Abstract
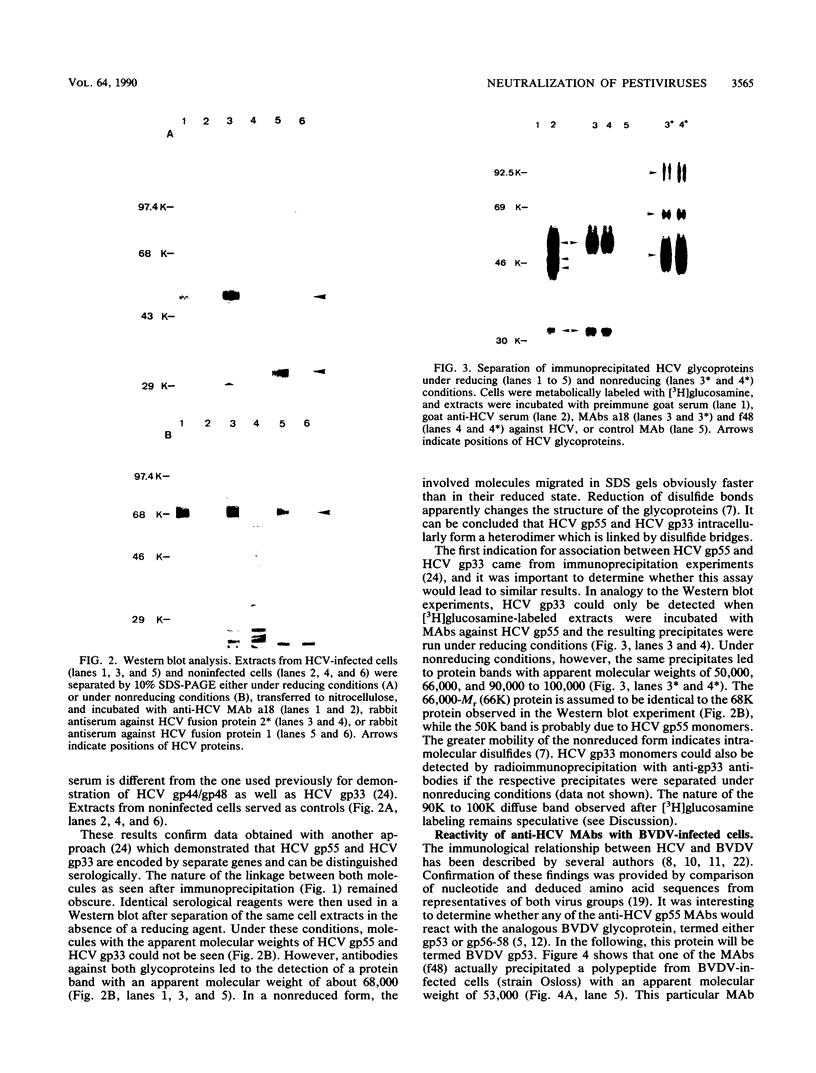
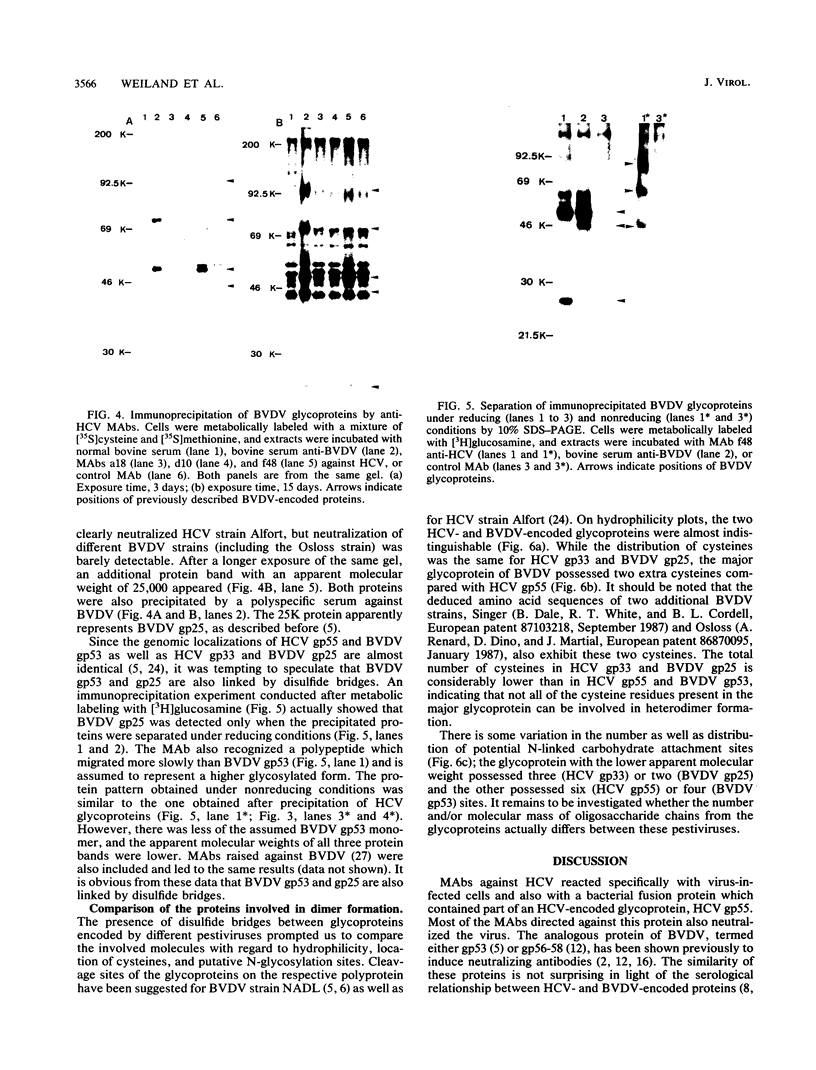
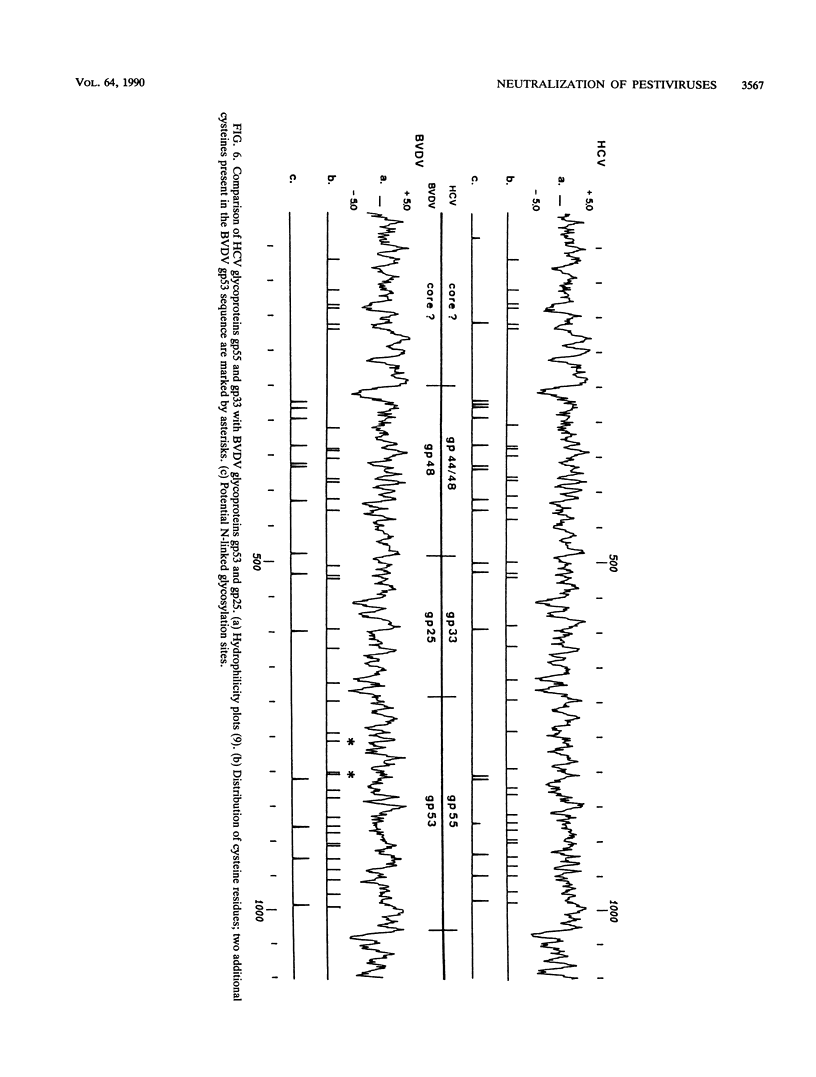
Neutralizing monoclonal antibodies directed against hog cholera virus (HCV) precipitated two HCV-encoded glycoproteins, HCV gp55 and HCV gp33. Immunoassay with bacterial fusion proteins and Western immunoblotting with extracts from infected cells revealed that the antibodies recognized only HCV gp55. Coprecipitation of HCV gp33 was shown to be due to intermolecular disulfide bridges. One of the antibodies also reacted with the major glycoprotein of another pestivirus, bovine viral diarrhea virus (BVDV). The analogous BVDV glycoproteins exhibited a distribution of cysteine residues which was almost identical to that of HCV gp55 and gp33. The two BVDV glycoproteins were also linked by disulfide bridges.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolin S., Moennig V., Kelso Gourley N. E., Ridpath J. Monoclonal antibodies with neutralizing activity segregate isolates of bovine viral diarrhea virus into groups. Brief report. Arch Virol. 1988;99(1-2):117–123. doi: 10.1007/BF01311029. [DOI] [PubMed] [Google Scholar]
- Colett M. S., Larson R., Gold C., Strick D., Anderson D. K., Purchio A. F. Molecular cloning and nucleotide sequence of the pestivirus bovine viral diarrhea virus. Virology. 1988 Jul;165(1):191–199. doi: 10.1016/0042-6822(88)90672-1. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Anderson D. K., Retzel E. Comparisons of the pestivirus bovine viral diarrhoea virus with members of the flaviviridae. J Gen Virol. 1988 Oct;69(Pt 10):2637–2643. doi: 10.1099/0022-1317-69-10-2637. