Abstract
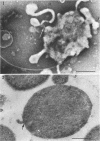
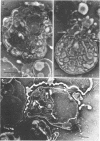
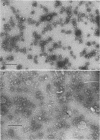
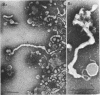
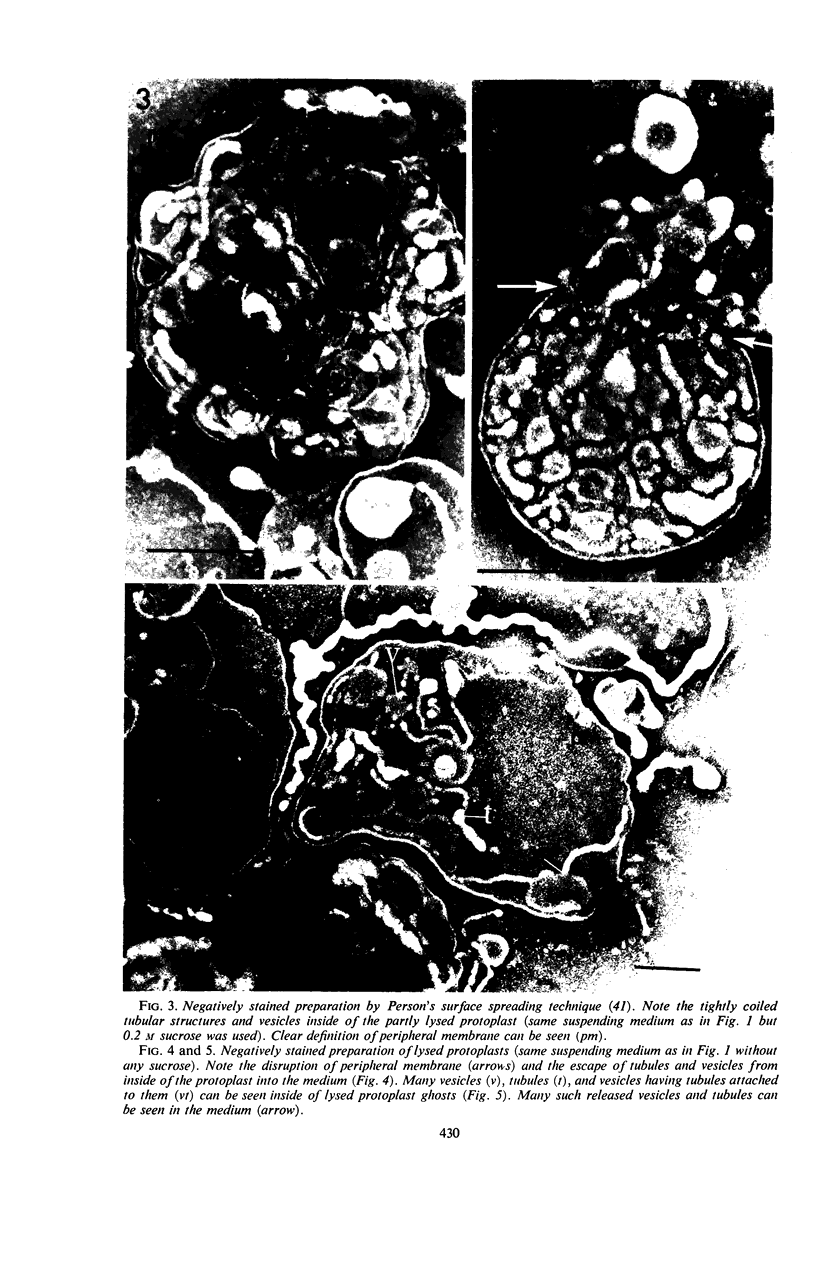
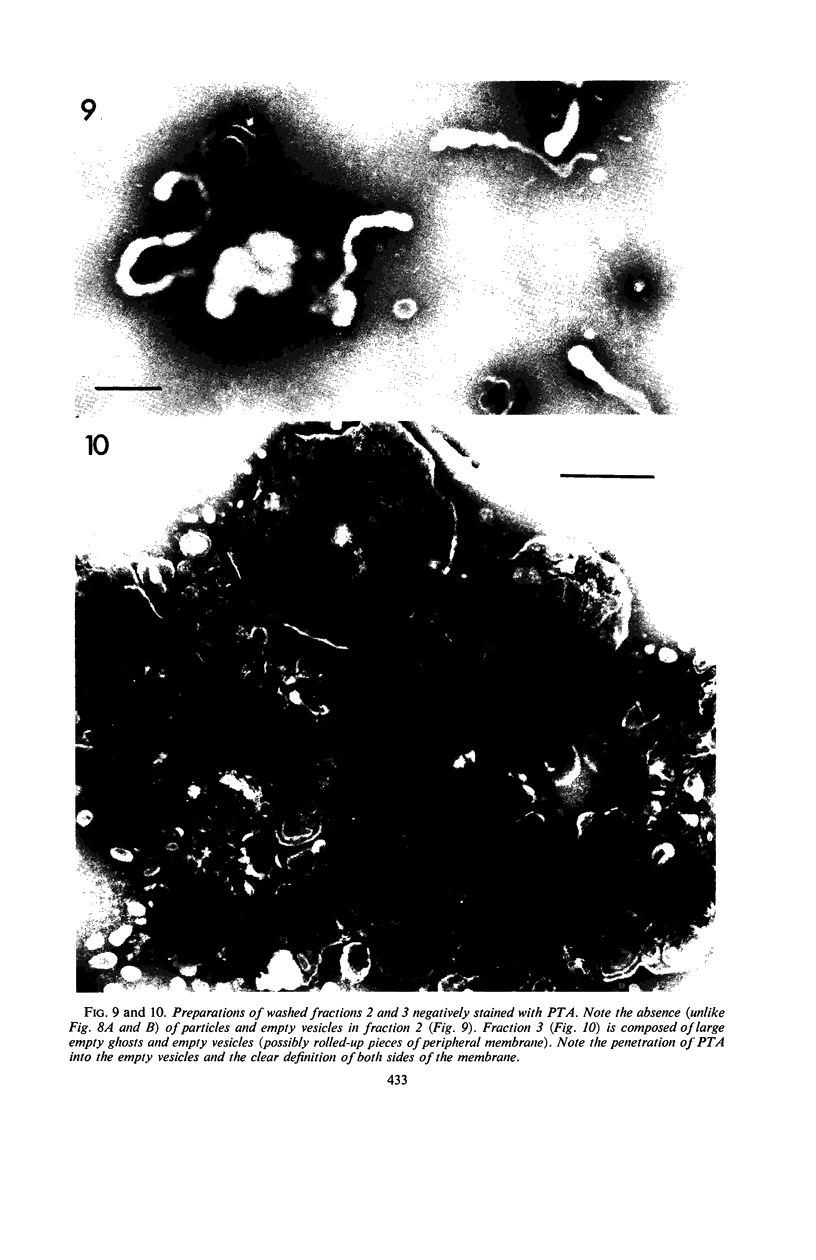
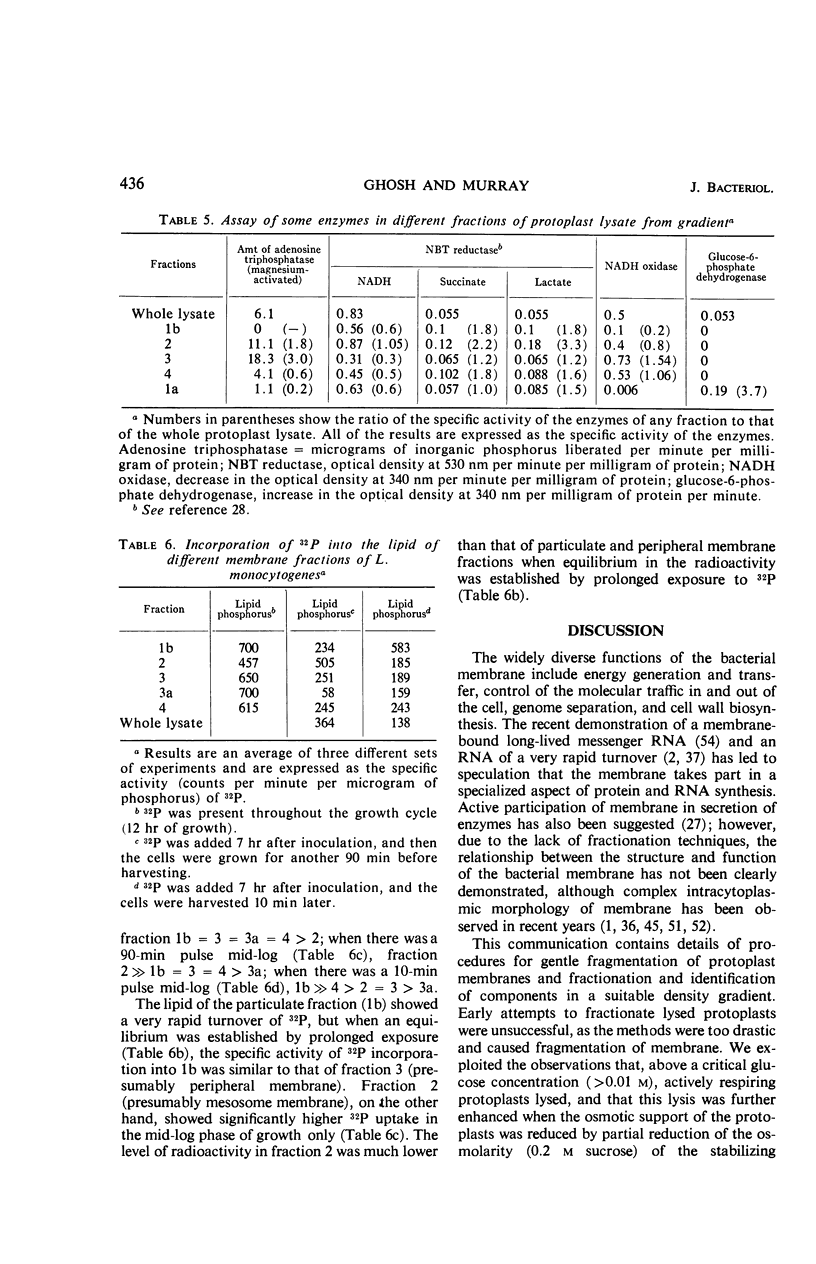
Protoplasts of Listeria monocytogenes strain 42 were fractionated after control lysis on a Ficoll (a polysucrose) density gradient. Visually, five zones could be recognized in the gradient. The first one was composed of amorphous cytoplasmic solutes (fraction 1a) and a mixture of particles (fraction 1b). These were: (i) light particles that were lipase-sensitive and composed of six subunits and (ii) heavy particles, sensitive to ribonuclease and devoid of fine structure. The second zone consisted of tubules and vesicles still harboring cytoplasmic components (fraction 2), whereas the third zone contained only empty vesicles and protoplast ghosts (fraction 3). The material congregating into the fourth zone was morphologically identical to that of the third (fraction 3a). The fifth and heaviest zone contained a mixture of (i) particles without any substructure and (ii) partly lysed protoplasts (fraction 4). Fractions 1b and 4 were the richest in nucleic acids (ribonucleic acid, 11.4 and 9.4%, respectively; deoxyribonucleic acid, 5.1 and 4.8%, respectively), whereas fraction 1b had the highest protein contents (74.6%). Phospholipids were mainly found in fractions 2 and 3. Except for fraction 1, all materials contained significant amounts of protein-bound phosphorus. The main concentrations of four enzymes were: glucose-6-phosphate dehydrogenase (fraction 1a); adenosine triphosphatase and reduced nicotinamide adenine diphosphate oxidase (fraction 3); nitro blue tetrazolium chloride reductase (fraction 2). Fractionation of strain 42 after addition of 32P during the mid-log phase of growth revealed