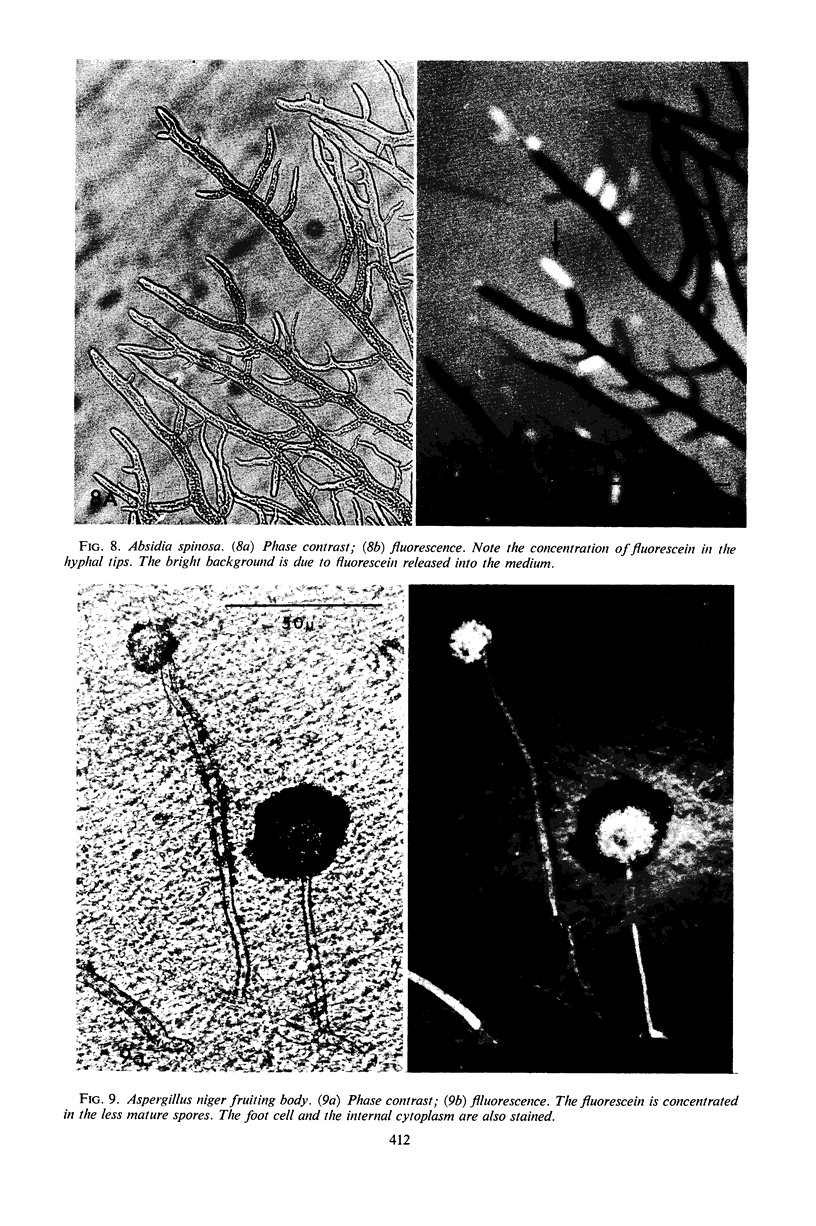
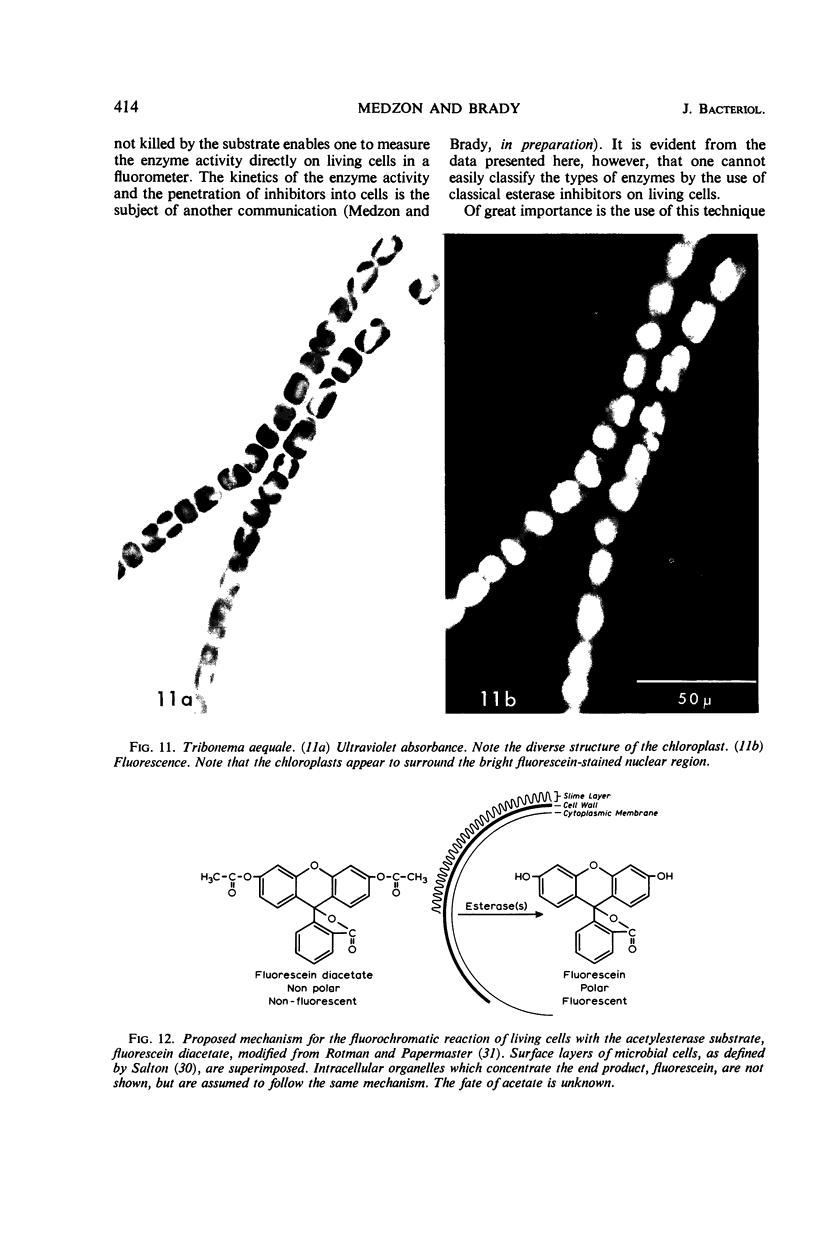
Abstract
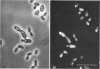
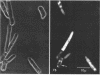
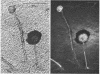
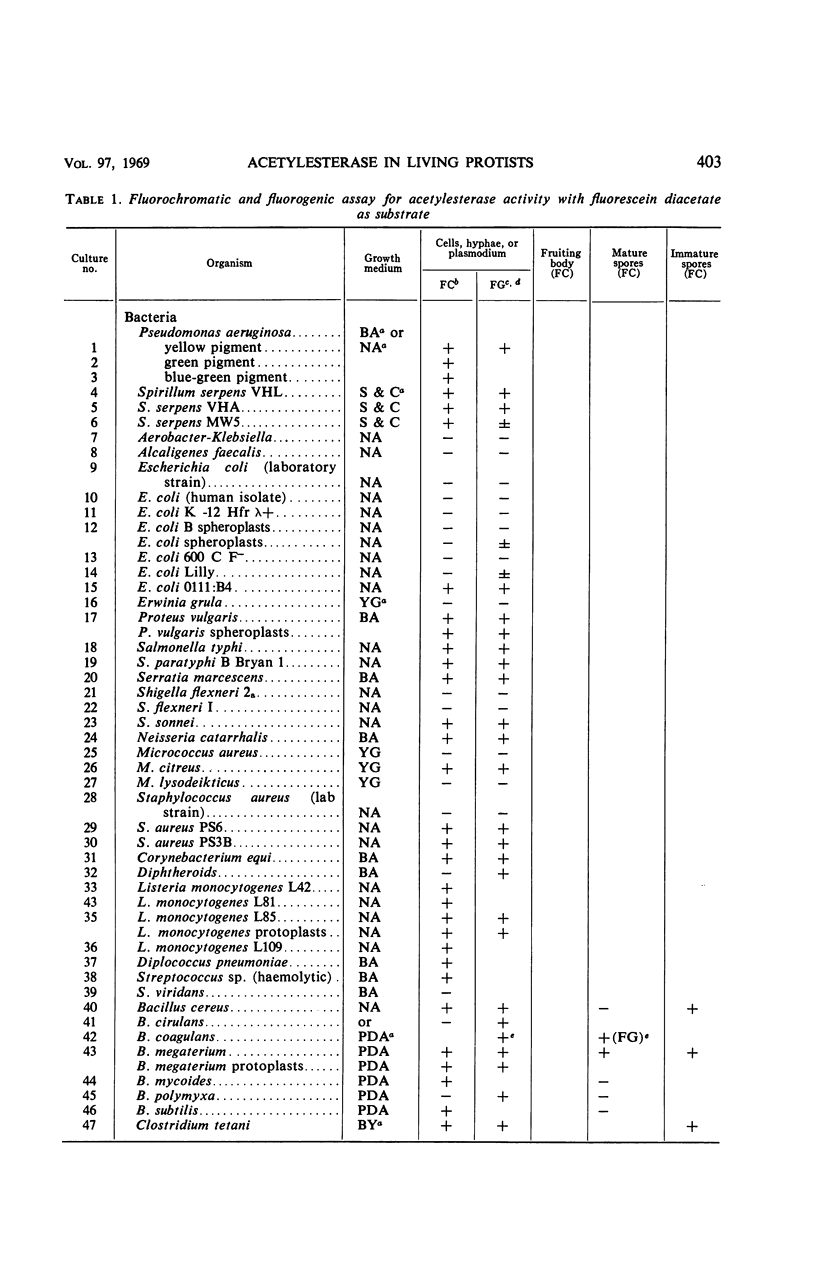
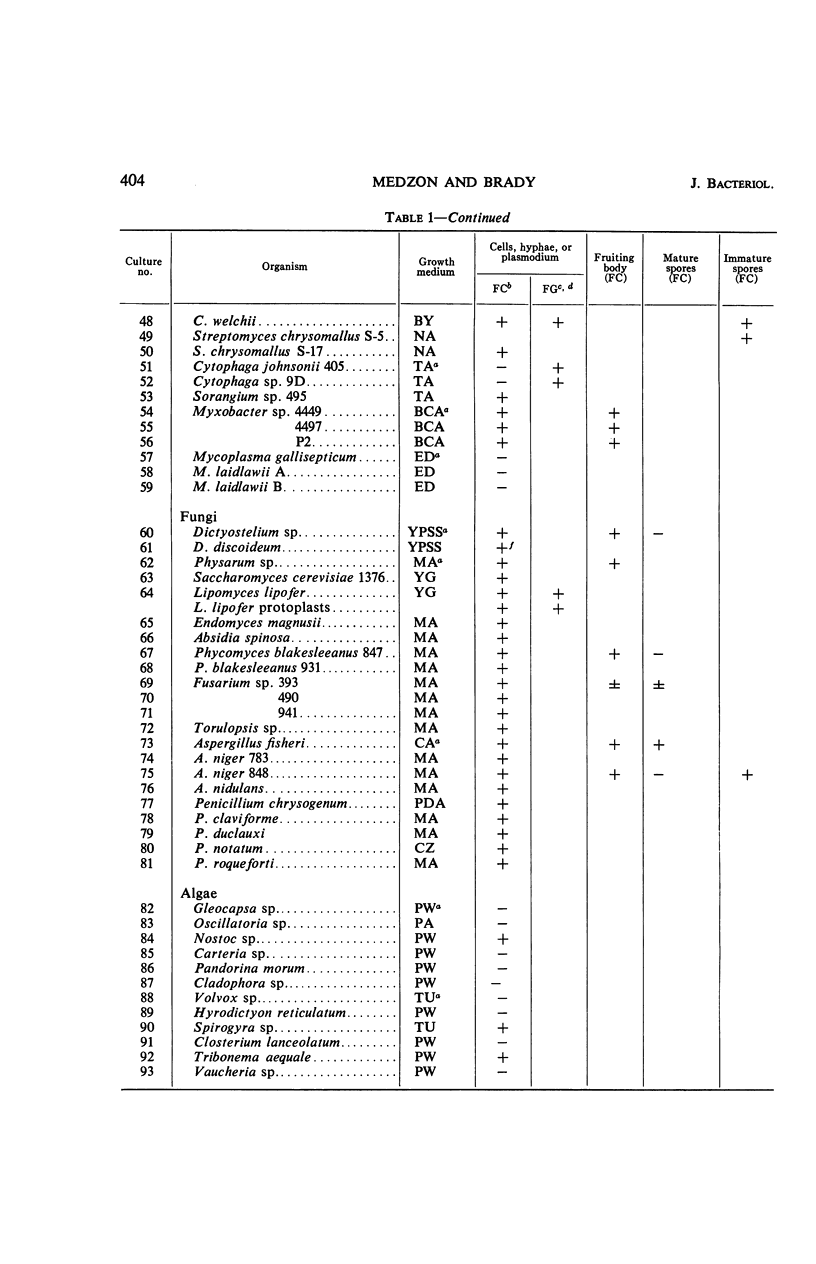
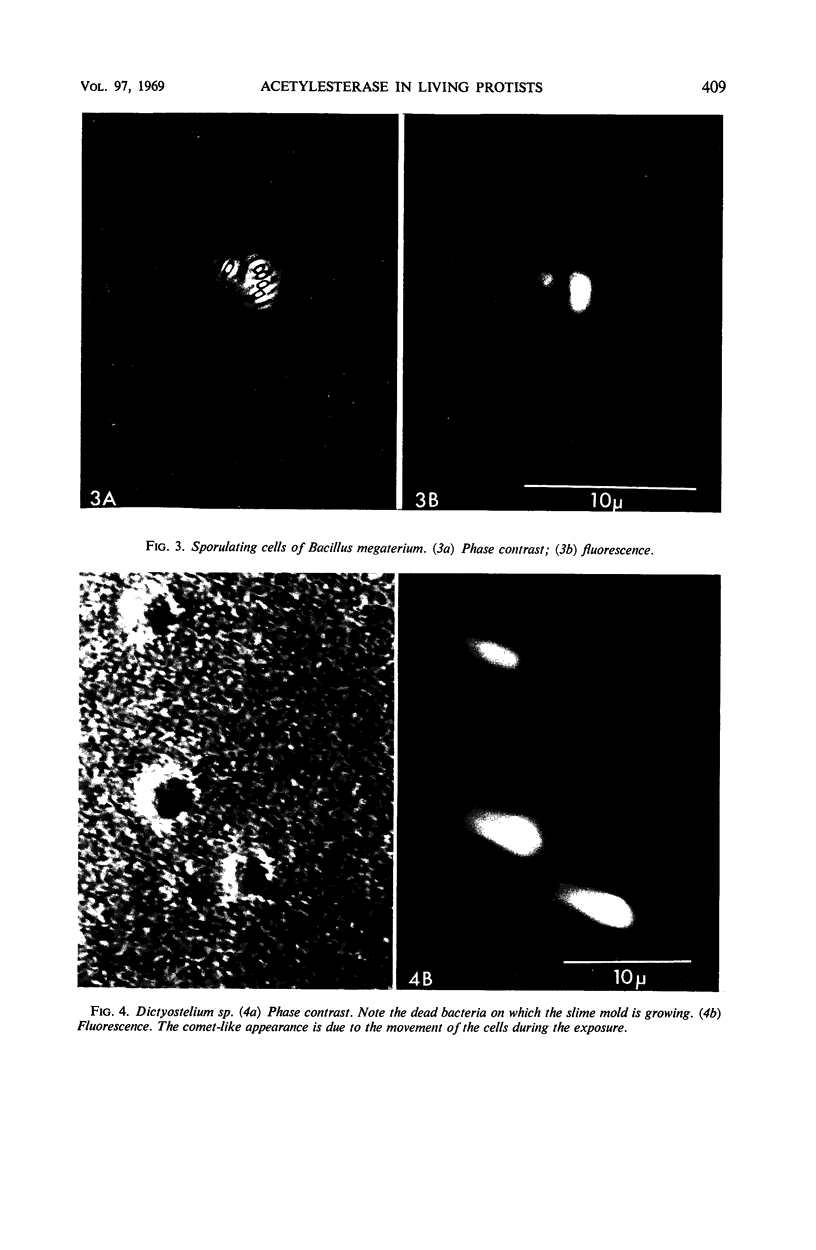
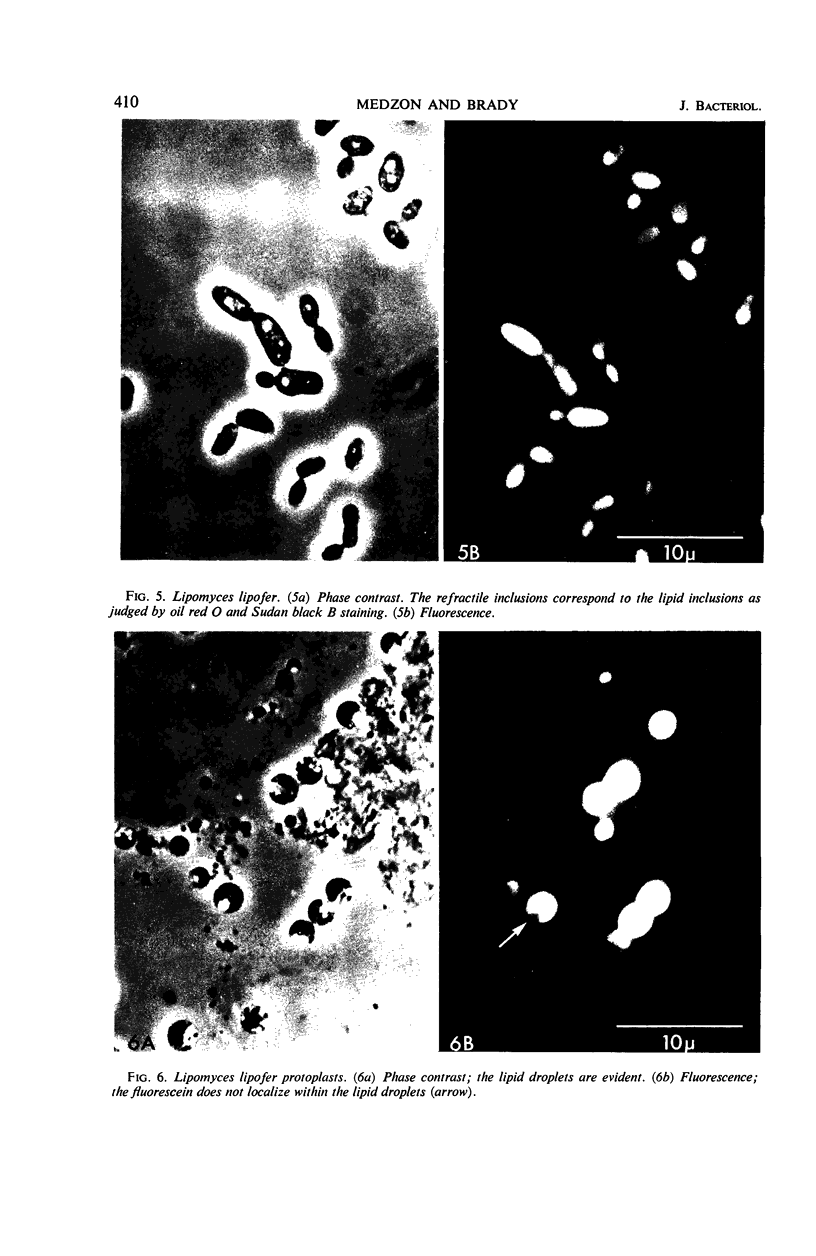
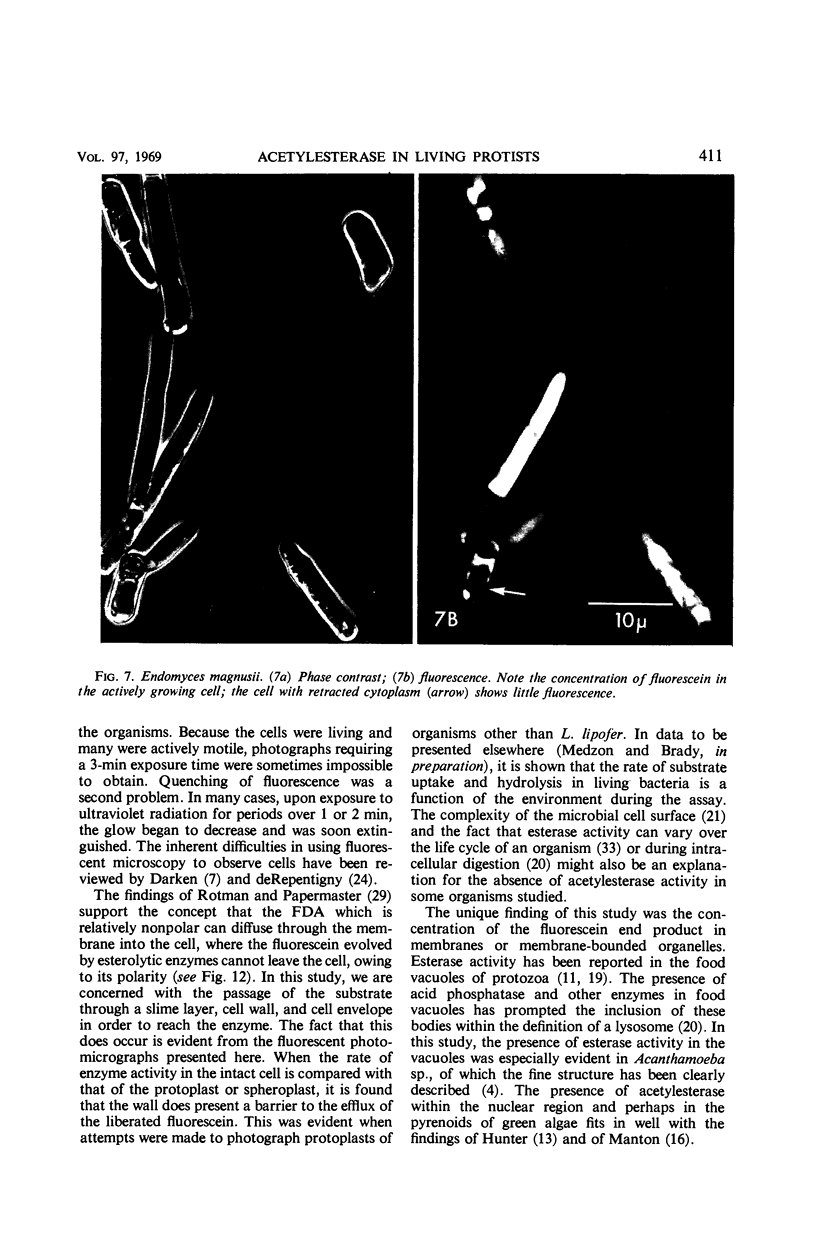
The fluorogenic acetylesterase (acetic ester hydrolase EC 3.1.1.6.) substrate, fluorescein diacetate, was used to measure enzyme activity in living protist cells. The visual enzyme assay was done by monitoring fluorochromasia by fluorescent microscopy. Quantitative fluorogenic assays were done by measuring the evolved fluorescein in a fluorometer. Of 59 strains of bacteria, 35 were fluorochromatically positive. Eight of the fluorochromatically