Abstract
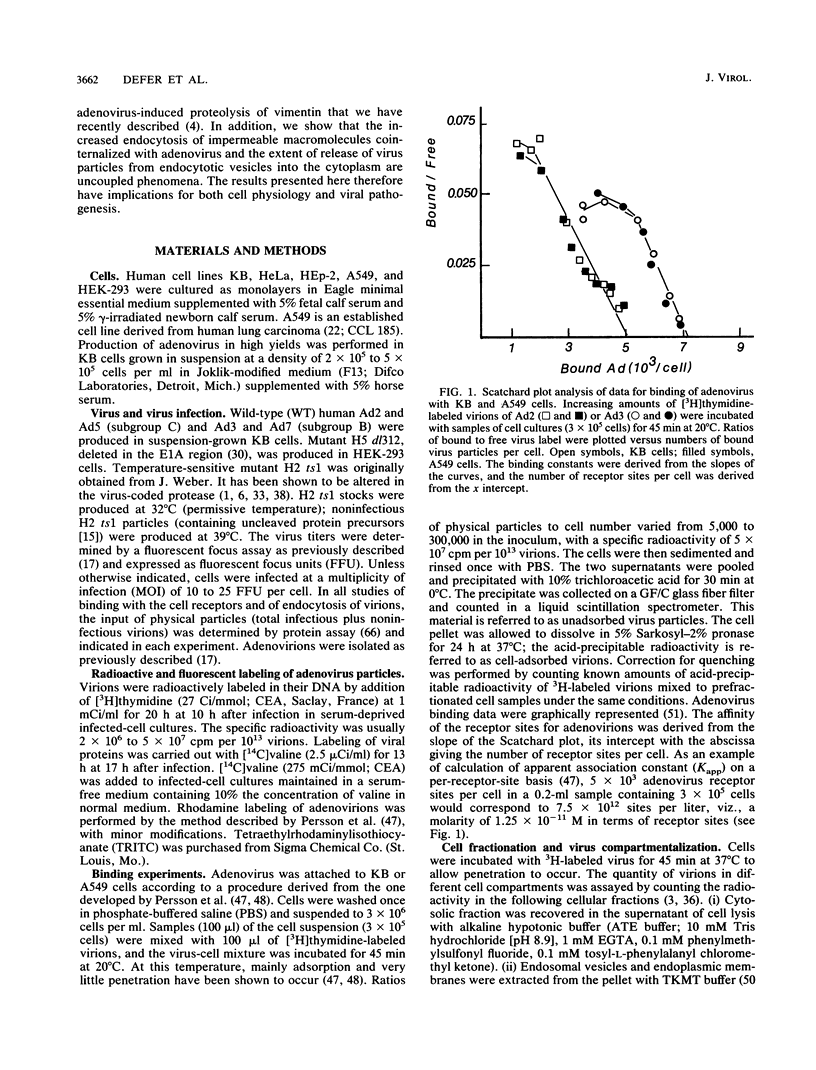
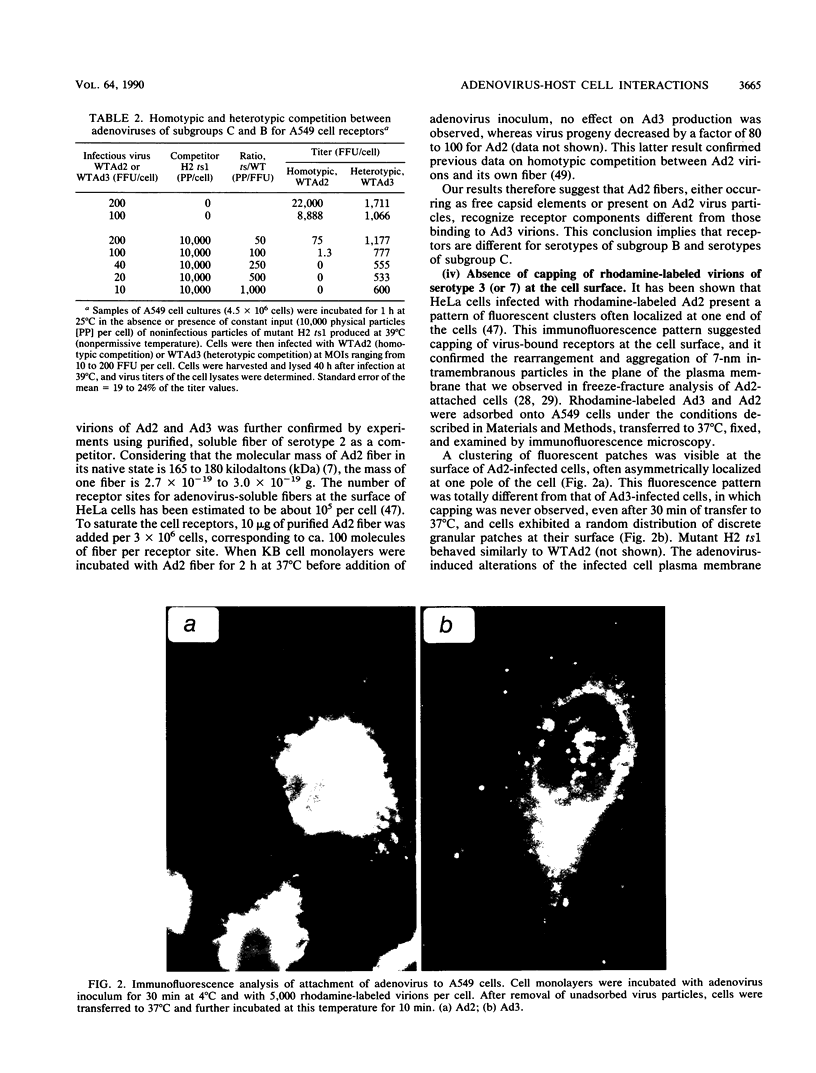
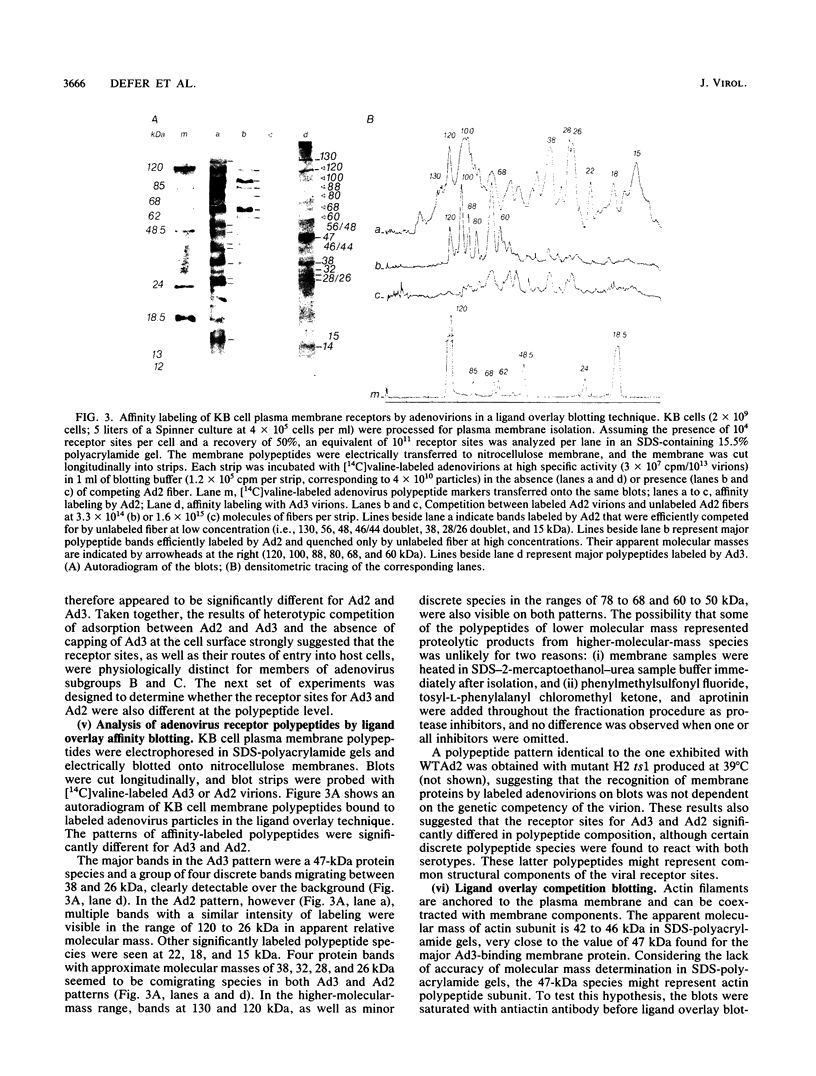
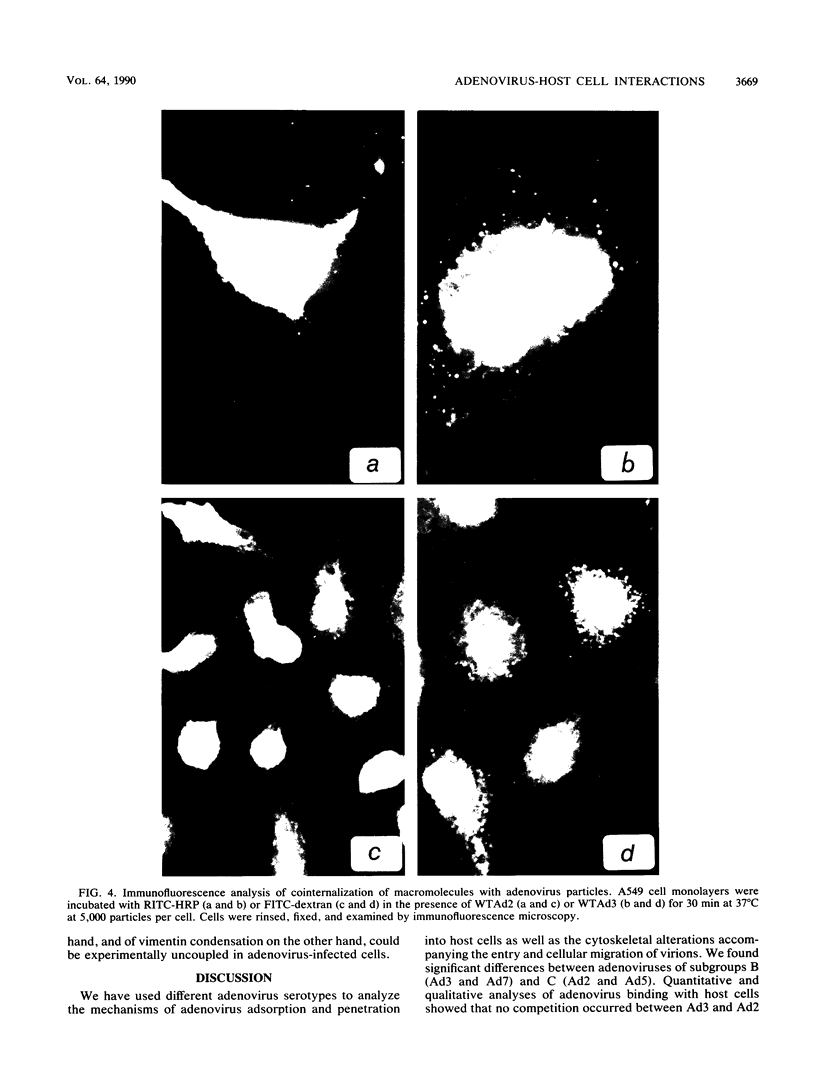
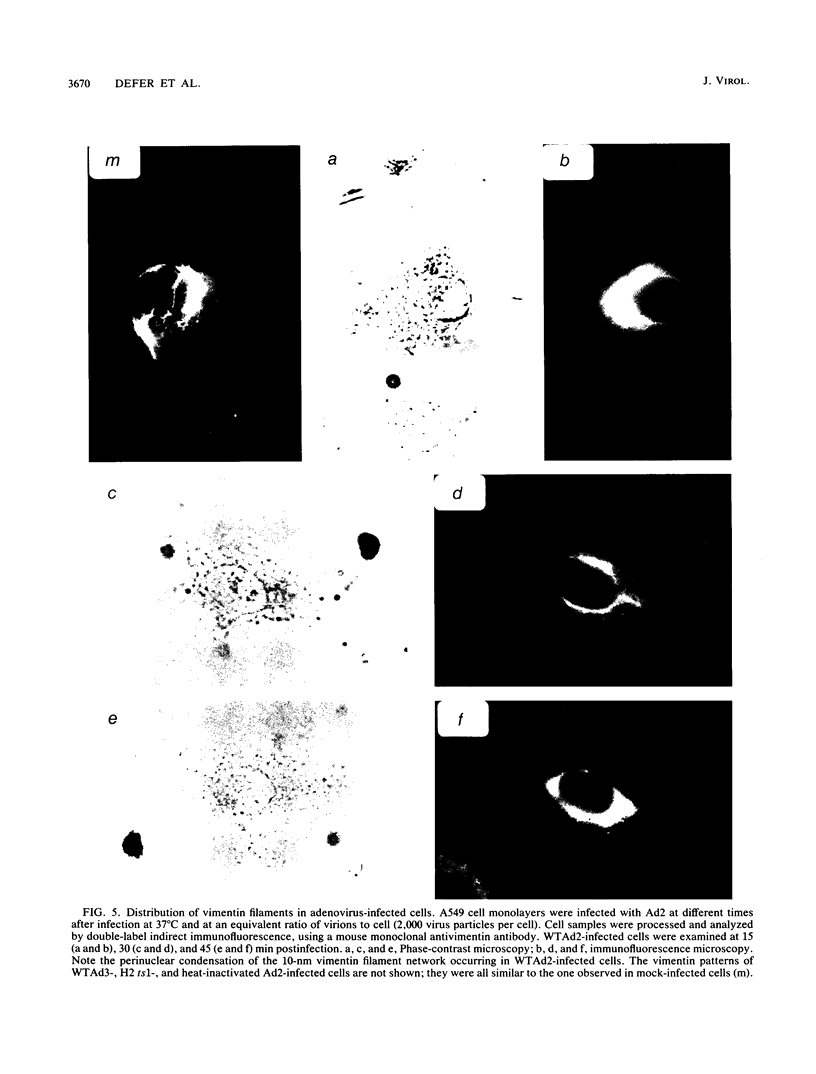
Host cell interactions of human adenovirus serotypes belonging to subgroups B (adenovirus type 3 [Ad3] and Ad7) and C (Ad2 and Ad5) were comparatively analyzed at three levels: (i) binding of virus particles with host cell receptors; (ii) cointernalization of macromolecules with adenovirions; and (iii) adenovirus-induced cytoskeletal alterations. The association constants with human cell receptors were found to be similar for Ad2 and Ad3 (8 x 10(9) to 9 x 10(9) M-1), and the number of receptor sites per cell ranged from 5,000 (Ad2) to 7,000 (Ad3). Affinity blottings, competition experiments, and immunofluorescence stainings suggested that the receptor sites for adenovirus were distinct for members of subgroups B and C. Adenovirions increased the permeability of cells to macromolecules. We showed that this global effect could be divided into two distinct events: (i) cointernalization of macromolecules and virions into endocytotic vesicles, a phenomenon that occurred in a serotype-independent way, and (ii) release of macromolecules into the cytoplasm upon adenovirus-induced lysis of endosomal membranes. The latter process was found to be type specific and to require unaltered and infectious virus particles of serotype 2 or 5. Perinuclear condensation of the vimentin filament network was observed at early stages of infection with Ad2 or Ad5 but not with Ad3, Ad7, and noninfectious particles of Ad2 or Ad5, obtained by heat inactivation of wild-type virions or with the H2 ts1 mutant. This phenomenon appeared to be a cytological marker for cytoplasmic transit of infectious virions within adenovirus-infected cells. It could be experimentally dissociated from vimentin proteolysis, which was found to be serotype dependent, occurring only with members of subgroup C, regardless of the infectivity of the input virus.