Abstract
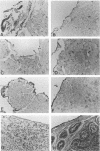
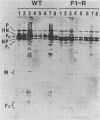
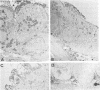
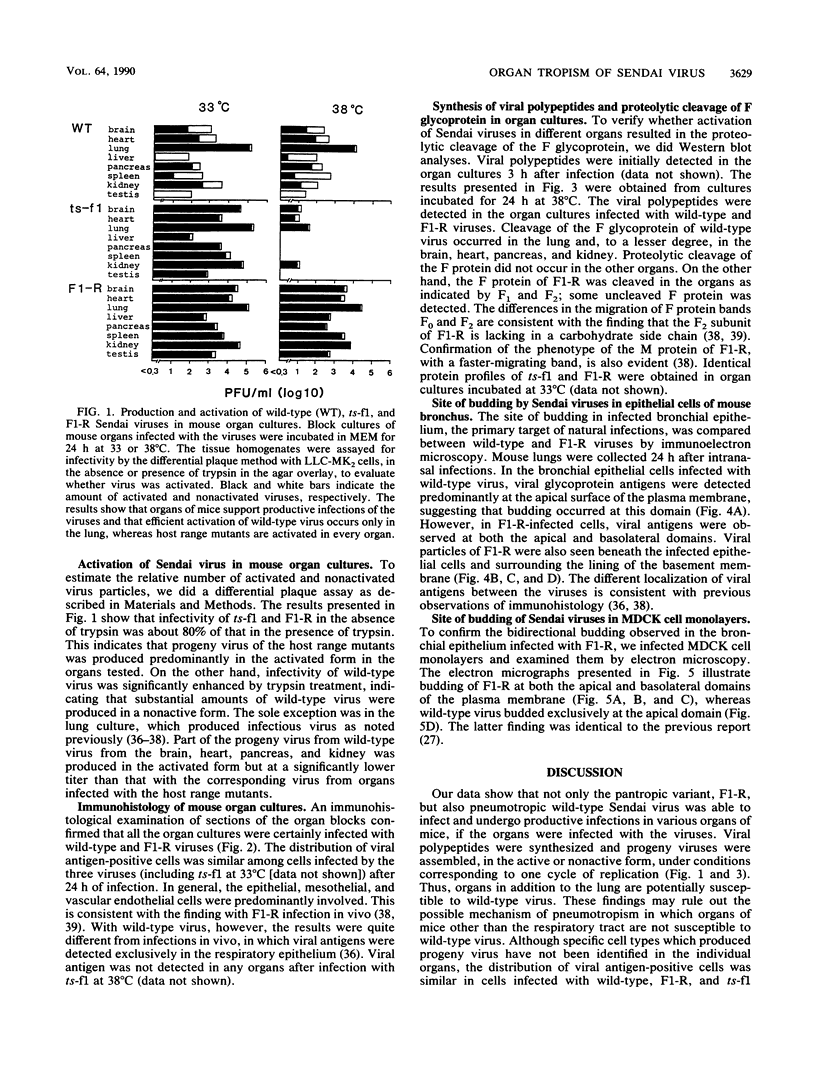
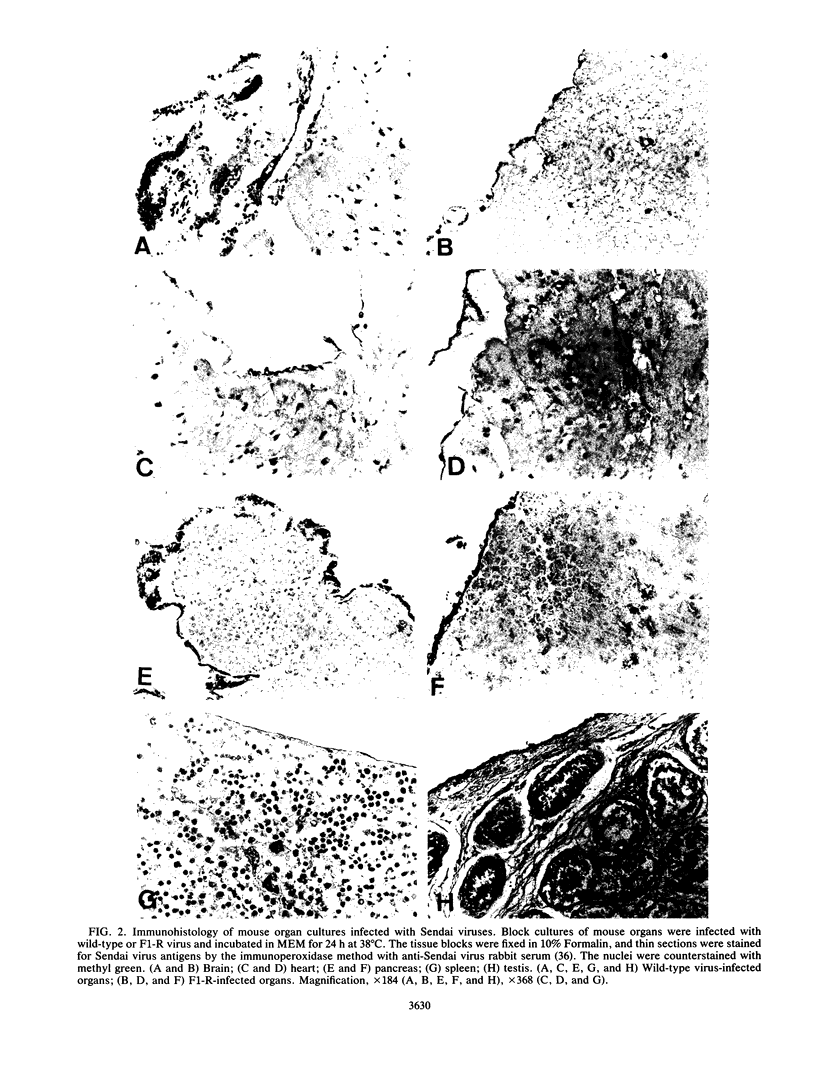
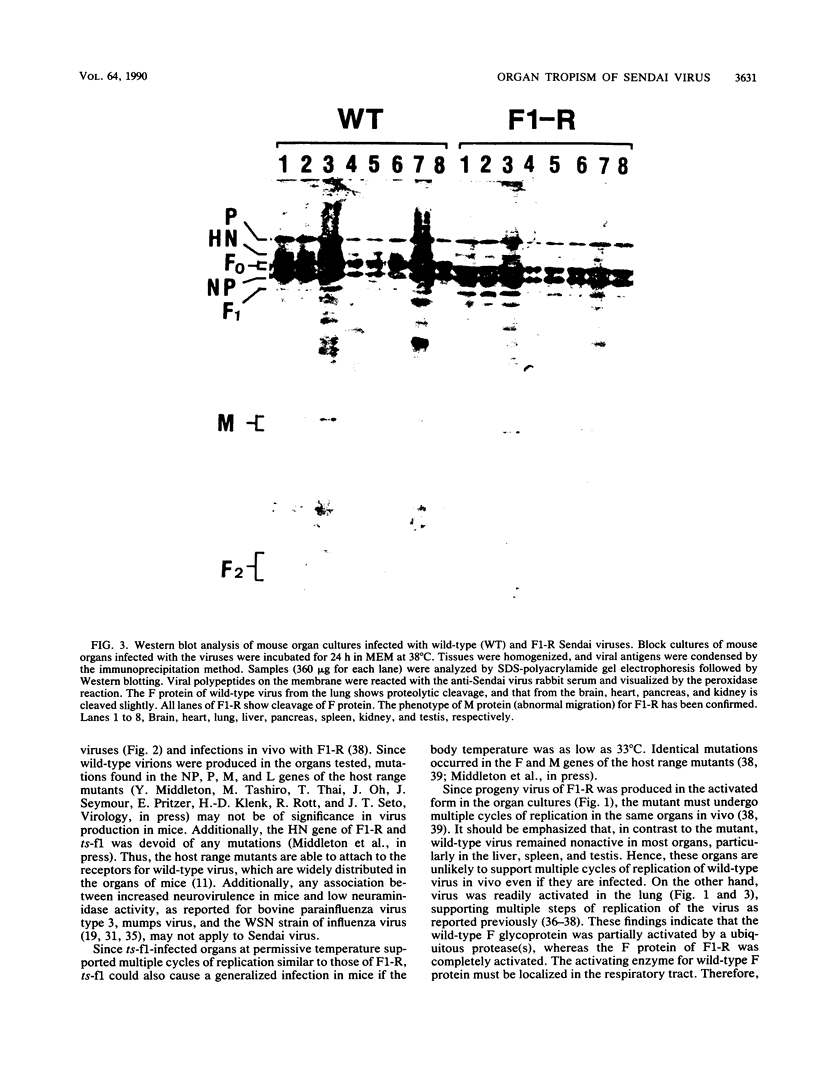
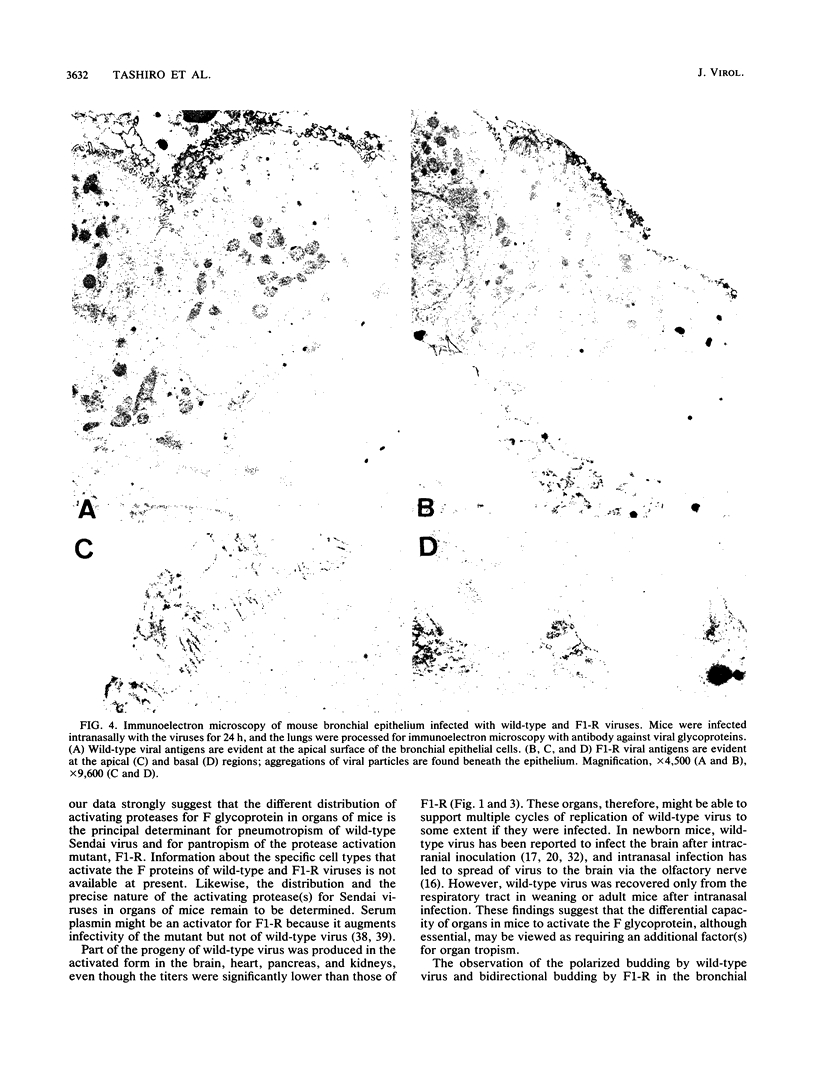
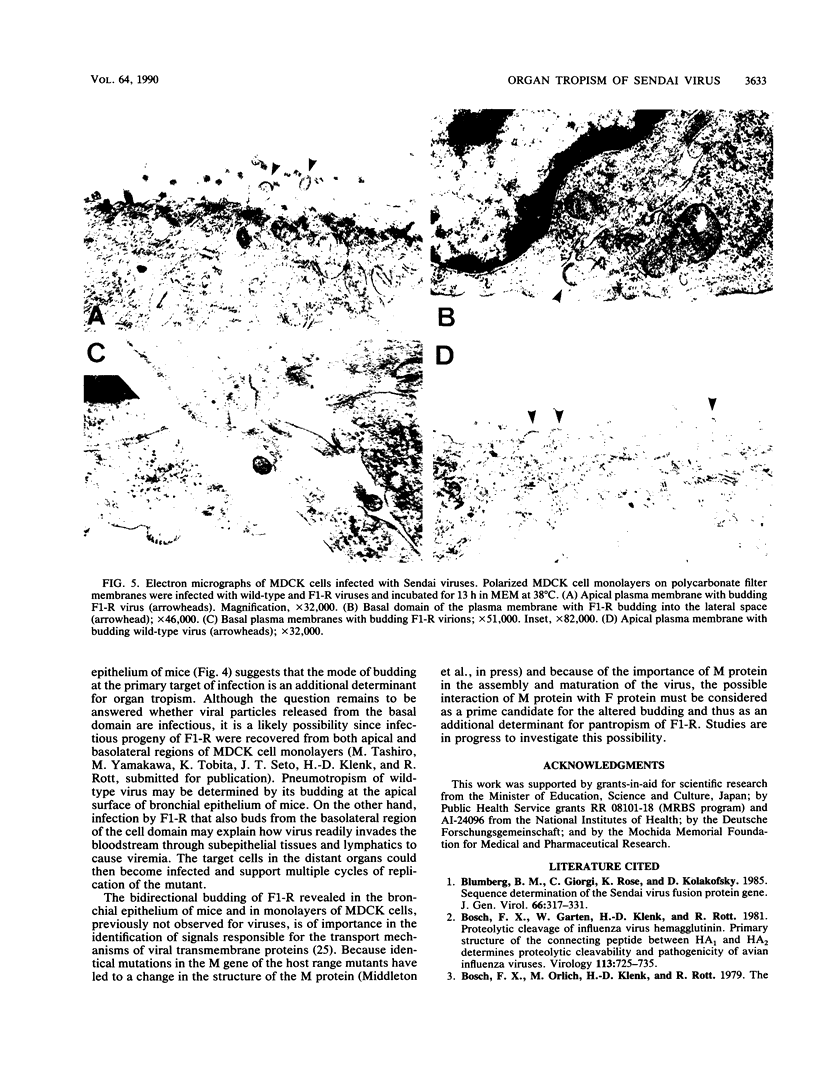
Wild-type Sendai virus is exclusively pneumotropic in mice, while a host range mutant, F1-R, is pantropic. The latter was attributed to structural changes in the fusion (F) glycoprotein, which was cleaved by ubiquitous proteases present in many organs (M. Tashiro, E. Pritzer, M. A. Khoshnan, M. Yamakawa, K. Kuroda, H.-D. Klenk, R. Rott, and J. T. Seto, Virology 165:577-583, 1988). These studies were extended by investigating, by use of an organ block culture system of mice, whether differences exist in the susceptibility of the lung and the other organs to the viruses and in proteolytic activation of the F protein of the viruses. Block cultures of mouse organs were shown to synthesize the viral polypeptides and to support productive infections by the viruses. These findings ruled out the possibility that pneumotropism of wild-type virus results because only the respiratory organs are susceptible to the virus. Progeny virus of F1-R was produced in the activated form as shown by infectivity assays and proteolytic cleavage of the F protein in the infected organ cultures. On the other hand, much of wild-type virus produced in cultures of organs other than lung remained nonactivated. The findings indicate that the F protein of wild-type virus was poorly activated by ubiquitous proteases which efficiently activated the F protein of F1-R. Thus, the activating protease for wild-type F protein is present only in the respiratory organs. These results, taken together