Abstract
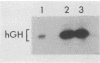
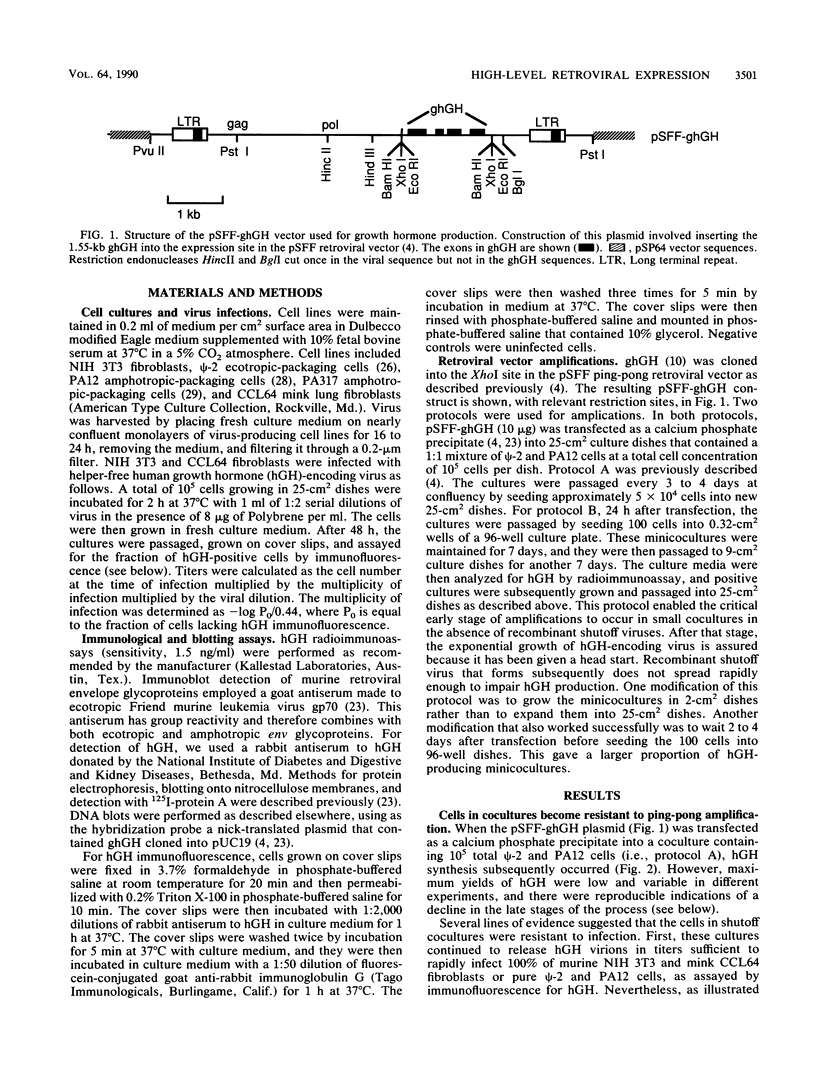
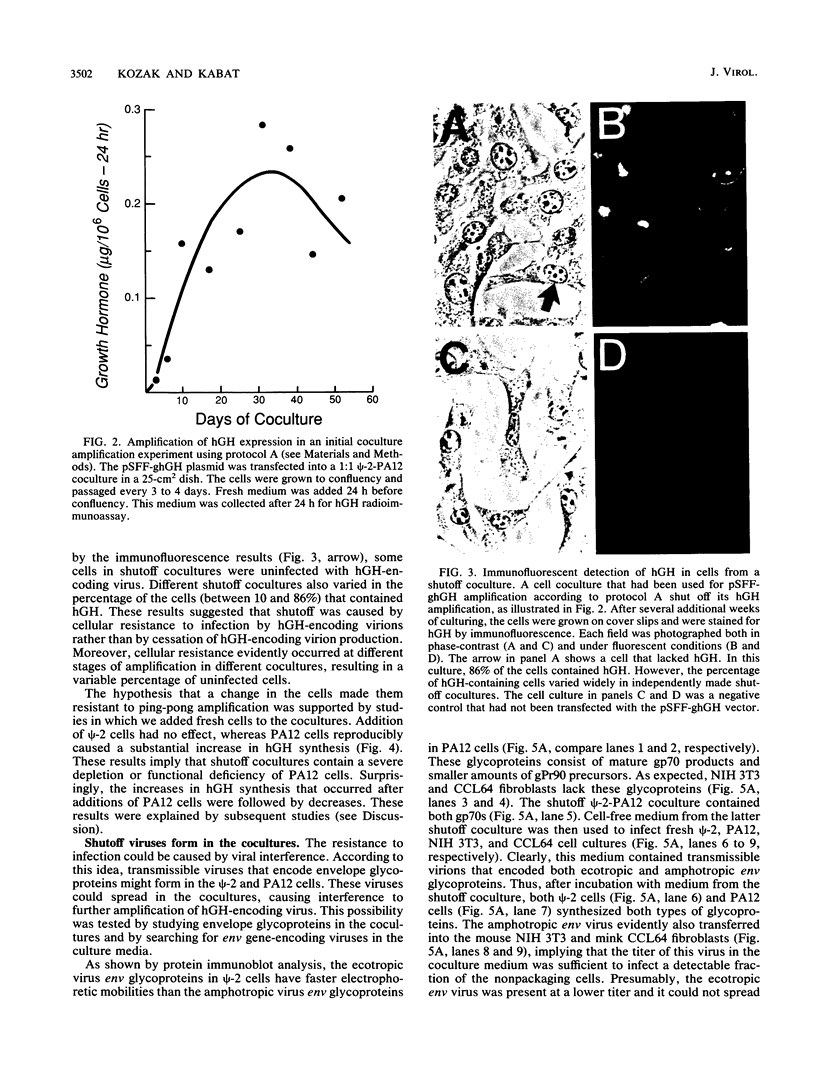
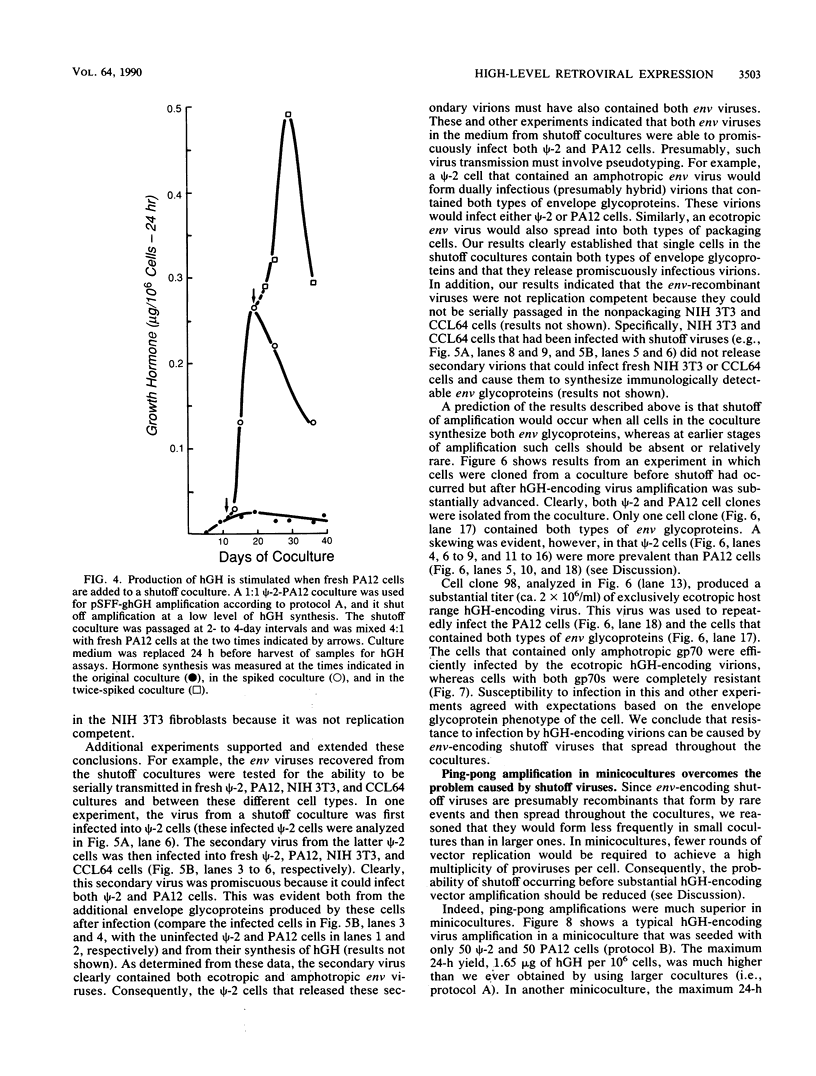
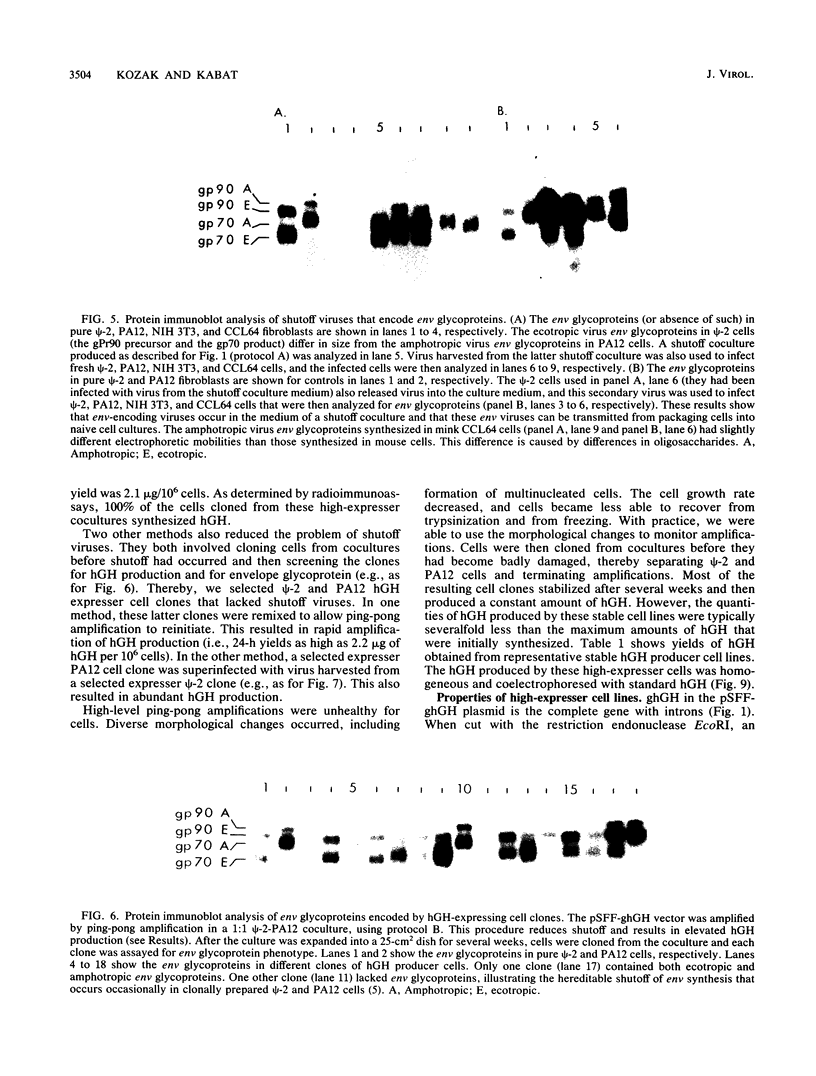
Retroviral vectors offer major advantages for gene transfer studies but have not been useful for producing proteins in large quantities. This deficiency has resulted in part from interference to superinfection, which limits the numbers of active proviruses in cells. Recently, we found that these vectors amplify when they are added as calcium phosphate precipitates to cocultures of cells that package retroviruses into ecotropic and amphotropic host range envelopes. Helper-free virions from either cell type can infect the other without interference, resulting in theoretically limitless back-and-forth (ping-pong) vector replication. In initial studies, however, amplifications of a vector that contained the human growth hormone gene ceased when the hormone produced was 0.3% or less of cellular protein synthesis. This limit was caused by two factors. First, recombinant shutoff viruses that are replication defective and encode envelope glycoproteins form at a low probability during any round of the vector replication cycle and these spread in cocultures, thereby establishing interference. Single cells in shutoff cocultures therefore synthesize both ecotropic and amphotropic envelope glycoproteins, and they release promiscuous (presumably hybrid) virions. The probability of forming shutoff viruses before the vector had amplified to a high multiplicity was reduced by using small cocultures. Second, cells with large numbers of proviruses are unhealthy and their proviral expression can be unstable. Stable expresser cell clones were obtained by selection. Thereby, cell lines were readily obtained that stably produce human growth hormone as 4 to 6% of the total protein synthesis. A ping-pong retroviral vector can be used for high-level protein production in vertebrate cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. E., Dawson K. M., Gull K., Kingsman S. M., Kingsman A. J. The expression of hybrid HIV:Ty virus-like particles in yeast. Nature. 