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akusjärvi G., Persson H. Gene and mRNA for precursor polypeptide VI from adenovirus type 2. J Virol. 1981 May;38(2):469–482. doi: 10.1128/jvi.38.2.469-482.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard A. K., Wadell G., Evander K. M., Lindman G. K. Specific properties of two enteric adenovirus 41 clones mapped within early region 1A. J Virol. 1985 Apr;54(1):145–150. doi: 10.1128/jvi.54.1.145-150.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin M. T., Boulanger P. Cytoskeletal proteins associated with intracytoplasmic human adenovirus at an early stage of infection. Exp Cell Res. 1985 Oct;160(2):356–370. doi: 10.1016/0014-4827(85)90182-x. [DOI] [PubMed] [Google Scholar]
- Belin M. T., Boulanger P. Processing of vimentin occurs during the early stages of adenovirus infection. J Virol. 1987 Aug;61(8):2559–2566. doi: 10.1128/jvi.61.8.2559-2566.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. doi: 10.1146/annurev.ge.20.120186.000401. [DOI] [PubMed] [Google Scholar]
- Bhatti A. R., Weber J. Protease of adenovirus type 2: partial characterization. Virology. 1979 Jul 30;96(2):478–485. doi: 10.1016/0042-6822(79)90105-3. [DOI] [PubMed] [Google Scholar]
- Boulanger P., Devaux C. Native molecular weight of adenovirus proteins: on the oligomeric structure of the fiber. Biochem Biophys Res Commun. 1983 Feb 10;110(3):913–918. doi: 10.1016/0006-291x(83)91049-5. [DOI] [PubMed] [Google Scholar]
- Brown D. T., Burlingham B. T. Penetration of host cell membranes by adenovirus 2. J Virol. 1973 Aug;12(2):386–396. doi: 10.1128/jvi.12.2.386-396.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco L., Esteban M. Modification of membrane permeability in vaccinia virus-infected cells. Virology. 1982 Feb;117(1):62–69. doi: 10.1016/0042-6822(82)90507-4. [DOI] [PubMed] [Google Scholar]