with a comparison of the predicted amino acid substitutions between the viruses, strongly suggest that the different efficiencies among mouse organs in the proteolytic activation of F protein must be the primary determinant for organ tropism of Sendai virus. Additionally, immunoelectron microscopic examination of the mouse bronchus indicated that the budding site of wild-type virus was restricted to the apical domain of the epithelium, whereas budding by F1-R occurred at the apical and basal domains. Bipolar budding was also observed in MDCK monolayers infected with F1-R. The differential budding site at the primary target of infection may be an additional determinant for organ tropism of Sendai virus in mice.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg B. M., Giorgi C., Rose K., Kolakofsky D. Sequence determination of the Sendai virus fusion protein gene. J Gen Virol. 1985 Feb;66(Pt 2):317–331. doi: 10.1099/0022-1317-66-2-317. [DOI] [PubMed] [Google Scholar]
- Bosch F. X., Garten W., Klenk H. D., Rott R. Proteolytic cleavage of influenza virus hemagglutinins: primary structure of the connecting peptide between HA1 and HA2 determines proteolytic cleavability and pathogenicity of Avian influenza viruses. Virology. 1981 Sep;113(2):725–735. doi: 10.1016/0042-6822(81)90201-4. [DOI] [PubMed] [Google Scholar]
- Choppin P. W., Scheid A. The role of viral glycoproteins in adsorption, penetration, and pathogenicity of viruses. Rev Infect Dis. 1980 Jan-Feb;2(1):40–61. doi: 10.1093/clinids/2.1.40. [DOI] [PubMed] [Google Scholar]
- Farr A. G., Nakane P. K. Immunohistochemistry with enzyme labeled antibodies: a brief review. J Immunol Methods. 1981;47(2):129–144. doi: 10.1016/0022-1759(81)90114-9. [DOI] [PubMed] [Google Scholar]