1987 Sep 3;329(6134):68–70. doi: 10.1038/329068a0. [DOI] [PubMed] [Google Scholar]
- Armentano D., Yu S. F., Kantoff P. W., von Ruden T., Anderson W. F., Gilboa E. Effect of internal viral sequences on the utility of retroviral vectors. J Virol. 1987 May;61(5):1647–1650. doi: 10.1128/jvi.61.5.1647-1650.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassin R. H., Ruscetti S., Ali I., Haapala D. K., Rein A. Normal DBA/2 mouse cells synthesize a glycoprotein which interferes with MCF virus infection. Virology. 1982 Nov;123(1):139–151. doi: 10.1016/0042-6822(82)90301-4. [DOI] [PubMed] [Google Scholar]
- Bestwick R. K., Kozak S. L., Kabat D. Overcoming interference to retroviral superinfection results in amplified expression and transmission of cloned genes. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5404–5408. doi: 10.1073/pnas.85.15.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestwick R. K., Machida C. A., Polonoff E., Kabat D. Frequent hereditable shutdown of murine retrovirus gene expression in murine cell lines. Mol Cell Biol. 1984 May;4(5):908–914. doi: 10.1128/mcb.4.5.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowtell D. D., Cory S., Johnson G. R., Gonda T. J. Comparison of expression in hemopoietic cells by retroviral vectors carrying two genes. J Virol. 1988 Jul;62(7):2464–2473. doi: 10.1128/jvi.62.7.2464-2473.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiswell D. J., Enrietto P. J., Evans S., Quade K., Wyke J. A. Molecular mechanisms involved in morphological variation of avian sarcoma virus-infected rat cells. Virology. 1982 Jan 30;116(2):428–440. doi: 10.1016/0042-6822(82)90137-4. [DOI] [PubMed] [Google Scholar]