- Carrasco L. Modification of membrane permeability induced by animal viruses early in infection. Virology. 1981 Sep;113(2):623–629. doi: 10.1016/0042-6822(81)90190-2. [DOI] [PubMed] [Google Scholar]
- Chardonnet Y., Dales S. Early events in the interaction of adenoviruses with HeLa cells. I. Penetration of type 5 and intracellular release of the DNA genome. Virology. 1970 Mar;40(3):462–477. doi: 10.1016/0042-6822(70)90189-3. [DOI] [PubMed] [Google Scholar]
- Chardonnet Y., Dales S. Early events in the interaction of adenoviruses with HeLa cells. II. Comparative observations on the penetration of types 1, 5, 7, and 12. Virology. 1970 Mar;40(3):478–485. doi: 10.1016/0042-6822(70)90190-x. [DOI] [PubMed] [Google Scholar]
- Chatterjee P. K., Flint S. J. Adenovirus type 2 endopeptidase: an unusual phosphoprotein enzyme matured by autocatalysis. Proc Natl Acad Sci U S A. 1987 Feb;84(3):714–718. doi: 10.1073/pnas.84.3.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Halluin J. C., Milleville M., Boulanger P. A., Martin G. R. Temperature-sensitive mutant of adenovirus type 2 blocked in virion assembly: accumulation of light intermediate particles. J Virol. 1978 May;26(2):344–356. doi: 10.1128/jvi.26.2.344-356.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALES S. An electron microscope study of the early association between two mammalian viruses and their hosts. J Cell Biol. 1962 May;13:303–322. doi: 10.1083/jcb.13.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Puentes C., Carrasco L. Viral infection permeabilizes mammalian cells to protein toxins. Cell. 1980 Jul;20(3):769–775. doi: 10.1016/0092-8674(80)90323-2. [DOI] [PubMed] [Google Scholar]
- FitzGerald D. J., Padmanabhan R., Pastan I., Willingham M. C. Adenovirus-induced release of epidermal growth factor and pseudomonas toxin into the cytosol of KB cells during receptor-mediated endocytosis. Cell. 1983 Feb;32(2):607–617. doi: 10.1016/0092-8674(83)90480-4. [DOI] [PubMed] [Google Scholar]