- Homma M., Ouchi M. Trypsin action on the growth of Sendai virus in tissue culture cells. 3. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J Virol. 1973 Dec;12(6):1457–1465. doi: 10.1128/jvi.12.6.1457-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma M. Trypsin action on the growth of Sendai virus in tissue culture cells. I. Restoration of the infectivity for L cells by direct action of tyrpsin on L cell-borne Sendai virus. J Virol. 1971 Nov;8(5):619–629. doi: 10.1128/jvi.8.5.619-629.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M., Choppin P. W. Analysis of Sendai virus mRNAs with cDNA clones of viral genes and sequences of biologically important regions of the fusion protein. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7732–7736. doi: 10.1073/pnas.81.24.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida N., Homma M. Sendai virus. Adv Virus Res. 1978;23:349–383. doi: 10.1016/s0065-3527(08)60103-7. [DOI] [PubMed] [Google Scholar]
- Ito Y., Yamamoto F., Takano M., Maeno K., Shimokata K., Iinuma M., Hara K., Iijima S. Detection of cellular receptors for Sendai virus in mouse tissue sections. Arch Virol. 1983;75(1-2):103–113. doi: 10.1007/BF01314130. [DOI] [PubMed] [Google Scholar]
- Itoh M., Shibuta H., Homma M. Single amino acid substitution of Sendai virus at the cleavage site of the fusion protein confers trypsin resistance. J Gen Virol. 1987 Nov;68(Pt 11):2939–2944. doi: 10.1099/0022-1317-68-11-2939. [DOI] [PubMed] [Google Scholar]
- Kawaoka Y., Naeve C. W., Webster R. G. Is virulence of H5N2 influenza viruses in chickens associated with loss of carbohydrate from the hemagglutinin? Virology. 1984 Dec;139(2):303–316. doi: 10.1016/0042-6822(84)90376-3. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Rott R. The molecular biology of influenza virus pathogenicity. Adv Virus Res. 1988;34:247–281. doi: 10.1016/S0065-3527(08)60520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensson K., Leestma J., Lundh B., Norrby E. Sendai virus infection in the mouse brain: virus spread and long-term effects. Acta Neuropathol. 1984;63(2):89–95. doi: 10.1007/BF00697190. [DOI] [PubMed] [Google Scholar]
- Kristensson K., Orvell C., Leestma J., Norrby E. Sendai virus infection in the brains of mice: distribution of viral antigens studied with monoclonal antibodies. J Infect Dis. 1983 Feb;147(2):297–301. doi: 10.1093/infdis/147.2.297. [DOI] [PubMed] [Google Scholar]
- Lawton P., Karimi Z., Mancinelli L., Seto J. T. Persistent infections with Sendai virus and Newcastle disease viruses. Arch Virol. 1986;89(1-4):225–233. doi: 10.1007/BF01309891. [DOI] [PubMed] [Google Scholar]
- Merz D. C., Wolinsky J. S. Biochemical features of mumps virus neuraminidases and their relationship with pathogenicity. Virology. 1981 Oct 15;114(1):218–227. doi: 10.1016/0042-6822(81)90267-1. [DOI] [PubMed] [Google Scholar]
- Mims C. A., Murphy F. A. Parainfluenza virus Sendai infection in macrophages, ependyma, choroid plexus, vascular endothelium and respiratory tract of mice. Am J Pathol. 1973 Mar;70(3):315–328. [PMC free article] [PubMed] [Google Scholar]
- Mochizuki Y., Tashiro M., Homma M. Pneumopathogenicity in mice of a Sendai virus mutant, TSrev-58, is accompanied by in vitro activation with trypsin. J Virol. 1988 Aug;62(8):3040–3042. doi: 10.1128/jvi.62.8.3040-3042.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu M., Homma M. Trypsin action on the growth of Sendai virus in tissue culture cells. V. An activating enzyme for Sendai virus in the chorioallantoic fluid of the embryonated chicken egg. Microbiol Immunol. 1980;24(2):113–122. doi: 10.1111/j.1348-0421.1980.tb00569.x. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Hamaguchi M., Toyoda T. Molecular biology of Newcastle disease virus. Prog Vet Microbiol Immunol. 1989;5:16–64. [PubMed] [Google Scholar]
