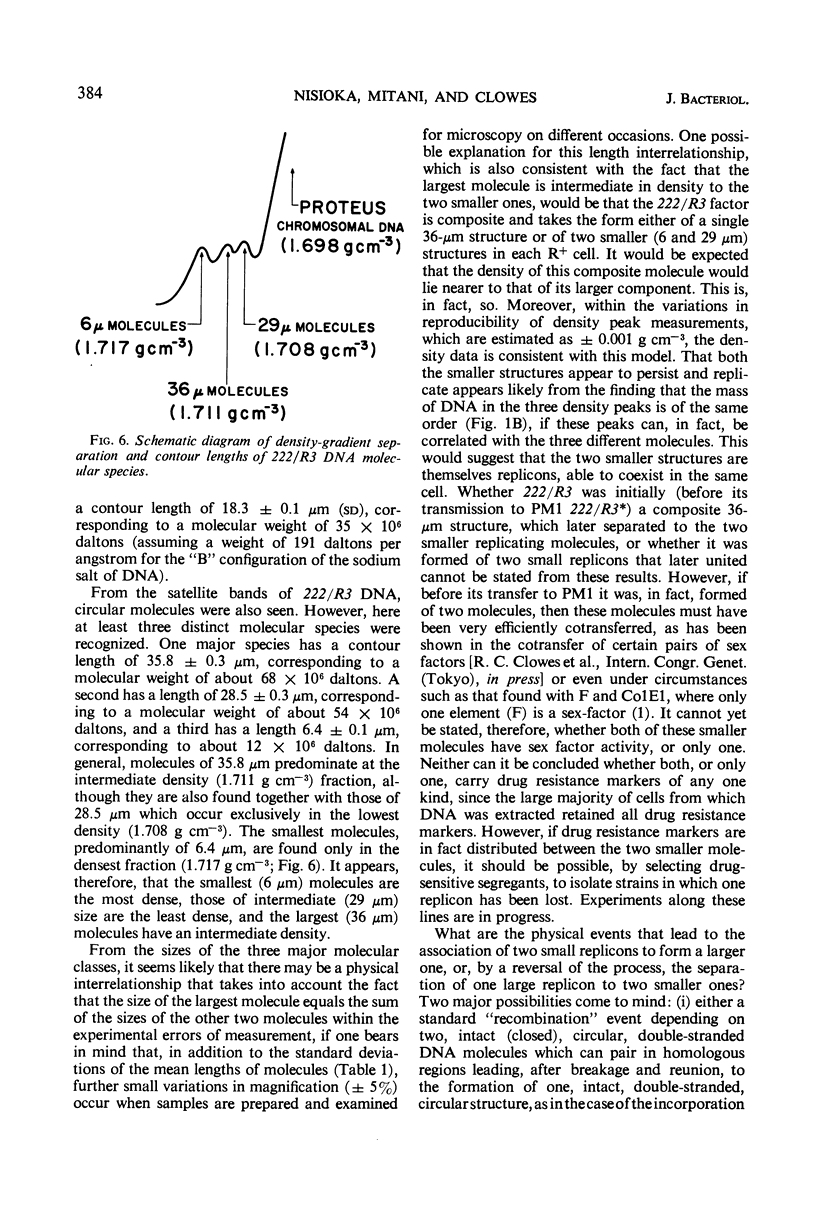
Two R factors, one (R15) conferring resistance to streptomycin and sulfonamide (SMrSUr) and the other (222/R3) to streptomycin, sulfonamide, and chloramphenicol (SMrSUrCMr), were transferred to a Proteus mirabilis strain, and deoxyribonucleic acid (DNA) extracted from these strains was subjected to density-gradient centrifugation. R15-DNA formed a single satellite band at a density of 1.709 g cm−3. Electron microscopy of samples from this band showed circular molecules of one type, with a contour length of 18 μm (35 × 106 daltons). 222/R3-DNA formed a satellite band with three peaks at densities 1.708, 1.711 and 1.717 g cm−3. Electron micrographs revealed circular structures from each band with contour lengths, respectively, of 29 (54 × 106 daltons), 36 (68 × 106 daltons), and 6 μm (12 × 106 daltons). “Supertwisted” forms of several molecular species were found. It is suggested that 222/R3 DNA comprises either a single 36-μm molecule or two individual molecules, 29 and 6 μm in length, and that this may reflect the evolutionary development of R factors.