- Geiduschek E. P., Tocchini-Valentini G. P. Transcription by RNA polymerase III. Annu Rev Biochem. 1988;57:873–914. doi: 10.1146/annurev.bi.57.070188.004301. [DOI] [PubMed] [Google Scholar]
- Gershoni J. M., Palade G. E. Protein blotting: principles and applications. Anal Biochem. 1983 May;131(1):1–15. doi: 10.1016/0003-2697(83)90128-8. [DOI] [PubMed] [Google Scholar]
- Giard D. J., Aaronson S. A., Todaro G. J., Arnstein P., Kersey J. H., Dosik H., Parks W. P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973 Nov;51(5):1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- Green M., Piña M., Kimes R. C. Biochemical studies on adenovirus multiplication. XII. Plaquing efficiencies of purified human adenoviruses. Virology. 1967 Mar;31(3):562–565. doi: 10.1016/0042-6822(67)90241-3. [DOI] [PubMed] [Google Scholar]
- Green M., Piña M., Kimes R., Wensink P. C., MacHattie L. A., Thomas C. A., Jr Adenovirus DNA. I. Molecular weight and conformation. Proc Natl Acad Sci U S A. 1967 May;57(5):1302–1309. doi: 10.1073/pnas.57.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennache B., Boulanger P. Biochemical study of KB-cell receptor for adenovirus. Biochem J. 1977 Aug 15;166(2):237–247. doi: 10.1042/bj1660237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennache B., Torpier G., Boulanger P. Adenovirus adsorption and sterol redistribution in KB cell plasma membrane. Exp Cell Res. 1982 Feb;137(2):459–463. doi: 10.1016/0014-4827(82)90052-0. [DOI] [PubMed] [Google Scholar]
- Hennache B., Torpier G., Boulanger P. Freeze-fracture study of adenovirus-induced KB cell surface alterations. Exp Cell Res. 1979 Nov;124(1):139–150. doi: 10.1016/0014-4827(79)90264-7. [DOI] [PubMed] [Google Scholar]
- Kidd A. H. Genome variants of adenovirus 41 (subgroup G) from children with diarrhoea in South Africa. J Med Virol. 1984;14(1):49–59. doi: 10.1002/jmv.1890140108. [DOI] [PubMed] [Google Scholar]