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Larson R., Belzer S. K., Retzel E. Proteins encoded by bovine viral diarrhea virus: the genomic organization of a pestivirus. Virology. 1988 Jul;165(1):200–208. doi: 10.1016/0042-6822(88)90673-3. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Moennig V., Horzinek M. C. Recent advances in pestivirus research. J Gen Virol. 1989 Feb;70(Pt 2):253–266. doi: 10.1099/0022-1317-70-2-253. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis R. O., Corapi W., Dubovi E. J. Neutralizing monoclonal antibodies to bovine viral diarrhoea virus bind to the 56K to 58K glycoprotein. J Gen Virol. 1988 Jan;69(Pt 1):77–86. doi: 10.1099/0022-1317-69-1-77. [DOI] [PubMed] [Google Scholar]
- Donis R. O., Dubovi E. J. Molecular specificity of the antibody responses of cattle naturally and experimentally infected with cytopathic and noncytopathic bovine viral diarrhea virus biotypes. Am J Vet Res. 1987 Nov;48(11):1549–1554. [PubMed] [Google Scholar]
- Hahn Y. S., Galler R., Hunkapiller T., Dalrymple J. M., Strauss J. H., Strauss E. G. Nucleotide sequence of dengue 2 RNA and comparison of the encoded proteins with those of other flaviviruses. Virology. 1988 Jan;162(1):167–180. doi: 10.1016/0042-6822(88)90406-0. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Use of protein A-bearing staphylococci for the immunoprecipitation and isolation of antigens from cells. Methods Enzymol. 1981;73(Pt B):442–459. doi: 10.1016/0076-6879(81)73084-2. [DOI] [PubMed] [Google Scholar]
- Laude H. Hog cholera virus: art and facts. Ann Rech Vet. 1987;18(2):127–138. [PubMed] [Google Scholar]