Full text
PDF

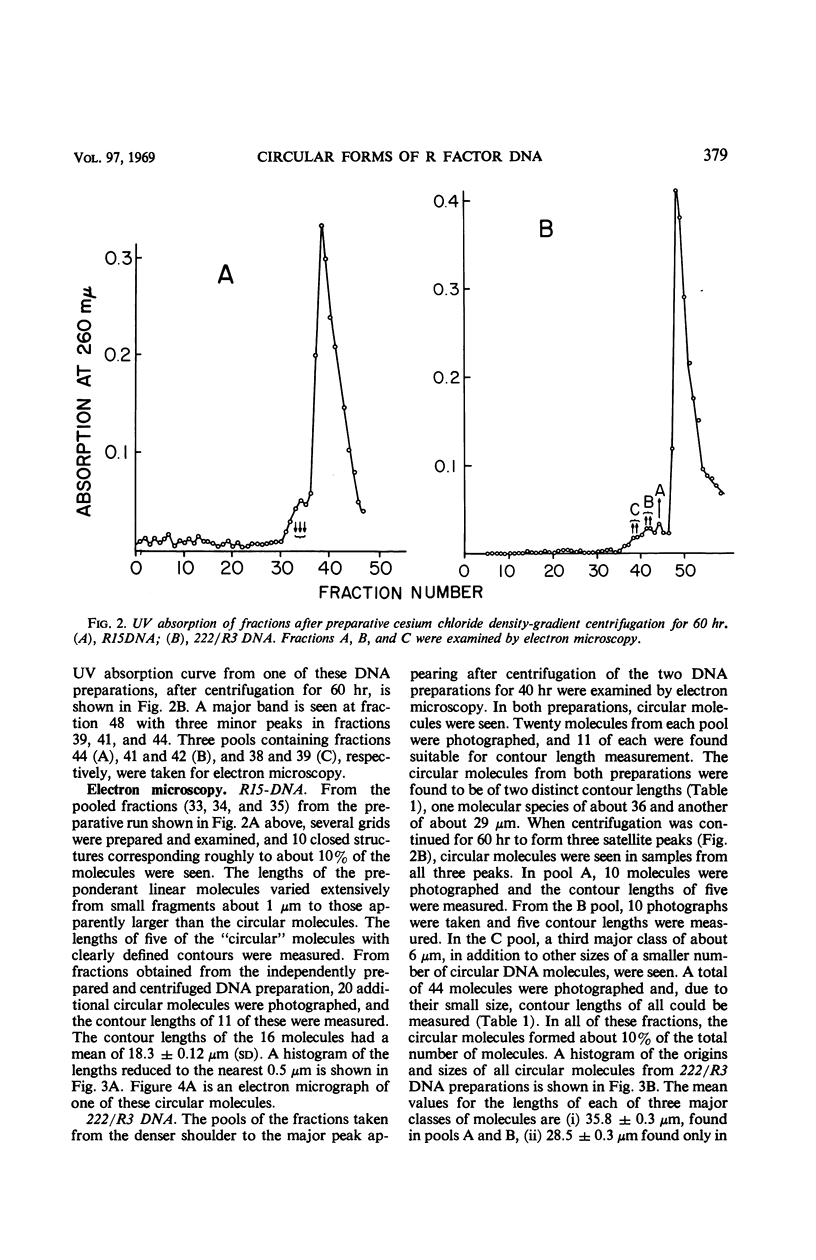

Images in this article
Selected References
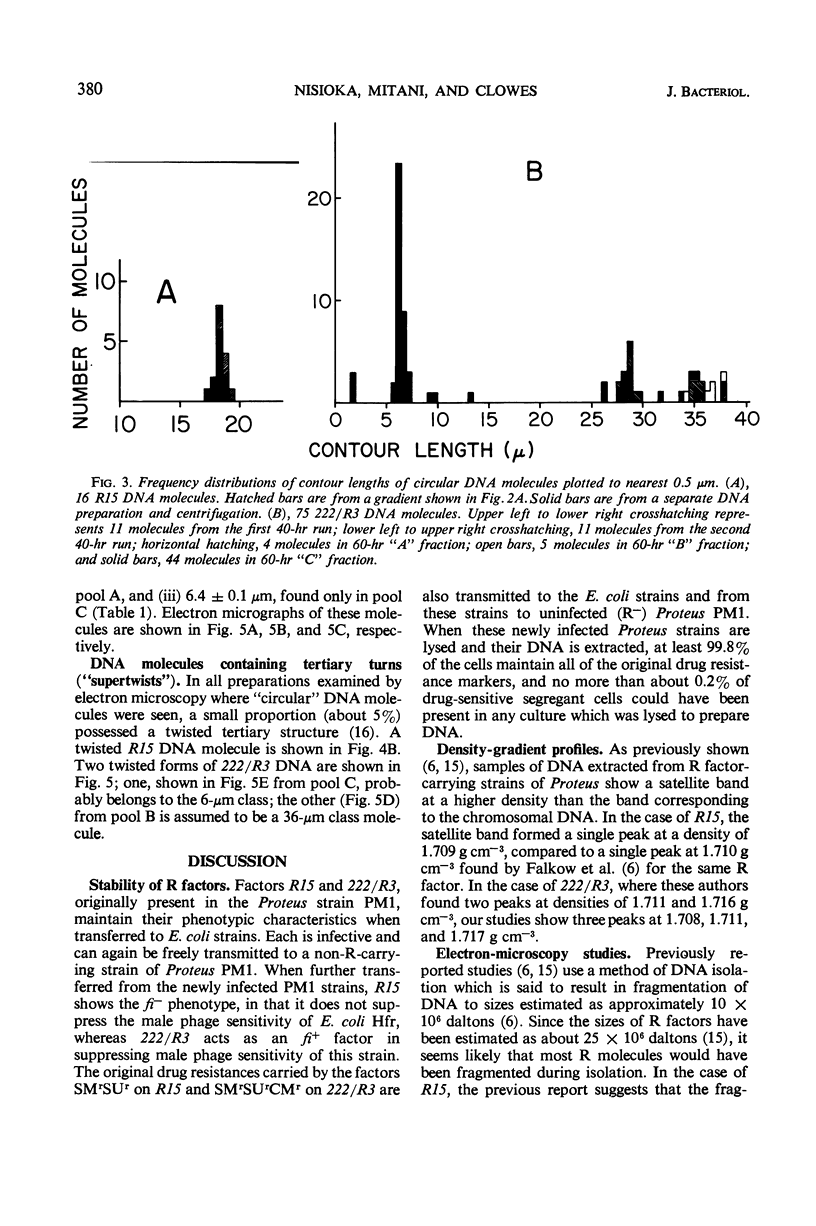
These references are in PubMed. This may not be the complete list of references from this article.
- ALFOLDI L., JACOB F., WOLLMAN E. L. Zygose létale dans des croisements entre souches colicinogènes et non colicinogènes d'Escherichia coli. C R Hebd Seances Acad Sci. 1957 Jun 12;244(24):2974–2977. [PubMed] [Google Scholar]
- Anderson E. S. Facteurs de transfert et résistance aux antibiotiques chez les entérobactéries. Ann Inst Pasteur (Paris) 1967 May;112(5):547–563. [PubMed] [Google Scholar]
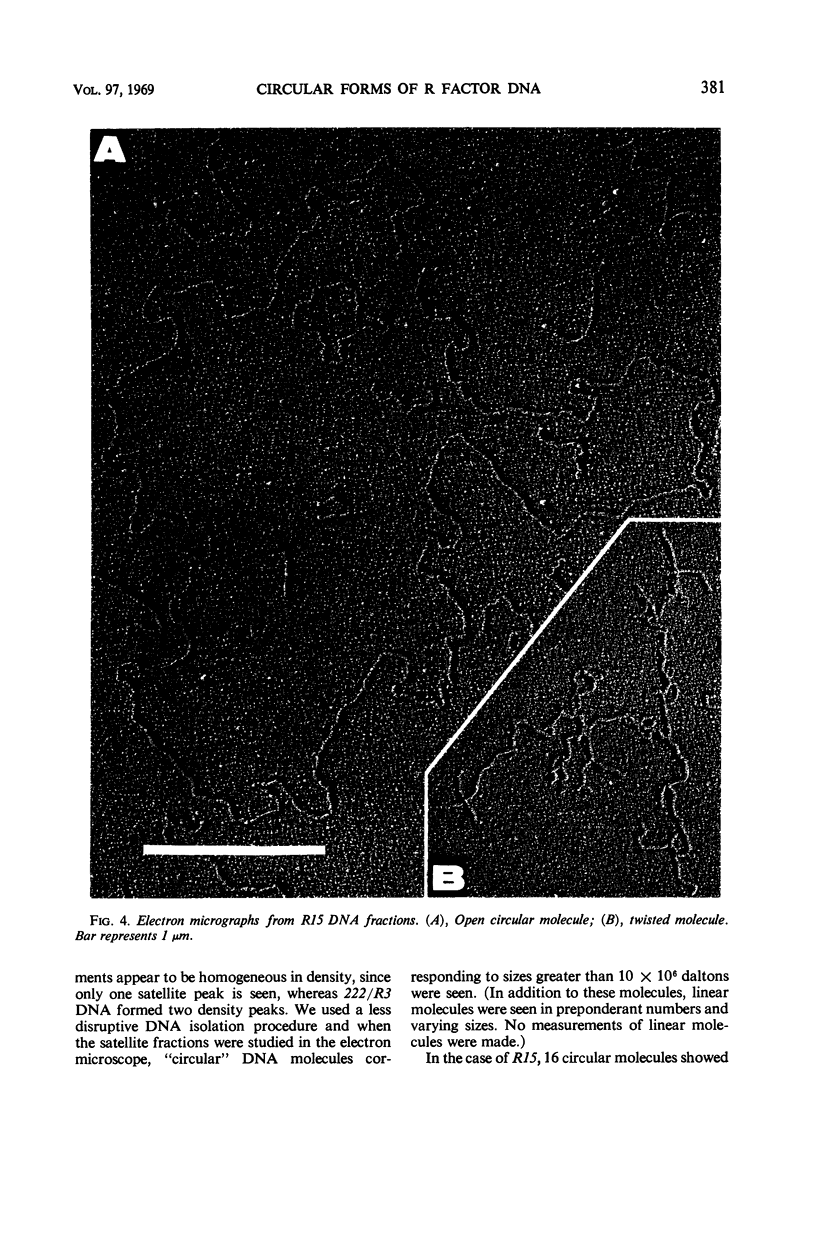
- CAVALLI-SFORZA L. L. La sessualità nei batteri. Boll Ist Sieroter Milan. 1950 Sep-Oct;29(9-10):281–289. [PubMed] [Google Scholar]
- Falkow S., Citarella R. V., Wohlhieter J. A. The molecular nature of R-factors. J Mol Biol. 1966 May;17(1):102–116. doi: 10.1016/s0022-2836(66)80097-9. [DOI] [PubMed] [Google Scholar]
- Gemski P., Jr, Wohlhieter J. A., Baron L. S. Chromosome transfer between Escherichia coli HFR strains and Proteus mirabilis. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1461–1467. doi: 10.1073/pnas.58.