Abstract
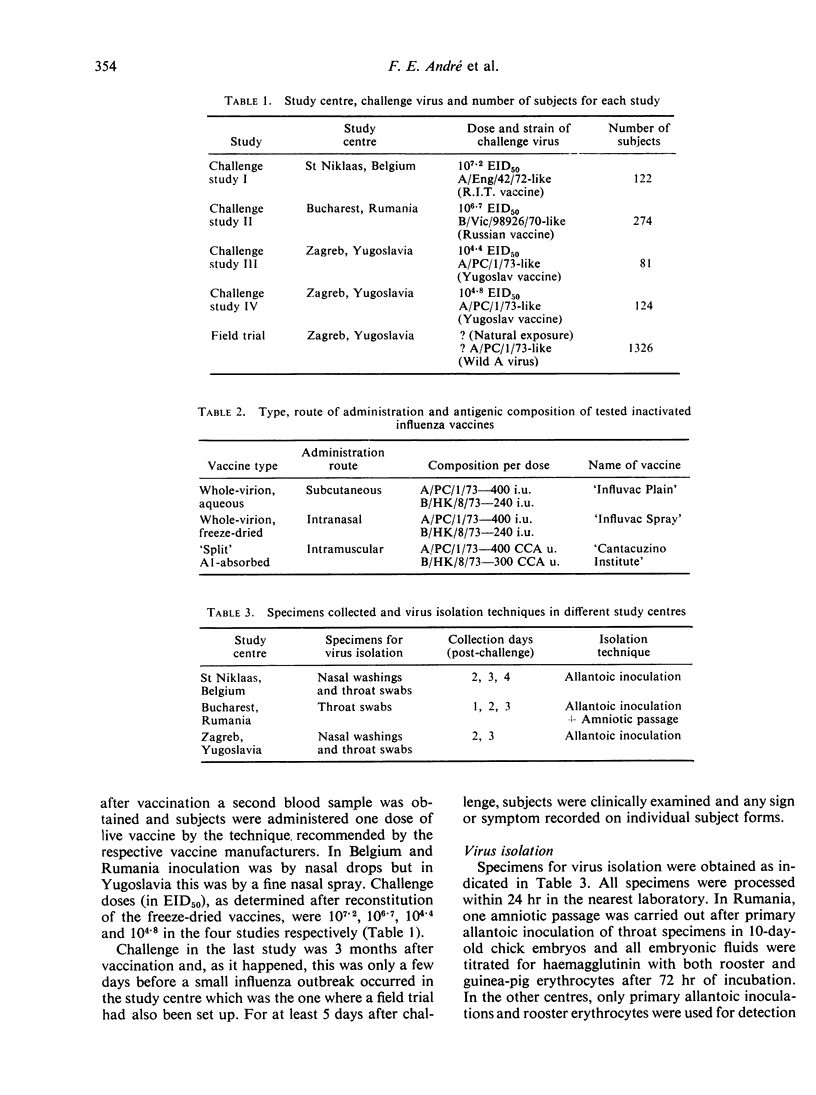
Four placebo-controlled double-blind studies on the protective efficacies of a freeze-dried aerosol and an injectable whole-virion inactivated influenza vaccine, each containing 400 i.u. A/Port Chalmers/1/73 (H3N2) and 240 i.u. B/Hong Kong/8/73 per dose, were carried out on a total of 601 subjects using three different live influenza vaccines as challenge virus. In the second of these studies a tween-ether ‘split’ aluminium-absorbed injectable vaccine containing 400 CCA units A/Port Chalmers/1/73 (H3N2) and 300 CCA (chick cell agglutination) units B/Hong Kong/8/73 was also tested. Challenge in the first three studies occurred 3 weeks after vaccination whereas in the last study it took place 3 months after vaccination. The live vaccines were recommended for the 1974-75 season in Belgium, Rumania and Yugoslavia in which countries the studies were performed and contained an A/England/42/72 (H3N2)-like strain, a B/Victoria/98926/70-like strain and an A/Port Chalmers/1/73 (H3N2)-like strain respectively. The latter vaccine was used in both of the last two studies. Infection with the vaccine strain was diagnosed by virus isolation and/or serological response after challenge since this produced negligible clinical signs and symptoms.
