Abstract
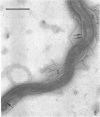
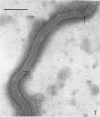
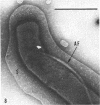
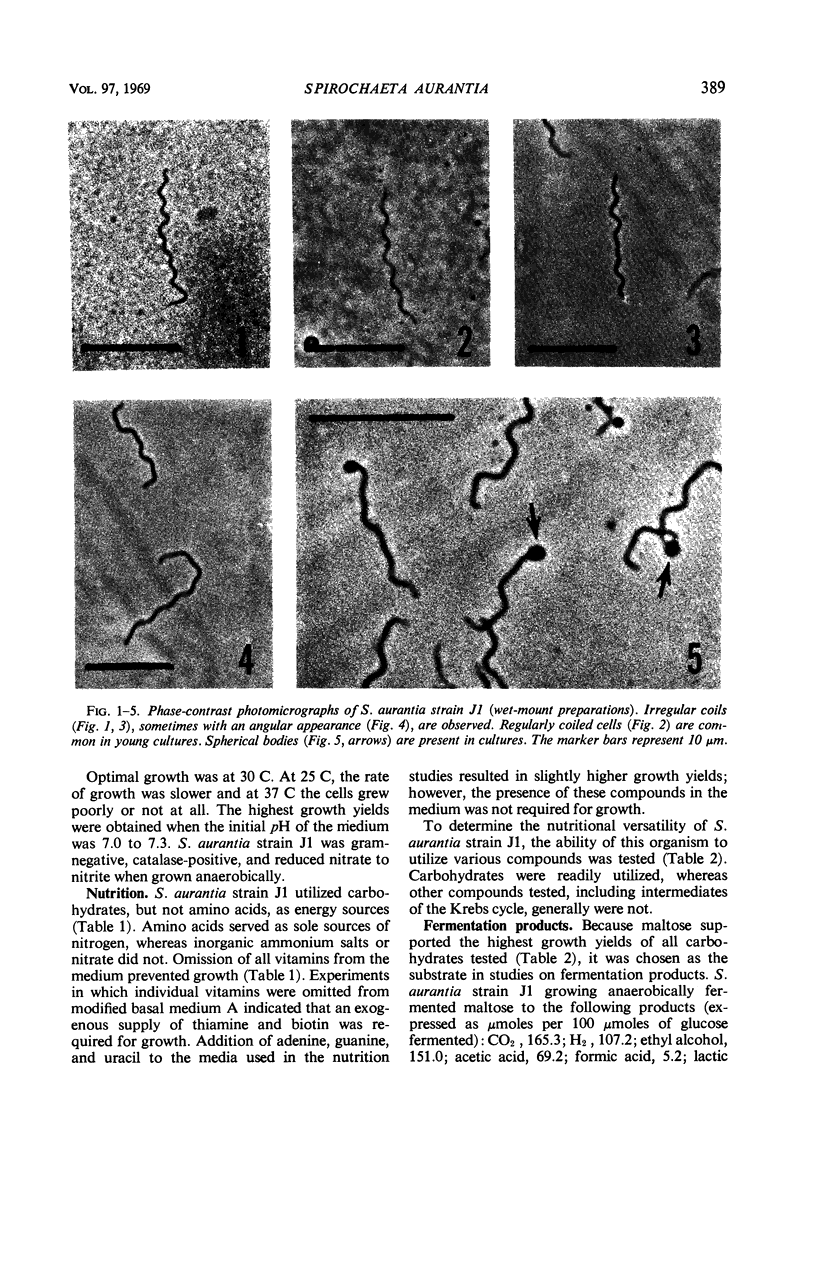
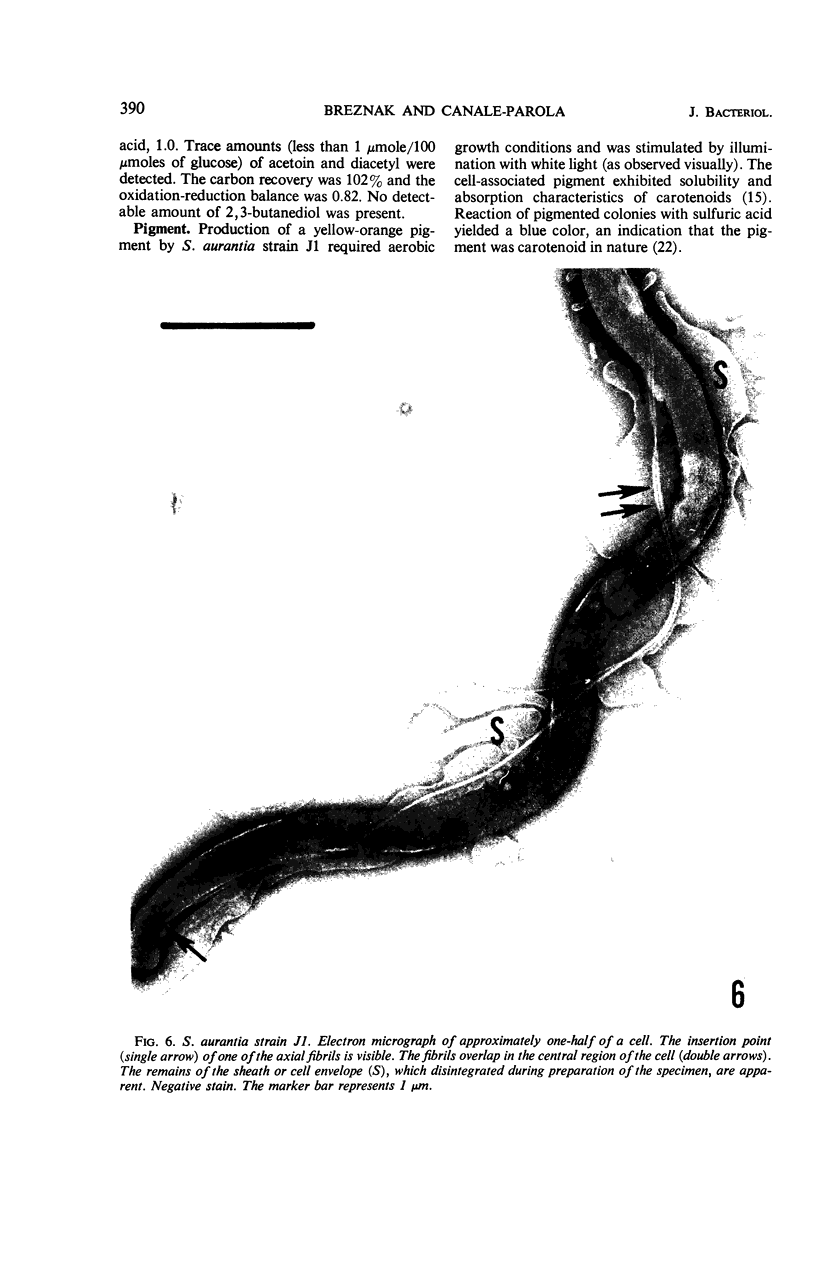
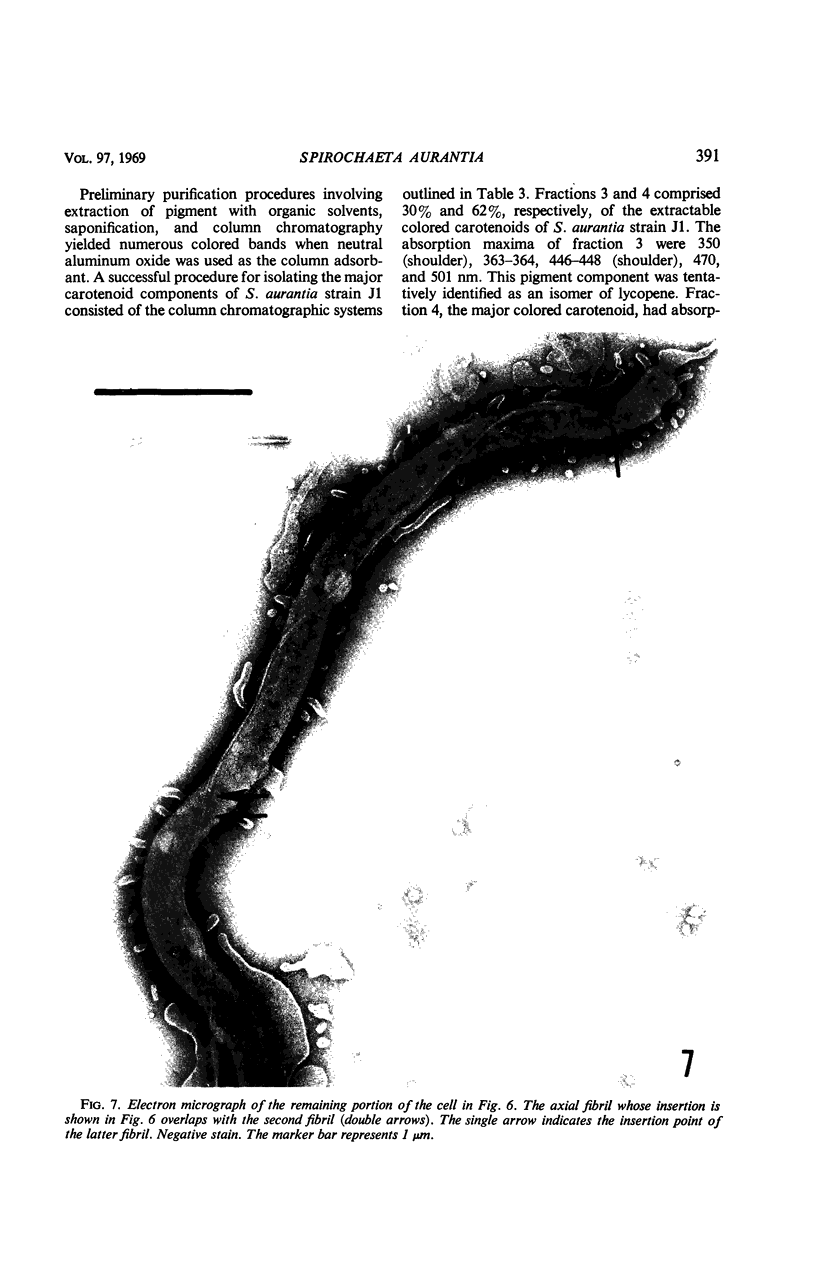
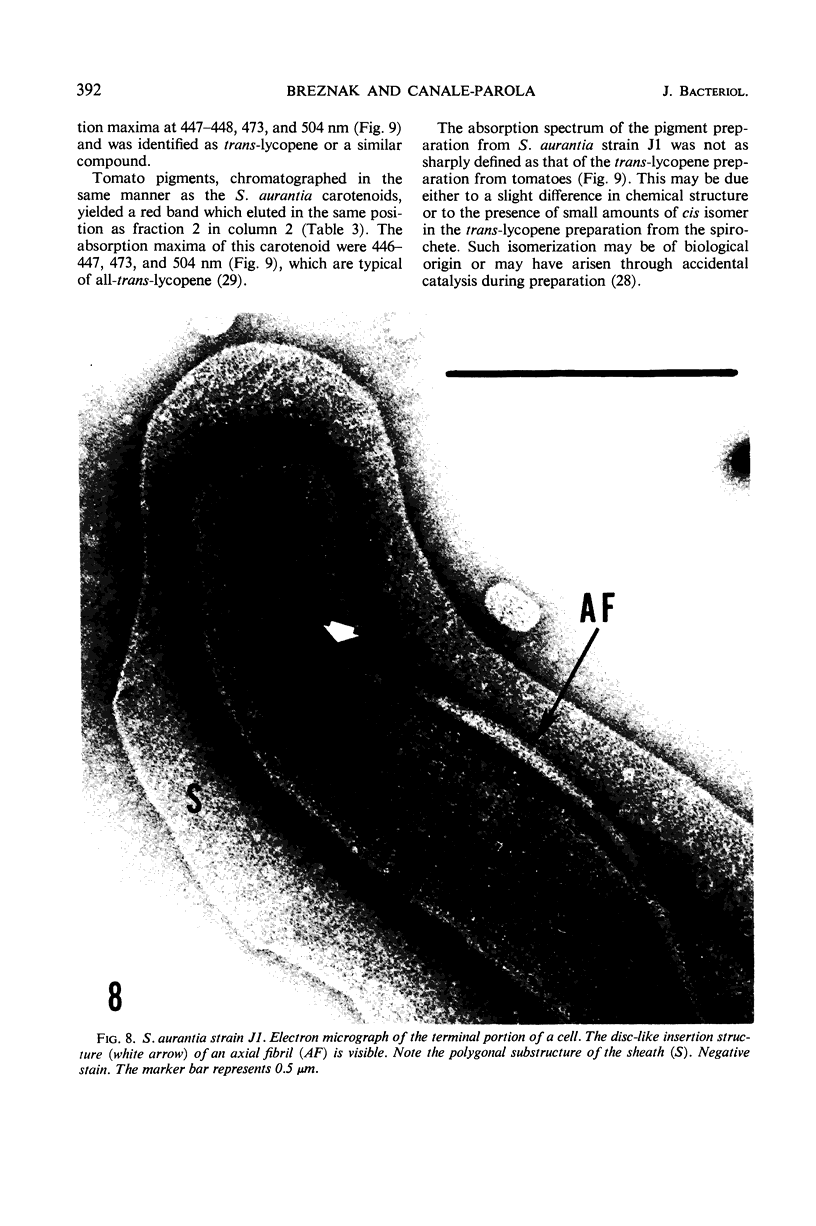
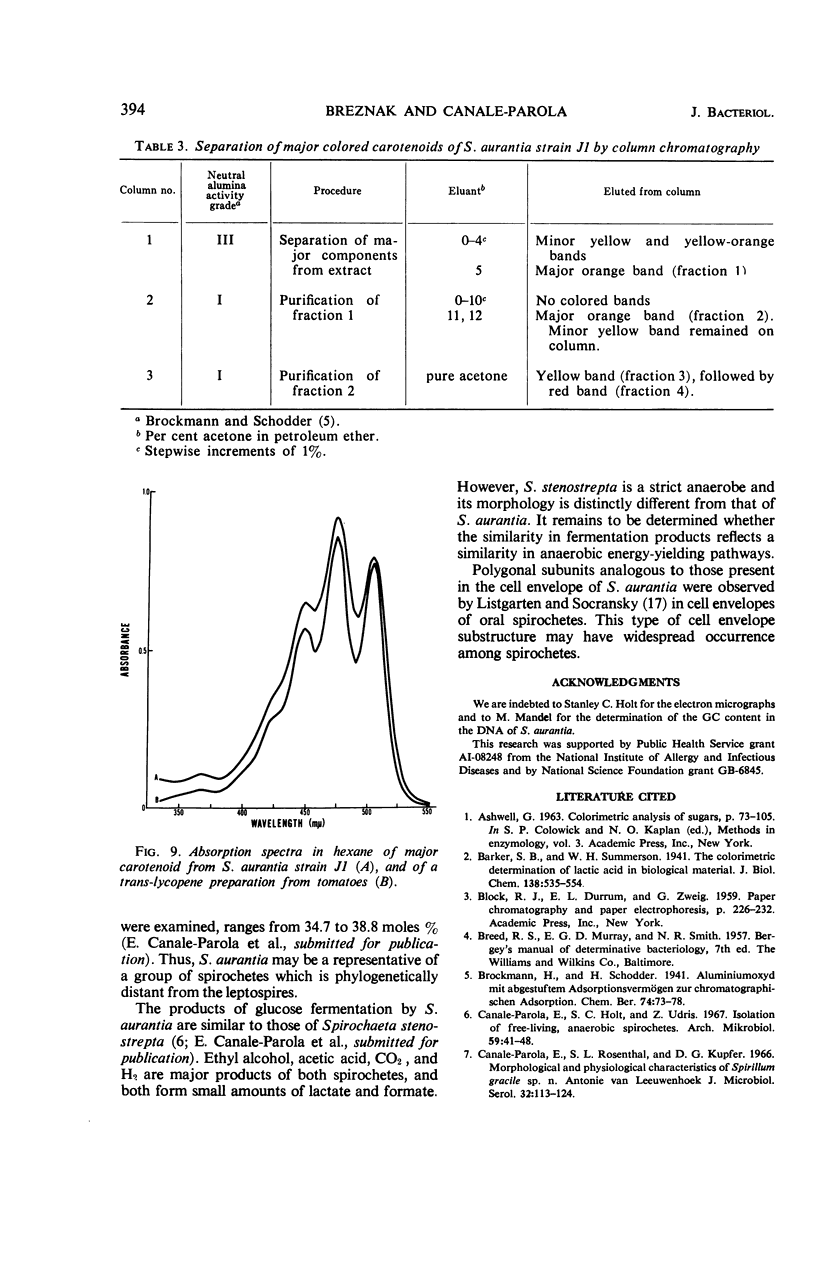
A strain of Spirochaeta aurantia was isolated from mud by a procedure involving migration of the organisms through cellulose ester filter discs (0.3-μm pore diameter) onto the surface of culture plates. The helical cells measured 0.3 by 10 to 20 μm during exponential growth. Electron microscopy showed the presence of two subterminally inserted axial fibrils partially overlapping in a 1-2-1 arrangement. An outer envelope, exhibiting a polygonal substructure, was observed. The