negative strains were fluorogenically positive. Of 22 strains of slime molds and fungi, all were fluorochromatically positive. Three out of 12 different algae were fluorochromatically positive. Several unidentified protozoa were also fluorochromatically positive. Four out of six protozoa were fluorochromatically positive. Structures of special interest showing acetylesterase activity were: the growing hyphal tips of fungi, the vacuolated areas of yeast and protozoa, newly formed bacterial spores or immature fungal spores, “mesosome-like” bodies in Bacillus megaterium, and the cell membrane and nuclear region of green algae. Yeast protoplasts and bacterial protoplasts and spheroplasts were fluorochromatically positive when derived from positive cells and negative when derived from negative cells. There was no correlation between the possession of a capsule and acetylesterase activity. There was no effect on the viability of bacterial cells incubated in the presence of fluorescein diacetate. Paraoxon inhibited bacterial and yeast enzyme at 10−5m. Eserine (10−5m) and Paraoxon (10−7m) inhibited B. megaterium enzyme. Sodium acetate at 10−2m did not inhibit bacterial enzyme. The implications of these findings on the location and expression of esterase activity in living cells are discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN S. L. Genetic control of the esterases in the Protozoan Tetrahymena pyriformis. Ann N Y Acad Sci. 1961 Nov 2;94:753–773. doi: 10.1111/j.1749-6632.1961.tb35571.x. [DOI] [PubMed] [Google Scholar]
- Baillie A., Thomson R. O., Batty I., Walker P. D. Some preliminary observations on the location of esterases in Bacillus cereus. J Appl Bacteriol. 1967 Aug;30(2):312–316. doi: 10.1111/j.1365-2672.1967.tb00302.x. [DOI] [PubMed] [Google Scholar]