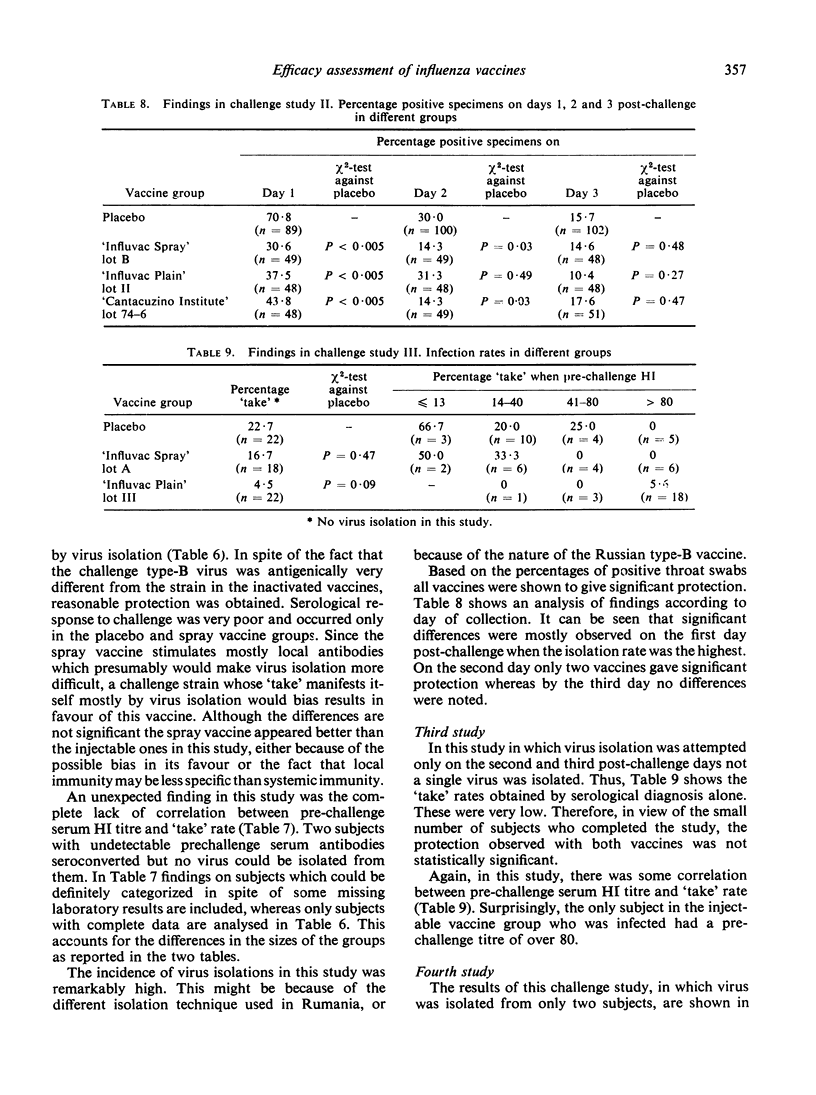
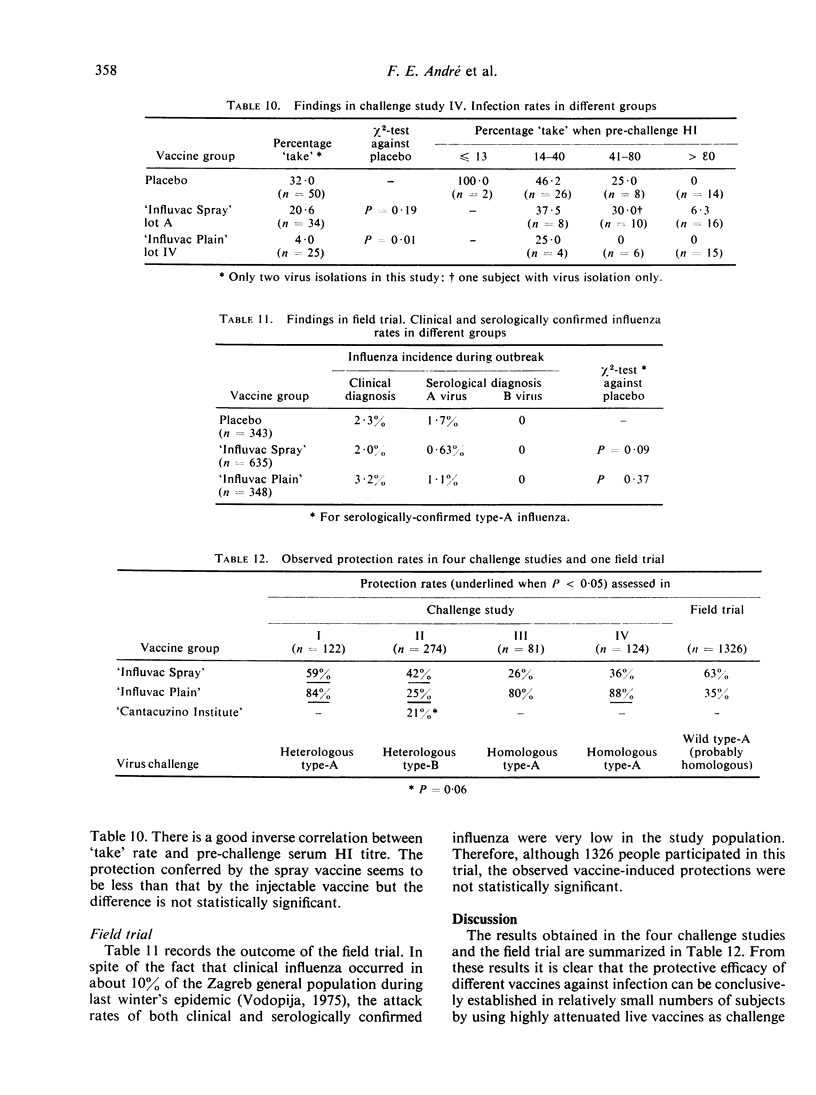
The aerosol vaccine showed infection protection rates of 59% (P = 0·0075), 42% (P < 0·005), 26% (P = 0·47) and 36% (P = 0·19) and the whole-virion vaccine rates of 84% (P < 0·005), 25% (P = 0·025), 80% (P = 0·09) and 88% (P = 0·01) respectively. The tween-ether ‘split’ vaccine included in the second study gave 21% (P = 0·06) protection against a very heterologous type-B virus.
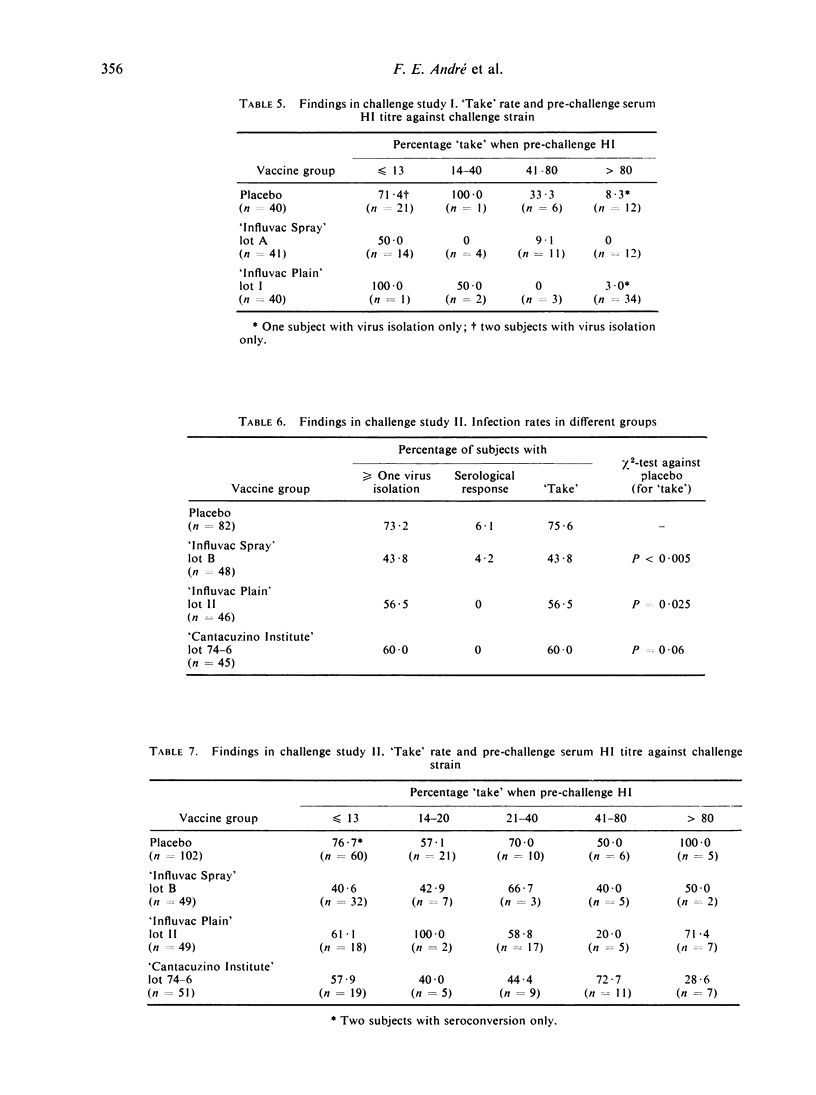
It is argued that results in such studies are biased in favour of injectable vaccines when infection is diagnosed by serology alone whereas the bias is in favour of an aerosol vaccine if this is done by virus isolation alone. When challenge was with the type-A vaccines most ‘takes’ were diagnosed only on the basis of a serological response. With these two vaccines an inverse relationship existed between pre-challenge serum HI (haemagglutination-inhibiting) antibody levels against the challenge strain and ‘take’ rate. With the type-B vaccine, on the other hand, virus isolation commonly occurred in the absence of sero-conversion and there was no correlation between level of serum HI antibodies and ‘take’ rate.