spirochete grew either aerobically or anaerobically, with aerobic yields of 9.8 × 108 cells per ml and anaerobic yields of 3.0 × 108 cells per ml. The organism used carbohydrates, but not amino acids, as energy sources. Amino acids served as sole nitrogen sources, whereas inorganic ammonium salts did not. The presence of biotin and thiamine in the medium was required for growth. Growing cells fermented maltose mainly to carbon dioxide, hydrogen, ethyl alcohol, and acetic acid. Small amounts of formic and lactic acids, acetoin, and diacetyl were produced. Cells of S. aurantia growing aerobically produced a yellow-orange pigment. Chemical analysis indicated that the pigment was carotenoid in nature, its main component being lycopene or a similar compound. S. aurantia is not closely related to the leptospires, since it lacks both the hemolytic antigen and the hooked ends typical of the latter organisms. Furthermore, the guanine plus cytosine content in the deoxyribonucleic acid of S. aurantia (66.8 moles%) differs drastically from that of leptospires.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COX C. D., ALEXANDER A. D., MURPHY L. C. Evaluation of the hemolytic test in the serodiagnosis of human leptospirosis. J Infect Dis. 1957 Sep-Oct;101(2):210–218. doi: 10.1093/infdis/101.2.210. [DOI] [PubMed] [Google Scholar]
- COX C. D. Standardization and stabilization of an extract from Leptospira biflexa and its use in the hemolytic test for leptospirosis. J Infect Dis. 1957 Sep-Oct;101(2):203–209. doi: 10.1093/infdis/101.2.203. [DOI] [PubMed] [Google Scholar]
