Abstract
In the intracellular death program, hetero- and homodimerization of different anti- and pro-apoptotic Bcl-2-related proteins are critical in the determination of cell fate. From a rat ovarian fusion cDNA library, we isolated a new pro-apoptotic Bcl-2 gene, Bcl-2-related ovarian killer (Bok). Bok had conserved Bcl-2 homology (BH) domains 1, 2, and 3 and a C-terminal transmembrane region present in other Bcl-2 proteins, but lacked the BH4 domain found only in anti-apoptotic Bcl-2 proteins. In the yeast two-hybrid system, Bok interacted strongly with some (Mcl-1, BHRF1, and Bfl-1) but not other (Bcl-2, Bcl-xL, and Bcl-w) anti-apoptotic members. This finding is in direct contrast to the ability of other pro-apoptotic members (Bax, Bak, and Bik) to interact with all of the anti-apoptotic proteins. In addition, negligible interaction was found between Bok and different pro-apoptotic members. In mammalian cells, overexpression of Bok induced apoptosis that was blocked by the baculoviral-derived cysteine protease inhibitor P35. Cell killing induced by Bok was also suppressed following coexpression with Mcl-1 and BHRF1 but not with Bcl-2, further indicating that Bok heterodimerized only with selective anti-apoptotic Bcl-2 proteins. Northern blot analysis indicated that Bok was highly expressed in the ovary, testis and uterus. In situ hybridization analysis localized Bok mRNA in granulosa cells, the cell type that underwent apoptosis during follicle atresia. Identification of Bok as a new pro-apoptotic Bcl-2 protein with restricted tissue distribution and heterodimerization properties could facilitate elucidation of apoptosis mechanisms in reproductive tissues undergoing hormone-regulated cyclic cell turnover.
Keywords: apoptosis, programmed cell death, ovary, testis, uterus
Apoptosis or programmed cell death is important during embryonic development, metamorphosis, tissue renewal, hormone-induced tissue atrophy, and many pathological conditions. In multicellular organisms, apoptosis ensures the elimination of superfluous cells including those that are generated in excess, have already completed their specific functions or are harmful to the whole organism. In reproductive tissues that are characterized by cyclic functional changes, massive cell death occurs under the control of hormonal signals. A growing body of evidence suggests that the intracellular “death program” activated during apoptosis is similar in different cell types and conserved during evolution (1, 2).
Apoptosis involves two essential steps. The Bcl-2 family of proteins that consists of different anti- and pro-apoptotic members is important in the “decision” step of apoptosis (3). In contrast, the “execution” phase of apoptosis is mediated by the activation of caspases, cysteine proteases homologous to the Caernorhabditis elegans protease ced-3, that induce cell death via the proteolytic cleavage of substrates vital for cellular homeostasis (4, 5). Bcl-2-related proteins act upstream from caspases in the cell death pathway (6). Recent studies demonstrated that another C. elegans gene, ced-4, or its mammalian homolog Apaf-1 can bridge between Bcl-2/ced-9 family members and caspases (7, 8).
The protooncogene Bcl-2 was isolated at the breakpoint of the t(14, 18) chromosomal translocation associated with follicular B-cell lymphoma (9). Overexpression of Bcl-2 suppresses apoptosis induced by a variety of agents both in vitro and in vivo (10). Subsequent studies identified a family of Bcl-2-related proteins possessing several conserved Bcl-2 homology (BH) domains important for homo- or heterodimerization between family members (11–13). In addition, a C-terminal membrane anchoring region is also conserved in most members. Based on their differential roles in regulating apoptosis, the Bcl-2-related proteins can be separated into anti-apoptotic (Bcl-2, Bcl-xL, Mcl-1, Bcl-w, and Bfl-1/A1) and pro-apoptotic members (Bax, BAD, Bak, Bik, Hrk, and BID). Through heterodimerization, the balance between pro- and anti-apoptotic Bcl-2 proteins presumably determines the cell fate (3, 13). It has also been found that the anti-apoptotic effect of Bcl-2 is not universal because Bcl-2 overexpression is not effective in blocking Fas-mediated apoptosis and the apoptosis of autoreactive thymocytes during negative selection (13, 14). Recent identification of multiple Bcl-2-related proteins suggests that selective Bcl-2 members may act in a tissue- and dimerization-specific manner.