- Magar R., Minocha H. C., Lecomte J. Bovine viral diarrhea virus proteins: heterogeneity of cytopathogenic and non-cytopathogenic strains and evidence of a 53K glycoprotein neutralization epitope. Vet Microbiol. 1988 Apr;16(4):303–314. doi: 10.1016/0378-1135(88)90012-0. [DOI] [PubMed] [Google Scholar]
- Mandl C. W., Guirakhoo F., Holzmann H., Heinz F. X., Kunz C. Antigenic structure of the flavivirus envelope protein E at the molecular level, using tick-borne encephalitis virus as a model. J Virol. 1989 Feb;63(2):564–571. doi: 10.1128/jvi.63.2.564-571.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl C. W., Heinz F. X., Kunz C. Sequence of the structural proteins of tick-borne encephalitis virus (western subtype) and comparative analysis with other flaviviruses. Virology. 1988 Sep;166(1):197–205. doi: 10.1016/0042-6822(88)90161-4. [DOI] [PubMed] [Google Scholar]
- Meyers G., Rümenapf T., Thiel H. J. Molecular cloning and nucleotide sequence of the genome of hog cholera virus. Virology. 1989 Aug;171(2):555–567. doi: 10.1016/0042-6822(89)90625-9. [DOI] [PubMed] [Google Scholar]
- Nowak T., Wengler G. Analysis of disulfides present in the membrane proteins of the West Nile flavivirus. Virology. 1987 Jan;156(1):127–137. doi: 10.1016/0042-6822(87)90443-0. [DOI] [PubMed] [Google Scholar]
- Rümenapf T., Meyers G., Stark R., Thiel H. J. Hog cholera virus--characterization of specific antiserum and identification of cDNA clones. Virology. 1989 Jul;171(1):18–27. doi: 10.1016/0042-6822(89)90506-0. [DOI] [PubMed] [Google Scholar]