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERSHEY A. D., BURGI E. COMPLEMENTARY STRUCTURE OF INTERACTING SITES AT THE ENDS OF LAMBDA DNA MOLECULES. Proc Natl Acad Sci U S A. 1965 Feb;53:325–328. doi: 10.1073/pnas.53.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson F. T., Roth T. F., Helinski D. R. Circular DNA forms of a bacterial sex factor. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1731–1738. doi: 10.1073/pnas.58.4.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D., Bujard H., Wolff B., Russell D. Electron microscopy of size and shape of viral DNA in solutions of different ionic strengths. J Mol Biol. 1967 Jan 28;23(2):163–181. doi: 10.1016/s0022-2836(67)80024-x. [DOI] [PubMed] [Google Scholar]
- MARMUR J., ROWND R., FALKOW S., BARON L. S., SCHILDKRAUT C., DOTY P. The nature of intergeneric episomal infection. Proc Natl Acad Sci U S A. 1961 Jul 15;47:972–979. doi: 10.1073/pnas.47.7.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONK M., CLOWES R. C. TRANSFER OF THE COLICIN I FACTOR IN ESCHERICHIA COLI K12 AND ITS INTERACTION WITH THE F FERTILITY FACTOR. J Gen Microbiol. 1964 Sep;36:365–384. doi: 10.1099/00221287-36-3-365. [DOI] [PubMed] [Google Scholar]