that the radio-activity was mainly detected in fraction 1b, when growth in the presence of the marker was allowed for 10 min, and in fraction 2, when growth was allowed for 90 min. The vesicles of fraction 2, often tubular, are probably of mesosomal origin, whereas those of fraction 3, which are always spherical, represent, most likely, the bulk of the cell plasma membrane. Our data showed slight chemical differences between these two fractions, but the differences in enzymatic activities and lipid-phosphorus incorporation during long pulse experiments were most dramatic.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAM D. ELECTRON MICROSCOPE OBSERVATIONS ON INTACT CELLS, PROTOPLASTS, AND THE CYTOPLASMIC MEMBRANE OF BACILLUS STEAROTHERMOPHILUS. J Bacteriol. 1965 Mar;89:855–873. doi: 10.1128/jb.89.3.855-873.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ABRAMS A., NIELSEN L., THAEMERT J. RAPIDLY SYNTHESIZED RIBONUCLEIC ACID IN MEMBRANE GHOSTS FROM STREPTOCOCCUS FECALIS PROTOPLASTS. Biochim Biophys Acta. 1964 Feb 17;80:325–337. doi: 10.1016/0926-6550(64)90104-5. [DOI] [PubMed] [Google Scholar]
- CHAPMAN G. B., HILLIER J. Electron microscopy of ultra-thin sections of bacteria I. Cellular division in Bacillus cereus. J Bacteriol. 1953 Sep;66(3):362–373. doi: 10.1128/jb.66.3.362-373.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS M. R., STEVENS R. W. FINE STRUCTURE OF LISTERIA MONOCYTOGENES. J Bacteriol. 1963 Sep;86:414–428. doi: 10.1128/jb.86.3.414-428.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Participation of the cytoplasmic membrane in the growth and spore fromation of bacilli. J Biophys Biochem Cytol. 1960 Oct;8:507–528. doi: 10.1083/jcb.8.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. FATE OF THE MESOSOMES OF BACILLUS MEGATERIUM DURING PROTOPLASTING. J Bacteriol. 1964 Jun;87:1483–1491. doi: 10.1128/jb.87.6.1483-1491.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandes B., Chaix P., Ryter A. Localisation des cytochromes de Bacillus subtilis dans les structures mésosomiques. C R Acad Sci Hebd Seances Acad Sci D. 1966 Nov 21;263(21):1632–1635. [PubMed] [Google Scholar]