- Nagai Y., Klenk H. D., Rott R. Proteolytic cleavage of the viral glycoproteins and its significance for the virulence of Newcastle disease virus. Virology. 1976 Jul 15;72(2):494–508. doi: 10.1016/0042-6822(76)90178-1. [DOI] [PubMed] [Google Scholar]
- Nayak D. P., Jabbar M. A. Structural domains and organizational conformation involved in the sorting and transport of influenza virus transmembrane proteins. Annu Rev Microbiol. 1989;43:465–501. doi: 10.1146/annurev.mi.43.100189.002341. [DOI] [PubMed] [Google Scholar]
- Portner A., Scroggs R. A., Naeve C. W. The fusion glycoprotein of Sendai virus: sequence analysis of an epitope involved in fusion and virus neutralization. Virology. 1987 Apr;157(2):556–559. doi: 10.1016/0042-6822(87)90301-1. [DOI] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Sabatini D. D. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Two disulfide-linked polypeptide chains constitute the active F protein of paramyxoviruses. Virology. 1977 Jul 1;80(1):54–66. doi: 10.1016/0042-6822(77)90380-4. [DOI] [PubMed] [Google Scholar]
- Shibuta H., Akami M., Matumoto M. Plaque formation by sendai virus of parainfluenza virus group, type 1 on monkey, calf kidney and chick embryo cell monolayers. Jpn J Microbiol. 1971 Mar;15(2):175–183. doi: 10.1111/j.1348-0421.1971.tb00567.x. [DOI] [PubMed] [Google Scholar]
- Shibuta H., Kanda T., Nozawa A., Sato S., Kumanishi T. Experimental parainfluenza virus infection in mice: growth and spread of a highly pathogenic variant of parainfluenza 3 virus in the mouse brain. Arch Virol. 1985;83(1-2):43–52. doi: 10.1007/BF01310963. [DOI] [PubMed] [Google Scholar]