In a placebo-controlled double-blind field trial conducted in parallel on 1326 subjects in the same population as the last challenge study, the aerosol vaccine gave 63% (P = 0·09) and the whole-virion vaccine only 35% (P = 0·37) protection against serologically confirmed influenza.
It is concluded that challenge studies using a live vaccine as challenge virus can yield statistically significant results and that the efficacy of inactivated vaccines can be validly compared if they are administered by the same route. Such studies can be conveniently conducted on large numbers of subjects and this method of assessing vaccine efficacy deserves to be further evaluated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beare A. S., Tyrrell D. A., Hobson D., Howells C. H., Pereira M. S., Pollock T. M., Tyler L. E. Live influenza B vaccine in volunteers. A report to the Medical Research Council by their Committee on Influenza and Other Respiratory Virus Vaccines. J Hyg (Lond) 1969 Mar;67(1):1–11. doi: 10.1017/s002217240004136x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch R. B. Assessment of immunity to influenza using artifical challenge of normal volunteers with influenza virus. Dev Biol Stand. 1975;28:295–306. [PubMed] [Google Scholar]
- Freestone D. S., Hamilton-Smith S., Schild G. C., Buckland R., Chinn S., Tyrrell D. A. Antibody responses and resistance to challenge in volunteers vaccinated with live attenuated, detergent split and oil adjuvant A2-Hong Kong-68 (H 3 N 2 ) influenza vaccines. A report to the Medical Research Council Committee on Influenza and other Respiratory Virus Vaccines. J Hyg (Lond) 1972 Sep;70(3):531–543. doi: 10.1017/s0022172400063117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson D., Curry R. L., Beare A. S., Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972 Dec;70(4):767–777. doi: 10.1017/s0022172400022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien K. S., Jacobs J., Marcus E. A., Strik R. V. The protective effect of intranasal immunization with inactivated influenza virus vaccine. Postgrad Med J. 1973 Mar;49(569):175–179. [PMC free article] [PubMed] [Google Scholar]
- Tyrrell D. A., Buckland R. A., Schild G. C., Freestone D. S., Chinn S., Slepushkin A. N. Nasal and circulating antibody responses to influenza vaccination and their importance in resistance to infection. Postgrad Med J. 1973 Mar;49(569):200–202. doi: 10.1136/pgmj.49.569.200. [DOI] [PMC free article] [PubMed] [Google Scholar]