- Clapham P., Nagy K., Weiss R. A. Pseudotypes of human T-cell leukemia virus types 1 and 2: neutralization by patients' sera. Proc Natl Acad Sci U S A. 1984 May;81(9):2886–2889. doi: 10.1073/pnas.81.9.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danos O., Mulligan R. C. Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6460–6464. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNoto F. M., Moore D. D., Goodman H. M. Human growth hormone DNA sequence and mRNA structure: possible alternative splicing. Nucleic Acids Res. 1981 Aug 11;9(15):3719–3730. doi: 10.1093/nar/9.15.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty J. P., Temin H. M. Determination of the rate of base-pair substitution and insertion mutations in retrovirus replication. J Virol. 1988 Aug;62(8):2817–2822. doi: 10.1128/jvi.62.8.2817-2822.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglitis M. A., Anderson W. F. Retroviral vectors for introduction of genes into mammalian cells. Biotechniques. 1988 Jul-Aug;6(7):608–614. [PubMed] [Google Scholar]
- Emerman M., Temin H. M. Quantitative analysis of gene suppression in integrated retrovirus vectors. Mol Cell Biol. 1986 Mar;6(3):792–800. doi: 10.1128/mcb.6.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischinger P. J., Blevins C. S., Dunlop N. M. Genomic masking of nondefective recombinant murine leukemia virus in Moloney virus stocks. Science. 1978 Aug 4;201(4354):457–459. doi: 10.1126/science.663667. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Nebert D. W., Hollander M. C., Luethy J. D., Papathanasiou M., Fargnoli J., Holbrook N. J. Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol Cell Biol. 1989 Oct;9(10):4196–4203. doi: 10.1128/mcb.9.10.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D. A., Hart K. A., Wyke J. A. Rearrangements of viral and cellular DNA are often associated with expression of Rous sarcoma virus in rat cells. Cell. 1985 May;41(1):279–287. doi: 10.1016/0092-8674(85)90081-9. [DOI] [PubMed] [Google Scholar]