Less than 1% of ovarian follicles endowed during early life can ovulate, whereas the remaining 99% undergo apoptosis (15). To elucidate the apoptotic mechanism during ovarian follicle demise, we examined the expression pattern of different anti-apoptotic Bcl-2 members in the ovary and found Mcl-1 to be highly expressed. Using Mcl-1 as bait to screen an ovarian fusion cDNA library in the yeast two-hybrid system, we isolated Bcl-2-related ovarian killer (Bok), a new Bcl-2 family member containing conserved BH1, -2, and -3 domains and a C-terminal transmembrane region. Overexpression of Bok induced apoptosis in mammalian cells that could be blocked by some, but not other, anti-apoptotic Bcl-2 members. Furthermore, Northern blot analysis showed that the Bok transcript is expressed mainly in the reproductive tissues including the ovary, testis, and uterus.
MATERIALS AND METHODS
Two-Hybrid Screening.
The full-length ORF of rat Mcl-1 cDNA was fused in frame with the GAL4-binding domain into the pGBT-9 yeast shuttle vector (CLONTECH). This vector was used to identify Mcl-1-interacting proteins by screening 1.5 million transformants from a GAL4-activation domain (AD)-tagged ovarian fusion cDNA library. The ovarian cDNAs were prepared from 27-day-old Sprague–Dawley rats primed for 36 h with 10 units equine gonadotropin. Yeast cells were first transformed with pGBT9-Mcl-1 and colonies selected in plates deficient for tryptophan. In the second step, cells were transformed with cDNAs from the ovarian library before selection of clones in plates lacking tryptophan, leucine, and histidine. Positive transformants were further selected for growth in media containing 5 mM 3-aminotriazole. Individual AD-fusion cDNAs were retrieved following transformation of Escherichia coli cells.
A total of 40 potential Mcl-1-interacting clones was rescreened against the empty vector or vectors encoding different Bcl-2 proteins to eliminate false positives. Three clones of Bok cDNAs were isolated based on their ability to interact with Mcl-1 in an HF7c yeast reporter strain (16). DNA sequence analysis and comparison with known genes using the blastx algorithm (17) revealed that the positive clones encode a polypeptide sharing high homology with Bcl-2 proteins. Further analysis of expressed sequence tags in the GenBank revealed that expressed sequence tag accession number AA103989 has >98% identity with the 5′sequence of cloned cDNA and contains extra 5′sequence of the murine Bok homolog. Full-length ORF and 5′untranslated sequence of rat Bok were obtained by PCR using the GAL4-AD-tagged ovarian Matchmaker cDNA library (CLONTECH) as the template and an upstream primer based on murine expressed sequence tags. Complementary DNA fragments with an identical ORF were also obtained in separate PCR using a rat brain cDNA library (Stratagene) as the template. Interactions between Bok and different Bcl-2 members were assessed in the yeast two-hybrid system using pGBT9 GAL4-binding domain and pGADGH GAL4-AD vectors (18). Specific binding of different protein pairs was evaluated based on the activation of GAL1-HIS3 and GAL4-lacZ reporter genes.
Cell Culture and Transfection with Plasmids.
For the expression of Bcl-2 proteins in eukaryotic cells, PCR-generated ORF of different cDNAs were subcloned into the pcDNA3 vector (Invitrogen). Following transfection of cDNAs, cell death was monitored (19). Chinese hamster ovary (CHO) cells (2 × 105 per well) were cultured in DMEM/F12 supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mM glutamine. One day later, cells were transfected using the lipofectamine procedure (Life Technologies, Gaithersburg, MD) with the empty pcDNA3 expression vector or the same vector containing different cDNAs, together with 1/10–1/20 fractions of an indicator plasmid pCMV-β-galactosidase (β-gal) to allow the identification of transfected cells. Inclusion of 10- to 20-fold excess of expression vectors as compared with the pCMV-β-gal reporter plasmid ensured that most of the β-gal-expressing cells also expressed the protein(s) under investigation. Cells were incubated with plasmids in a serum-free medium for 4 h, followed by addition of fetal bovine serum to a final concentration of 5% and further incubation for 14 h. After an additional culture in fresh medium for 18 h, cells were fixed by 0.25% glutaraldehyde and stained with 5-bromo-4-chloro-3-indolyl β-d-galactoside (0.4 mg/ml) to detect β-gal expression. The number of blue cells was counted by microscopic examination (19). To verify the nature of cell death, total cellular DNA was extracted for 3′end labeling of DNA ends at 18 h after transfection with 32P-ddATP before gel fractionation to identify internucleosomal DNA fragmentation (20). Statistical differences among treatment groups were analyzed using one-way ANOVA and Scheffe F test.
Northern and Southern Blots and in Situ Analyses.