- Shimokata K., Nishiyama Y., Ito Y., Kimura Y., Nagata I. Pathogenesis of Sendai virus infection in the central nervous system of mice. Infect Immun. 1976 May;13(5):1497–1502. doi: 10.1128/iai.13.5.1497-1502.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda T., Iwasaki K., Shibuta H. Determination of the complete nucleotide sequence of the Sendai virus genome RNA and the predicted amino acid sequences of the F, HN and L proteins. Nucleic Acids Res. 1986 Feb 25;14(4):1545–1563. doi: 10.1093/nar/14.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S. M., Scheid A., Choppin P. W. Loss on serial passage of rhesus monkey kidney cells of proteolytic activity required for Sendai virus activation. Infect Immun. 1978 Apr;20(1):235–241. doi: 10.1128/iai.20.1.235-241.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro M., Homma M. Evidence of proteolytic activation of Sendai virus in mouse lung. Arch Virol. 1983;77(2-4):127–137. doi: 10.1007/BF01309262. [DOI] [PubMed] [Google Scholar]
- Tashiro M., Homma M. Pneumotropism of Sendai virus in relation to protease-mediated activation in mouse lungs. Infect Immun. 1983 Feb;39(2):879–888. doi: 10.1128/iai.39.2.879-888.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro M., Pritzer E., Khoshnan M. A., Yamakawa M., Kuroda K., Klenk H. D., Rott R., Seto J. T. Characterization of a pantropic variant of Sendai virus derived from a host range mutant. Virology. 1988 Aug;165(2):577–583. doi: 10.1016/0042-6822(88)90601-0. [DOI] [PubMed] [Google Scholar]
- Toyoda T., Sakaguchi T., Imai K., Inocencio N. M., Gotoh B., Hamaguchi M., Nagai Y. Structural comparison of the cleavage-activation site of the fusion glycoprotein between virulent and avirulent strains of Newcastle disease virus. Virology. 1987 May;158(1):242–247. doi: 10.1016/0042-6822(87)90261-3. [DOI] [PubMed] [Google Scholar]