- Fitz-James P. C. DISCUSSION. Bacteriol Rev. 1965 Sep;29(3):293–298. doi: 10.1128/br.29.3.293-298.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz-James P., Hancock R. The initial structural lesion of penicillin action in Bacillus megaterium. J Cell Biol. 1965 Aug;26(2):657–667. doi: 10.1083/jcb.26.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B. K., Carroll K. K. Isolation, composition, and structure of membrane of Listeria monocytogenes. J Bacteriol. 1968 Feb;95(2):688–699. doi: 10.1128/jb.95.2.688-699.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B. K., Murray R. G. Fine structure of Listeria monocytogenes in relation to protoplast formation. J Bacteriol. 1967 Jan;93(1):411–426. doi: 10.1128/jb.93.1.411-426.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOKIN L. E., HOKIN M. R. Phosphoinositides and protein secretion in pancreas slices. J Biol Chem. 1958 Oct;233(4):805–810. [PubMed] [Google Scholar]
- LESTER R. L., SMITH A. L. Studies on the electron transport system. 28. The mode of reduction of tetrazolium salts by beef heart mitochondria; role of coenzyme Q and other lipids. Biochim Biophys Acta. 1961 Mar 4;47:475–496. doi: 10.1016/0006-3002(61)90543-1. [DOI] [PubMed] [Google Scholar]
- LOGAN J. E., MANNELL W. A., ROSSITER R. J. Estimation of nucleic acids in tissue from the nervous system. Biochem J. 1952 Jul;51(4):470–479. doi: 10.1042/bj0510470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MATHEWS M. M., SISTROM W. R. Intracellular location of carotenoid pigments and some respiratory enzymes in Sarcina lutea. J Bacteriol. 1959 Dec;78:778–787. doi: 10.1128/jb.78.6.778-787.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUDD S., KAWATA T., PAYNE J. I., SALL T., TAKAGI A. Plasma membranes and mitochondrial equivalents as functionally coordinated structures. Nature. 1961 Jan 7;189:79–80. doi: 10.1038/189079a0. [DOI] [PubMed] [Google Scholar]