- Kohn A. Early interactions of viruses with cellular membranes. Adv Virus Res. 1979;24:223–276. doi: 10.1016/s0065-3527(08)60395-4. [DOI] [PubMed] [Google Scholar]
- Kruijer W., van Schaik F. M., Sussenbach J. S. Nucleotide sequence analysis of a region of adenovirus 5 DNA encoding a hitherto unidentified gene. Nucleic Acids Res. 1980 Dec 20;8(24):6033–6042. doi: 10.1093/nar/8.24.6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langanger G., de Mey J., Moeremans M., Daneels G., de Brabander M., Small J. V. Ultrastructural localization of alpha-actinin and filamin in cultured cells with the immunogold staining (IGS) method. J Cell Biol. 1984 Oct;99(4 Pt 1):1324–1334. doi: 10.1083/jcb.99.4.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenstra J. A., Bloemendal H. The major proteins from HeLa cells. Identification and intracellular localization. Eur J Biochem. 1983 Feb 1;130(2):419–426. doi: 10.1111/j.1432-1033.1983.tb07168.x. [DOI] [PubMed] [Google Scholar]
- Li Q. G., Wadell G. Comparison of 17 genome types of adenovirus type 3 identified among strains recovered from six continents. J Clin Microbiol. 1988 May;26(5):1009–1015. doi: 10.1128/jcm.26.5.1009-1015.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Crowell R. L., Philipson L. Unrelated animal viruses share receptors. Nature. 1976 Feb 26;259(5545):679–681. doi: 10.1038/259679a0. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early interaction between animal viruses and cells. Monogr Virol. 1974;9:1–148. [PubMed] [Google Scholar]
- Madshus I. H., Olsnes S., Sandvig K. Mechanism of entry into the cytosol of poliovirus type 1: requirement for low pH. J Cell Biol. 1984 Apr;98(4):1194–1200. doi: 10.1083/jcb.98.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles B. D., Luftig R. B., Weatherbee J. A., Weihing R. R., Weber J. Quantitation of the interaction between adenovirus types 2 and 5 and microtubules inside infected cells. Virology. 1980 Aug;105(1):265–269. doi: 10.1016/0042-6822(80)90177-4. [DOI] [PubMed] [Google Scholar]