- COHEN J. A., OOSTERBAAN R. A., JANSZ H. S., BERENDS F. The active site of esterases. J Cell Comp Physiol. 1959 Dec;54:231–244. doi: 10.1002/jcp.1030540419. [DOI] [PubMed] [Google Scholar]
- DARKEN M. A. Microbiological process report. Natural and induced fluorescence in microscopic organisms. Appl Microbiol. 1961 Jul;9:354–360. doi: 10.1128/am.9.4.354-360.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Participation of the cytoplasmic membrane in the growth and spore fromation of bacilli. J Biophys Biochem Cytol. 1960 Oct;8:507–528. doi: 10.1083/jcb.8.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B. K., Murray R. G. Fine structure of Listeria monocytogenes in relation to protoplast formation. J Bacteriol. 1967 Jan;93(1):411–426. doi: 10.1128/jb.93.1.411-426.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEICK H. M., STEWART H. B. PREPARATION AND PROPERTIES OF PROTOPLASTS FROM LIPOMYCES LIPOFER. Can J Biochem. 1965 May;43:549–560. doi: 10.1139/o65-065. [DOI] [PubMed] [Google Scholar]
- KESTON A. S., BRANDT R. THE FLUOROMETRIC ANALYSIS OF ULTRAMICRO QUANTITIES OF HYDROGEN PEROXIDE. Anal Biochem. 1965 Apr;11:1–5. doi: 10.1016/0003-2697(65)90034-5. [DOI] [PubMed] [Google Scholar]
- Lederberg J. BACTERIAL PROTOPLASTS INDUCED BY PENICILLIN. Proc Natl Acad Sci U S A. 1956 Sep;42(9):574–577. doi: 10.1073/pnas.42.9.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLER M., TOTH J., TORO I. Studies on feeding and digestion in protozoa. IV. Acid phosphatase and nonspecific esterase activity of food vacuoles in amoeba proteus. Acta Biol Acad Sci Hung. 1962;13:105–116. [PubMed] [Google Scholar]