- Matile P., Wiemken A. The vacuole as the lysosome of the yeast cell. Arch Mikrobiol. 1967 Feb 20;56(2):148–155. doi: 10.1007/BF00408765. [DOI] [PubMed] [Google Scholar]
- Munoz E., Freer J. H., Ellar D. J., Salton M. R. Membrane-associated ATPase activity from Micrococcus lysodeikticus. Biochim Biophys Acta. 1968 Apr 29;150(3):531–533. doi: 10.1016/0005-2736(68)90156-9. [DOI] [PubMed] [Google Scholar]
- Murray E. G., Ghosh B. K., Murray R. G. The interaction of guanofuracin and Listeria monocytogenes. Can J Microbiol. 1966 Apr;12(2):285–297. doi: 10.1139/m66-039. [DOI] [PubMed] [Google Scholar]
- NORTH R. J. SOME STRUCTURAL ASPECTS OF LISTERIA MONOCYTOGENES. J Ultrastruct Res. 1963 Oct;59:187–197. doi: 10.1016/s0022-5320(63)80001-5. [DOI] [PubMed] [Google Scholar]
- OGUR M., ROSEN G. The nucleic acids of plant tissues; the extraction and estimation of desoxypentose nucleic acid and pentose nucleic acid. Arch Biochem. 1950 Feb;25(2):262–276. [PubMed] [Google Scholar]
- Pangborn J., Marr A. G., Robrish S. A. LOCALIZATION OF RESPIRATORY ENZYMES IN INTRACYTOPLASMIC MEMBRANES OF AZOTOBACTER AGILIS. J Bacteriol. 1962 Oct;84(4):669–678. doi: 10.1128/jb.84.4.669-678.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons D. F., Williams G. R., Chance B. Characteristics of isolated and purified preparations of the outer and inner membranes of mitochondria. Ann N Y Acad Sci. 1966 Jul 14;137(2):643–666. doi: 10.1111/j.1749-6632.1966.tb50188.x. [DOI] [PubMed] [Google Scholar]
- RYTER A., JACOB F. ETUDE AU MICROSCOPE 'ELECTRONIQUE DE LA LIAISON ENTRE NOYAU ET M'ESOSOME CHEZ BACILLUS SUBTILIS. Ann Inst Pasteur (Paris) 1964 Sep;107:384–400. [PubMed] [Google Scholar]