- Mirza M. A., Weber J. Uncoating of adenovirus type 2. J Virol. 1979 May;30(2):462–471. doi: 10.1128/jvi.30.2.462-471.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogier G., Chardonnet Y., Doerfler W. The fate of type 7 adenovirions in lysosomes of HeLa cells. Virology. 1977 Mar;77(1):67–77. doi: 10.1016/0042-6822(77)90406-8. [DOI] [PubMed] [Google Scholar]
- Otero M. J., Carrasco L. Proteins are cointernalized with virion particles during early infection. Virology. 1987 Sep;160(1):75–80. doi: 10.1016/0042-6822(87)90046-8. [DOI] [PubMed] [Google Scholar]
- Otto J. J. Detection of vinculin-binding proteins with an 125I-vinculin gel overlay technique. J Cell Biol. 1983 Oct;97(4):1283–1287. doi: 10.1083/jcb.97.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson R., Svensson U., Everitt E. Virus receptor interaction in the adenovirus system. II. Capping and cooperative binding of virions on HeLa cells. J Virol. 1983 Jun;46(3):956–963. doi: 10.1128/jvi.46.3.956-963.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson R., Wohlfart C., Svensson U., Everitt E. Virus-receptor interaction in the adenovirus system: characterization of the positive cooperative binding of virions on HeLa cells. J Virol. 1985 Apr;54(1):92–97. doi: 10.1128/jvi.54.1.92-97.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L., Lonberg-Holm K., Pettersson U. Virus-receptor interaction in an adenovirus system. J Virol. 1968 Oct;2(10):1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P. M. Cellular and adenovirus dl312 DNA metabolism in cycling or mitotic human cultures exposed to supralethal gamma radiation. J Cell Biol. 1989 Nov;109(5):1993–2002. doi: 10.1083/jcb.109.5.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger R. W. Adenoviruses: the nature of the virion and of controlling factors in productive or abortive infection and tumorigenesis. Adv Virus Res. 1969;14:1–61. doi: 10.1016/s0065-3527(08)60556-4. [DOI] [PubMed] [Google Scholar]