- Meynell E., Meynell G. G., Datta N. Phylogenetic relationships of drug-resistance factors and other transmissible bacterial plasmids. Bacteriol Rev. 1968 Mar;32(1):55–83. doi: 10.1128/br.32.1.55-83.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rownd R., Nakaya R., Nakamura A. Molecular nature of the drug-resistance factors of the Enterobacteriaceae. J Mol Biol. 1966 Jun;17(2):376–393. doi: 10.1016/s0022-2836(66)80149-3. [DOI] [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T. Episome-mediated transfer of drug resistance in Enterobacteriaceae. I. Transfer of resistance factors by conjugation. J Bacteriol. 1961 May;81:669–678. doi: 10.1128/jb.81.5.669-678.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T. Episome-mediated transfer of drug resistance in Enterobacteriaceae. II. Elimination of resistance factors with acridine dyes. J Bacteriol. 1961 May;81:679–683. doi: 10.1128/jb.81.5.679-683.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., NISHIDA H., OGATA C., ARAI T., SATO S. EPISOME-MEDIATED TRANSFER OF DRUG RESISTANCE IN ENTEROBACTERIACEAE. VII. TWO TYPES OF NATURALLY OCCURRING R FACTORS. J Bacteriol. 1964 Sep;88:716–726. doi: 10.1128/jb.88.3.716-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]