For Northern blot analysis of Bok expression, tissues were collected from 27-day-old Sprague–Dawley rats (Simonsen Laboratories, Gilroy, CA). For Bok expression in ovarian cells, ovaries were obtained from 26-day-old rats implanted for two days with a diethylstilbestrol capsule to stimulate development of multiple early antral follicles (21). Granulosa cells were prepared by needle puncture. For the extraction of total RNA, tissues were homogenized in Tri-Reagent solution (Molecular Research Center, Cincinnati) and at least two pools from each treatment group were used. In addition, poly(A)+ RNA was isolated using the Oligotex oligo-dT resin (Qiagen, Chatsworth, CA). Aliquots of each sample were denatured and fractionated in 1% agarose gels containing formaldehyde before Northern blot analysis. Membranes were prehybridized for 4 h at 65°C in a solution containing 50% formamide, 5× sodium phosphate buffer, 5× Denhardt’s solution, 0.5% SDS, and 500 μg/ml yeast tRNA. This was followed by overnight hybridization in the same conditions but with 1 × 106 cpm/ml of 32P-labeled Bok or glyceraldehyde-3-phosphate dehydrogenase cRNA probe. After hybridization, the membranes were washed twice in 2× standard saline citrate (SSC, 1× SSC = 0.15 M sodium chloride/0.015 M sodium citrate, pH 7) and 1% SDS at room temperature, followed by two washes in 0.1× SSC and 1% SDS at 65°C before exposure to Kodak RX films. For studies on the conservation of the Bok gene, the Zoo blot (CLONTECH) containing genomic DNA from different vertebrates was hybridized with a 32P-labeled rat Bok cDNA probe under stringent conditions.
For in situ hybridization analysis of Bok mRNA expression, ovaries from 26-day-old rats pretreated with 10 units equine chorionic gonadotropin 2 days earlier were isolated and fixed at 4°C for 4 h in 4% paraformaldehyde in PBS (pH 7.4), followed by overnight dehydration in 0.5 M sucrose. Tissue blocks were embedded in Tissue-Tek solution (Sakura Finetek USA, Torrence, CA) and snapped frozen in liquid nitrogen. Cryosections (12 μm thick) were mounted on charged microscopic slides (Fisher Scientific), postfixed in 4% paraformaldehyde and stored at −70°C for up to 1 month. Hybridization and washes of cryosections were as described (22). After 2 weeks of exposure under NTB2 emulsion (Kodak), the slides were developed, counterstained, and mounted with Permount (Fisher Scientific) for photography using a Nikon Optiphot microscope.
RESULTS
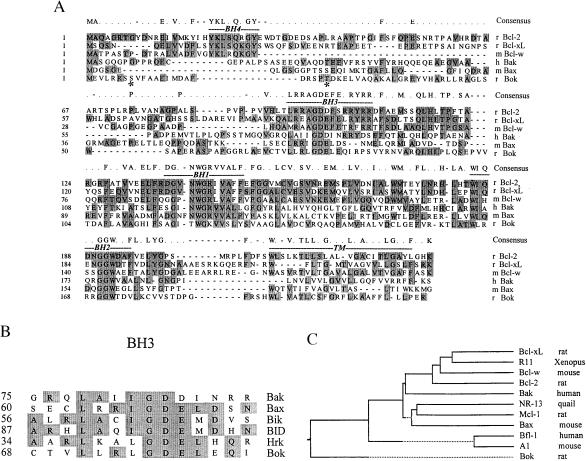
Using the anti-apoptotic protein Mcl-1 as bait, we screened an ovarian fusion cDNA library and isolated three Bok clones. Subsequent DNA sequencing and identification of homologous murine-expressed sequence tags allowed isolation of full-length Bok cDNAs following PCR of ovarian and brain cDNA libraries. The ORF of Bok encoded a protein of 213 amino acids showing no identity with any known gene. The novel protein has a predicted molecular mass of 23.5 kDa and a pI of 9.1. The methionine initiation codon conformed to the consensus Kozak sequence and hydrophobicity analysis predicted the presence of a C-terminal transmembrane domain. In addition, two potential phosphorylation sites were found near the N-terminal region. Comparison of DNA sequences among different Bcl-2 proteins indicated that Bok was a novel member of this family showing conserved BH1, -2, and -3 domains (Fig. 1A). However, the BH4 domain, known to be important for the anti-apoptotic function of mammalian Bcl-2 proteins, was missing in Bok. Closer comparison indicated the core BH1 domain of Bok (TWGK) was less conserved as compared with other Bcl-2 proteins (NWGR). For the BH3 domain found in pro-apoptotic members (Fig. 1B), the core sequence (GDE) was conserved in Bok but the flanking sequences were different. Furthermore, analysis of the phylogenetic relatedness of the different Bcl-2 members suggests that during evolution Bok diverged early from other Bcl-2 proteins (Fig. 1C).