- Reaveley D. A. The isolation and characterisation of cytoplasmic membranes and mesosomes of Bacillus licheniformis 6346. Biochem Biophys Res Commun. 1968 Mar 27;30(6):649–655. doi: 10.1016/0006-291x(68)90562-7. [DOI] [PubMed] [Google Scholar]
- SALTON M. R., CHAPMAN J. A. Isolation of the membranemesosome structures from Micrococcus lysodeikticus. J Ultrastruct Res. 1962 Jun;6:489–498. doi: 10.1016/s0022-5320(62)80004-5. [DOI] [PubMed] [Google Scholar]
- TERAI T., KAMAHORA T., YAMAMURA Y. Tellurite reductase from Mycobacterium avium. J Bacteriol. 1958 May;75(5):535–539. doi: 10.1128/jb.75.5.535-539.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN ITERSON, LEENE W. A CYTOCHEMICAL LOCALIZATION OF REDUCTIVE SITES IN A GRAM-POSITIVE BACTERIUM. TELLURITE REDUCTION IN BACILLUS SUBTILIS. J Cell Biol. 1964 Mar;20:361–375. doi: 10.1083/jcb.20.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANDERWINKEL E., MURRAY R. G. [Bacterial intracytoplasmic organelles and the site of oxidation-reduction activity]. J Ultrastruct Res. 1962 Aug;7:185–199. doi: 10.1016/s0022-5320(62)80035-5. [DOI] [PubMed] [Google Scholar]
- WEIBULL C. PLASMOLYSIS IN BACILLUS MEGATERIUM. J Bacteriol. 1965 Apr;89:1151–1154. doi: 10.1128/jb.89.4.1151-1154.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkin M. D. Protein synthesis by long-lived messenger ribonucleic acid in bacteria. Biochem J. 1966 Aug;100(2):501–506. doi: 10.1042/bj1000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Iterson W., Hoeniger J. F., Nijman van Zanten E. A "microtubule" in a bacterium. J Cell Biol. 1967 Jan;32(1):1–10. doi: 10.1083/jcb.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Iterson W. Symposium on the fine structure and replication of bacteria and their parts. II. Bacterial cytoplasm. Bacteriol Rev. 1965 Sep;29(3):299–325. doi: 10.1128/br.29.3.299-325.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]