- Schmitz H., Wigand R., Heinrich W. Worldwide epidemiology of human adenovirus infections. Am J Epidemiol. 1983 Apr;117(4):455–466. doi: 10.1093/oxfordjournals.aje.a113563. [DOI] [PubMed] [Google Scholar]
- Seth P., Fitzgerald D., Ginsberg H., Willingham M., Pastan I. Evidence that the penton base of adenovirus is involved in potentiation of toxicity of Pseudomonas exotoxin conjugated to epidermal growth factor. Mol Cell Biol. 1984 Aug;4(8):1528–1533. doi: 10.1128/mcb.4.8.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P., Pastan I., Willingham M. C. Adenovirus-dependent increase in cell membrane permeability. J Biol Chem. 1985 Aug 15;260(17):9598–9602. [PubMed] [Google Scholar]
- Silver L., Anderson C. W. Interaction of human adenovirus serotype 2 with human lymphoid cells. Virology. 1988 Aug;165(2):377–387. doi: 10.1016/0042-6822(88)90582-x. [DOI] [PubMed] [Google Scholar]
- Stillman B. Functions of the adenovirus E1B tumour antigens. Cancer Surv. 1986;5(2):389–404. [PubMed] [Google Scholar]
- Stone D. K., Xie X. S., Racker E. An ATP-driven proton pump in clathrin-coated vesicles. J Biol Chem. 1983 Apr 10;258(7):4059–4062. [PubMed] [Google Scholar]
- Svensson U., Persson R. Entry of adenovirus 2 into HeLa cells. J Virol. 1984 Sep;51(3):687–694. doi: 10.1128/jvi.51.3.687-694.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson U., Persson R., Everitt E. Virus-receptor interaction in the adenovirus system I. Identification of virion attachment proteins of the HeLa cell plasma membrane. J Virol. 1981 Apr;38(1):70–81. doi: 10.1128/jvi.38.1.70-81.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson U. Role of vesicles during adenovirus 2 internalization into HeLa cells. J Virol. 1985 Aug;55(2):442–449. doi: 10.1128/jvi.55.2.442-449.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiff H. E., Straus S. E., Garon C. F. Propagation and in vitro studies of previously non-cultivable enteral adenoviruses in 293 cells. Lancet. 1981 Oct 17;2(8251):832–834. doi: 10.1016/s0140-6736(81)91104-1. [DOI] [PubMed] [Google Scholar]