Figure 1.
Sequence comparison between Bok and different Bcl-2 family members. (A) Sequence alignment of Bok with several members of the Bcl-2 family proteins. The ORF for Bok predicts a protein of 213 amino acids in length. Asterisks indicate two potential phosphorylation sites. Shaded residues are identical in at least three of the six Bcl-2 proteins shown. Consensus sequences for these proteins are listed on top of the sequence alignment. The conserved BH domains (1–4) and the C-terminal transmembrane region are indicated. The GenBank accession number for Bok is AF027954. r: rat, m: mouse, h: human. (B) Comparison of BH3 domain sequences in different pro-apoptotic Bcl-2 proteins. (C) Phylogenetic relatedness of different Bcl-2 family members. Full-length polypeptides for all Bcl-2 members except Mcl-1 (amino acids 121–330 only) are used for comparison.
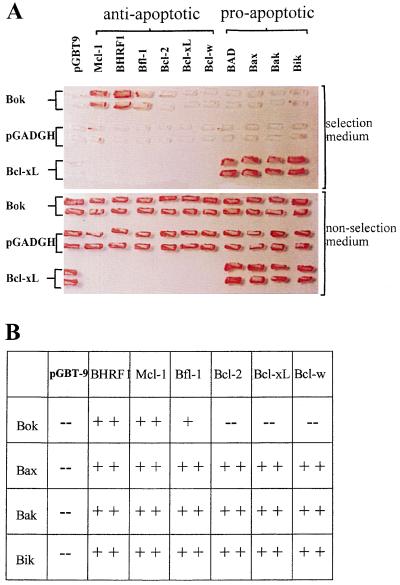
Using the yeast two-hybrid system, we investigated interactions between Bok and different anti- and pro-apoptotic Bcl-2 proteins. As shown in Fig. 2A, Bok interacted strongly with anti-apoptotic proteins Mcl-1 and BHRF1, minimally with Bfl-1 and negligibly with Bcl-2, Bcl-xL, and Bcl-w. We further tested interactions between Bok and several pro-apoptotic Bcl-2 proteins (Fig. 2A). Of interest, Bok did not interact with any pro-apoptotic members tested. To demonstrate that the lack of interactions between Bok and pro-apoptotic Bcl-2 proteins was not due to the killing of yeast cells by these apoptosis agonists, we also tested the growth of yeast cells cotransformed with Bcl-xL and different pro-apoptotic proteins (Fig. 2A). Although Bcl-xL showed negligible interaction with Bok, it interacted strongly with all the pro-apoptotic members tested.
Figure 2.
Bok only interacts with selective anti-apoptotic Bcl-2 proteins in the yeast two-hybrid system. (A) Upper: yeast cells were grown in the selective media containing 5 mM 3-aminotriazole and without tryptophan, leucine, and histidine. Prominent growth of yeast colonies expressing Bok fused to the GAL4 activation domain together with Mcl-1, BHRF1, or Bfl-1 fused to the GAL4 binding domain could be seen. Minimal growth of yeast colonies was found in cells that express the same Bok expressing vector together with Bcl-2, Bcl-xL, Bcl-w, BAD, Bax, Bak, or Bik fused to the GAL4 binding domain. In addition, prominent growth of colonies expressing Bcl-xL and different pro-apoptotic Bcl-2 proteins indicated that the lack of growth in yeast cells expressing Bok and different pro-apoptotic family members was not due to suppression of cell growth by these pro-apoptotic proteins. Lower: growth of yeast colonies transformed with the same vector pairs maintained in a nonselective media. (B) Summary of protein-protein interactions between pairs of pro-apoptotic proteins (Bok, Bak, Bik, and Bax) and different anti-apoptotic Bcl-2 members. The positive signs indicate prominent (++) or moderate (+) yeast cell growth whereas the negative signs (−) indicate the absence of reporter gene expression.
To further study the restricted dimerization property of Bok with selective anti-apoptotic proteins, we tested the growth of yeast cells that were cotransformed with different pairs of pro- and anti-apoptotic Bcl-2 proteins. Several pro-apoptotic proteins (Bak, Bik, and Bax), unlike Bok, all interacted strongly with diverse anti-apoptotic proteins tested (Fig. 2B), suggesting the restricted heterodimerization property of Bok was unique.