- Stark R., Rümenapf T., Meyers G., Thiel H. J. Genomic localization of hog cholera virus glycoproteins. Virology. 1990 Jan;174(1):286–289. doi: 10.1016/0042-6822(90)90076-4. [DOI] [PubMed] [Google Scholar]
- Strandström H., Veijalainen P., Moennig V., Hunsmann G., Schwarz H., Schäfer W. C-type particles produced by a permanent cell line from a leukemic pig. I. Origin and properties of the host cells and some evidence for the occurrence of C-type-like particles. Virology. 1974 Jan;57(1):175–178. doi: 10.1016/0042-6822(74)90118-4. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland E., Thiel H. J., Hess G., Weiland F. Development of monoclonal neutralizing antibodies against bovine viral diarrhea virus after pretreatment of mice with normal bovine cells and cyclophosphamide. J Virol Methods. 1989 Apr-May;24(1-2):237–243. doi: 10.1016/0166-0934(89)90026-8. [DOI] [PubMed] [Google Scholar]
- Wengler G., Castle E., Leidner U., Nowak T., Wengler G. Sequence analysis of the membrane protein V3 of the flavivirus West Nile virus and of its gene. Virology. 1985 Dec;147(2):264–274. doi: 10.1016/0042-6822(85)90129-1. [DOI] [PubMed] [Google Scholar]
- Wensvoort G., Terpstra C., Boonstra J., Bloemraad M., Van Zaane D. Production of monoclonal antibodies against swine fever virus and their use in laboratory diagnosis. Vet Microbiol. 1986 Jul;12(2):101–108. doi: 10.1016/0378-1135(86)90072-6. [DOI] [PubMed] [Google Scholar]
- Westaway E. G., Brinton M. A., Gaidamovich SYa, Horzinek M. C., Igarashi A., Käriäinen L., Lvov D. K., Porterfield J. S., Russell P. K., Trent D. W. Togaviridae. Intervirology. 1985;24(3):125–139. doi: 10.1159/000149632. [DOI] [PubMed] [Google Scholar]