- Haas M., Patch V. Genomic masking and rescue of dual-tropic murine leukemia viruses: role of pseudotype virions in viral lymphomagenesis. J Virol. 1980 Sep;35(3):583–591. doi: 10.1128/jvi.35.3.583-591.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W. G., Ham R. G. Clonal growth of chinese hamster cell lines in protein-free media. In Vitro. 1977 Sep;13(9):537–547. doi: 10.1007/BF02627849. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Sugimura H. Fv-4 resistance gene: a truncated endogenous murine leukemia virus with ecotropic interference properties. J Virol. 1989 Dec;63(12):5405–5412. doi: 10.1128/jvi.63.12.5405-5412.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner A. L., Bernstein A. Retrovirus transduction: segregation of the viral transforming function and the herpes simplex virus tk gene in infectious Friend spleen focus-forming virus thymidine kinase vectors. Mol Cell Biol. 1983 Dec;3(12):2191–2202. doi: 10.1128/mcb.3.12.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat D. Molecular biology of Friend viral erythroleukemia. Curr Top Microbiol Immunol. 1989;148:1–42. doi: 10.1007/978-3-642-74700-7_1. [DOI] [PubMed] [Google Scholar]
- Li J. P., Bestwick R. K., Spiro C., Kabat D. The membrane glycoprotein of Friend spleen focus-forming virus: evidence that the cell surface component is required for pathogenesis and that it binds to a receptor. J Virol. 1987 Sep;61(9):2782–2792. doi: 10.1128/jvi.61.9.2782-2792.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. P., D'Andrea A. D., Lodish H. F., Baltimore D. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature. 1990 Feb 22;343(6260):762–764. doi: 10.1038/343762a0. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Markowitz D., Goff S., Bank A. Construction and use of a safe and efficient amphotropic packaging cell line. Virology. 1988 Dec;167(2):400–406. [PubMed] [Google Scholar]