- Matile P., Wiemken A. The vacuole as the lysosome of the yeast cell. Arch Mikrobiol. 1967 Feb 20;56(2):148–155. doi: 10.1007/BF00408765. [DOI] [PubMed] [Google Scholar]
- Peberdy J. F., Turner M. The esterases of Mortierella ramanniana in relation to taxonomy. J Gen Microbiol. 1968 Apr;51(2):303–312. doi: 10.1099/00221287-51-2-303. [DOI] [PubMed] [Google Scholar]
- RODWELL A. W., ABBOT A. The function of glycerol, cholesterol and long-chain fatty acids in the nutrition of Mycoplasma mycoides. J Gen Microbiol. 1961 Jun;25:201–214. doi: 10.1099/00221287-25-2-201. [DOI] [PubMed] [Google Scholar]
- Roberts T. L., Rosenkrantz H. Acetyl esterase in Bacillus cereus spores. Can J Biochem. 1966 Jun;44(6):671–675. doi: 10.1139/o66-084. [DOI] [PubMed] [Google Scholar]
- Roberts T. L., Rosenkrantz H. Esterase activity in the green alga Trebouxia. Tex Rep Biol Med. 1967 Fall;25(3):432–436. [PubMed] [Google Scholar]
- Roberts T. L., Rosenkrantz H. Lipase and acetyl esterase activities in intact Bacillus coagulans spores. Can J Biochem. 1966 Jun;44(6):677–685. doi: 10.1139/o66-085. [DOI] [PubMed] [Google Scholar]
- Rotman B., Papermaster B. W. Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proc Natl Acad Sci U S A. 1966 Jan;55(1):134–141. doi: 10.1073/pnas.55.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIERRA G. Studies on bacterial esterases. I. Differentiation of a lipase and two ali-esterases during the growth of Pseudomonas aeruginosa and some observations on growth and esterase inhibition. Antonie Van Leeuwenhoek. 1957;23(3-4):241–265. doi: 10.1007/BF02545877. [DOI] [PubMed] [Google Scholar]
- STOCK A. H., URIEL J., GRABAR P. Esterase in extracellular concentrates of group A streptococci and the homologous antibody. Nature. 1961 Nov 4;192:434–435. doi: 10.1038/192434a0. [DOI] [PubMed] [Google Scholar]
- Salton M. R. Structure and function of bacterial cell membranes. Annu Rev Microbiol. 1967;21:417–442. doi: 10.1146/annurev.mi.21.100167.002221. [DOI] [PubMed] [Google Scholar]
- Sierra G. Dissociatiion of esterase from proteinase activity of Bacillus subtilis spores. Can J Microbiol. 1967 Jun;13(6):673–678. doi: 10.1139/m67-089. [DOI] [PubMed] [Google Scholar]
- WEIBULL C. The isolation of protoplasts from Bacillus megaterium by controlled treatment with lysozyme. J Bacteriol. 1953 Dec;66(6):688–695. doi: 10.1128/jb.66.6.688-695.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P. D., Thomson R. O., Baillie A. Fine structure of clostridia with special reference to the location of antigens and enzymes. J Appl Bacteriol. 1967 Dec;30(3):444–449. doi: 10.1111/j.1365-2672.1967.tb00322.x. [DOI] [PubMed] [Google Scholar]