- Canale-Parola E., Holt S. C., Udris Z. Isolation of free-living, anaerobic spirochetes. Arch Mikrobiol. 1967;59(1):41–48. doi: 10.1007/BF00406315. [DOI] [PubMed] [Google Scholar]
- Canale-Parola E., Rosenthal S. L., Kupfer D. G. Morphological and physiological characteristics of Spirillum gracile sp. n. Antonie Van Leeuwenhoek. 1966;32(2):113–124. doi: 10.1007/BF02097451. [DOI] [PubMed] [Google Scholar]
- JENSEN S. L., COHEN-BAZIRE G., NAKAYAMA T. O., STANIER R. Y. The path of carotenoid synthesis in a photosynthetic bacterium. Biochim Biophys Acta. 1958 Sep;29(3):477–498. doi: 10.1016/0006-3002(58)90003-9. [DOI] [PubMed] [Google Scholar]
- LISTGARTEN M. A., SOCRANSKY S. S. ELECTRON MICROSCOPY OF AXIAL FIBRILS, OUTER ENVELOPE, AND CELL DIVISION OF CERTAIN ORAL SPIROCHETES. J Bacteriol. 1964 Oct;88:1087–1103. doi: 10.1128/jb.88.4.1087-1103.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M. Deoxyribonucleic acid base composition in the genus Pseudomonas. J Gen Microbiol. 1966 May;43(2):273–292. doi: 10.1099/00221287-43-2-273. [DOI] [PubMed] [Google Scholar]
- Schmidt K., Pfennig N., Jensen S. L. Carotenoids of Thiorhodaceae IV. The carotenoid composition of 25 pure isolates. Arch Mikrobiol. 1965 Oct 14;52(2):132–146. [PubMed] [Google Scholar]
- VELDKAMP H. Isolation and characteristics of Treponema zuelzerae nov. spec., and anaerobic, free-living spirochete. Antonie Van Leeuwenhoek. 1960;26:103–125. doi: 10.1007/BF02538999. [DOI] [PubMed] [Google Scholar]
- Weeks O. B., Garner R. J. Biosynthesis of carotenoids in Flavobacterium dehydrogenans Arnaudi. Arch Biochem Biophys. 1967 Jul;121(1):35–49. doi: 10.1016/0003-9861(67)90007-0. [DOI] [PubMed] [Google Scholar]