Abstract
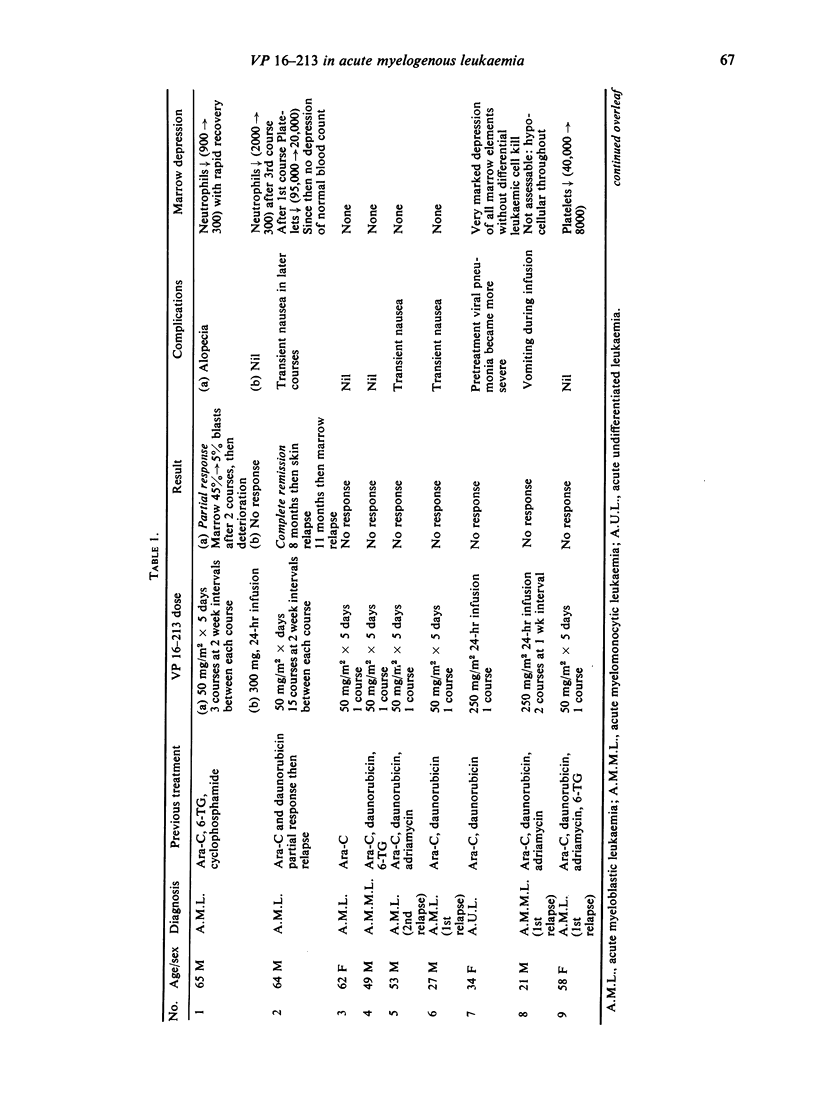
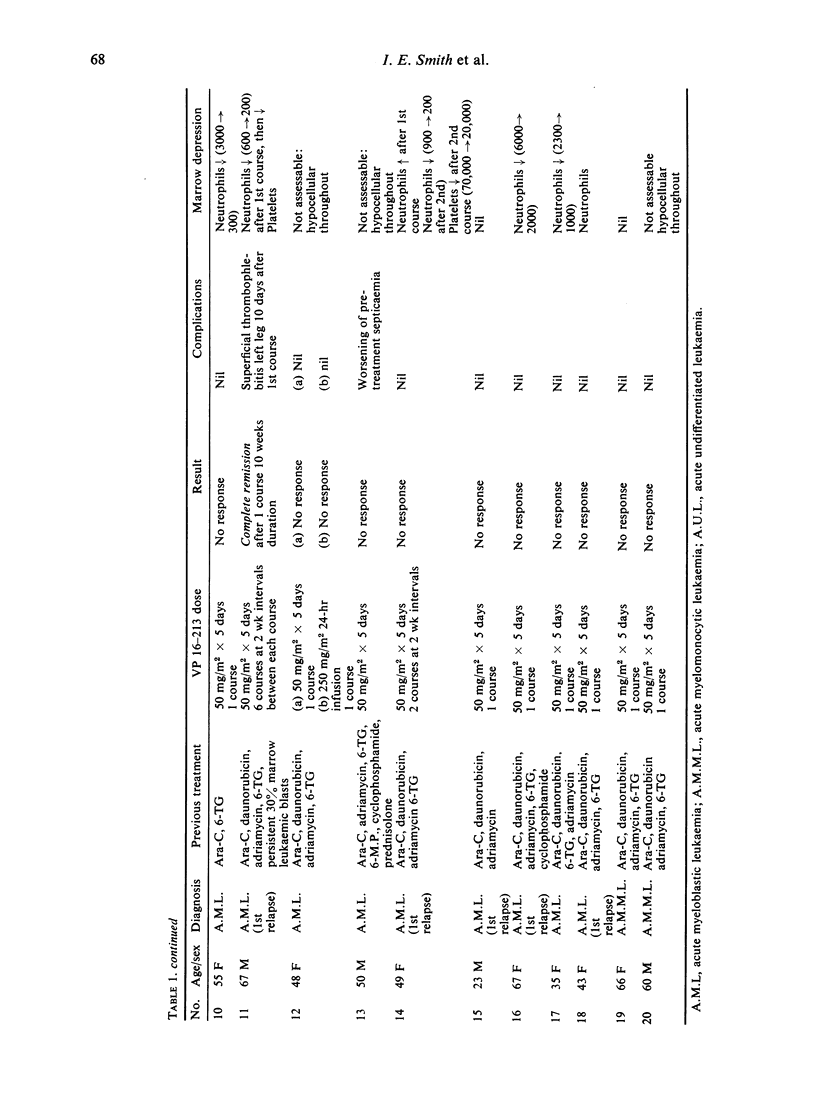
The use of the epipodophyllotoxin VP 16-213 is described in twenty patients with acute myelogenous leukaemia resistant to other chemotherapy. The drug was usually given in 5-day courses of 50 mg/m2 daily and occasionally in 24-hr infusions of 250 mg/m2, on the basis of its phase-activity.
Complete remission was achieved in only two patients: in one of these, remission was maintained with VP 16-213 for 8 months, and in the other for 10 weeks. A partial response was achieved in one other patient. Seventeen patients showed no response. No responses or remissions were achieved when the drug was used in a 24-hr infusion. Side effects were minimal, and the degree of marrow depression much less than for most other agents known to be active in acute myelogenous leukaemia. It was of interest that remission was achieved in one patient without the customary period of marrow hypoplasia. It is suggested that, although VP 16-213 appears to have minimal activity in the dosage used here, improvement might be sought by increasing the dosage, by scheduling the drug in a different way, or by using it in a combination chemotherapy regime.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruce W. R., Meeker B. E., Valeriote F. A. Comparison of the sensitivity of normal hematopoietic and transplanted lymphoma colony-forming cells to chemotherapeutic agents administered in vivo. J Natl Cancer Inst. 1966 Aug;37(2):233–245. [PubMed] [Google Scholar]
- Goldie J. H., Price L. A., Harrap K. R. Methotrexate toxicity: correlation with duration of administration, plasma levels, dose and excretion pattern. Eur J Cancer. 1972 Aug;8(4):409–414. doi: 10.1016/0014-2964(72)90125-9. [DOI] [PubMed] [Google Scholar]
- Stähelin H. 4'-Demethyl-epipodophyllotoxin thenylidene glucoside (VM 26), a podophyllum compound with a new mechanism of action. Eur J Cancer. 1970 Aug;6(4):303–311. doi: 10.1016/0014-2964(70)90095-2. [DOI] [PubMed] [Google Scholar]