- Uhnoo I., Wadell G., Svensson L., Johansson M. E. Importance of enteric adenoviruses 40 and 41 in acute gastroenteritis in infants and young children. J Clin Microbiol. 1984 Sep;20(3):365–372. doi: 10.1128/jcm.20.3.365-372.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. Genetic analysis of adenovirus type 2 III. Temperature sensitivity of processing viral proteins. J Virol. 1976 Feb;17(2):462–471. doi: 10.1128/jvi.17.2.462-471.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker J. R., Granum P. E. An absolute method for protein determination based on difference in absorbance at 235 and 280 nm. Anal Biochem. 1980 Nov 15;109(1):156–159. doi: 10.1016/0003-2697(80)90024-x. [DOI] [PubMed] [Google Scholar]
- Whyte P., Ruley H. E., Harlow E. Two regions of the adenovirus early region 1A proteins are required for transformation. J Virol. 1988 Jan;62(1):257–265. doi: 10.1128/jvi.62.1.257-265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigand R. Pitfalls in the identification of adenoviruses. J Virol Methods. 1987 Jun;16(3):161–169. doi: 10.1016/0166-0934(87)90001-2. [DOI] [PubMed] [Google Scholar]
- Yamashiro D. J., Fluss S. R., Maxfield F. R. Acidification of endocytic vesicles by an ATP-dependent proton pump. J Cell Biol. 1983 Sep;97(3):929–934. doi: 10.1083/jcb.97.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh-Kai L., Akusjärvi G., Aleström P., Pettersson U., Tremblay M., Weber J. Genetic identification of an endoproteinase encoded by the adenovirus genome. J Mol Biol. 1983 Jun 15;167(1):217–222. doi: 10.1016/s0022-2836(83)80044-8. [DOI] [PubMed] [Google Scholar]
- Yoshimura A. Adenovirus-induced leakage of co-endocytosed macromolecules into the cytosol. Cell Struct Funct. 1985 Dec;10(4):391–404. doi: 10.1247/csf.10.391. [DOI] [PubMed] [Google Scholar]