- Metsikkö K., Garoff H. Role of heterologous and homologous glycoproteins in phenotypic mixing between Sendai virus and vesicular stomatitis virus. J Virol. 1989 Dec;63(12):5111–5118. doi: 10.1128/jvi.63.12.5111-5118.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Law M. F., Verma I. M. Generation of helper-free amphotropic retroviruses that transduce a dominant-acting, methotrexate-resistant dihydrofolate reductase gene. Mol Cell Biol. 1985 Mar;5(3):431–437. doi: 10.1128/mcb.5.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Miller C. K., Temin H. M. Insertion of several different DNAs in reticuloendotheliosis virus strain T suppresses transformation by reducing the amount of subgenomic mRNA. J Virol. 1986 Apr;58(1):75–80. doi: 10.1128/jvi.58.1.75-80.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson J. C., Colley K. J. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem. 1989 Oct 25;264(30):17615–17618. [PubMed] [Google Scholar]
- Sitbon M., Nishio J., Wehrly K., Chesebro B. Pseudotyping of dual-tropic recombinant viruses generated by infection of mice with different ecotropic murine leukemia viruses. Virology. 1985 Jan 15;140(1):144–151. doi: 10.1016/0042-6822(85)90453-2. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Retrovirus vectors: promise and reality. Science. 1989 Nov 24;246(4933):983–983. doi: 10.1126/science.2686028. [DOI] [PubMed] [Google Scholar]
- Xu L., Yee J. K., Wolff J. A., Friedmann T. Factors affecting long-term stability of Moloney murine leukemia virus-based vectors. Virology. 1989 Aug;171(2):331–341. doi: 10.1016/0042-6822(89)90600-4. [DOI] [PubMed] [Google Scholar]
- Yan S. C., Grinnell B. W., Wold F. Post-translational modifications of proteins: some problems left to solve. Trends Biochem Sci. 1989 Jul;14(7):264–268. doi: 10.1016/0968-0004(89)90060-1. [DOI] [PubMed] [Google Scholar]
- Young R. A., Elliott T. J. Stress proteins, infection, and immune surveillance. Cell. 1989 Oct 6;59(1):5–8. doi: 10.1016/0092-8674(89)90861-1. [DOI] [PubMed] [Google Scholar]