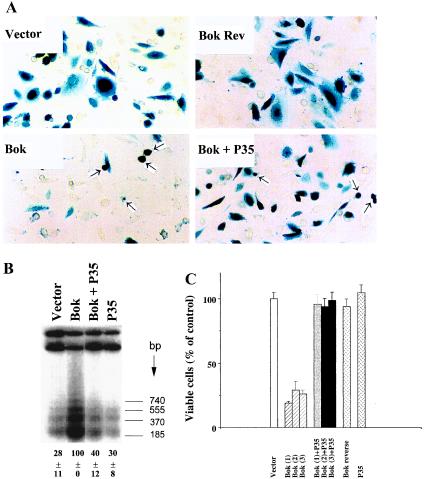
The ability of Bok to regulate apoptosis in mammalian cells was investigated. In CHO cells, transfection with expression vectors encoding Bok for 36 h induced cell death (Fig. 3A). The pro-apoptotic effect of Bok was specific because transfection of the empty plasmid or the same plasmid containing Bok cDNA in reverse orientation did not affect cell survival. Furthermore, coexpression of P35, a cysteine protease inhibitor derived from the baculovirus (23), prevented Bok-induced cell killing as indicated by increases in the number of viable cells (Fig. 3A). The 3′end labeling of genomic DNA fragments at an earlier time point (18 h) further demonstrated the induction of internucleosomal DNA fragmentation following Bok overexpression, confirming the induction of apoptosis (Fig. 3B). The observed DNA fragmentation was blocked by coexpression with P35. Quantitative analysis also indicated that Bok overexpression decreased viable cell number by 75% whereas coexpression of P35 completely reversed Bok killing (Fig. 3C), substantiating the involvement of caspases in Bok action.
Figure 3.
Overexpression of Bok promotes apoptosis in mammalian cells. (A) Morphology of CHO cells transfected with an expression vector encoding Bok. Normal cell morphology was found in cells transiently transfected with the empty pcDNA3 expression vector (2.1 μg DNA per 35-mm dish) or the vector containing Bok cDNA in reverse orientation (Bok Rev). Cells were also transfected with the Bok expression vector without or with an equal amount of the P35-expressing construct. Arrowheads indicate apoptotic cells whereas darkly stained cells represent transfected cells. (B) Internucleosomal DNA fragmentation induced by Bok and partial inhibition by the baculoviral protease inhibitor P35. CHO cells were treated as described in A. At 18 h after transfection, cellular DNA was extracted for analysis of DNA fragmentation using a 3′end labeling method. Quantitative estimation of low molecular weight DNA is shown at the bottom of the figure (mean ± SEM, n = 3). (C) Quantitative analysis of cell killing by Bok and the inhibitory effects of P35. The number of β-gal-expressing cells (mean ± SEM, n = 3) was determined at 36 h after transfection. Data from cells transfected with three independent clones (1, 2, and 3) encoding Bok are presented as a percentage of viable cells in the control group. CHO cells were transfected with a total of 2.1 μg plasmid DNA including 2.0 μg of pcDNA3 expression constructs and 0.1 μg of the pCMV-β-gal reporter. In cells transfected with two different pcDNA3 expression plasmids, 1.0 μg each was used. Similar results were obtained in three separate experiments.
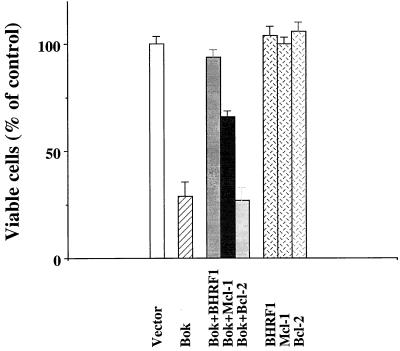
We further tested if the restricted heterodimerization of Bok with selective anti-apoptotic Bcl-2 members found in the yeast two-hybrid system could be substantiated in mammalian cells. Bok was coexpressed with Mcl-1, BHRF1, or Bcl-2 in CHO cells. As shown in Fig. 4, Bok-induced apoptosis was attenuated following coexpression with Mcl-1 or BHRF1; but coexpression with Bcl-2 was ineffective in blocking Bok action. Transfection of the same Bcl-2 expression vector was, however, capable of blocking apoptosis induced by staurosporin (data not shown).
Figure 4.
Suppression of Bok-induced apoptosis by selective anti-apoptotic Bcl-2 members in CHO cells. Cell killing by Bok and the antagonistic effects of Mcl-1 and BHRF1 were analyzed. Cell transfection and estimation of apoptosis were as described in the Fig. 3 legend. Coexpression of Bcl-2 was ineffective in suppressing Bok-induced apoptosis.
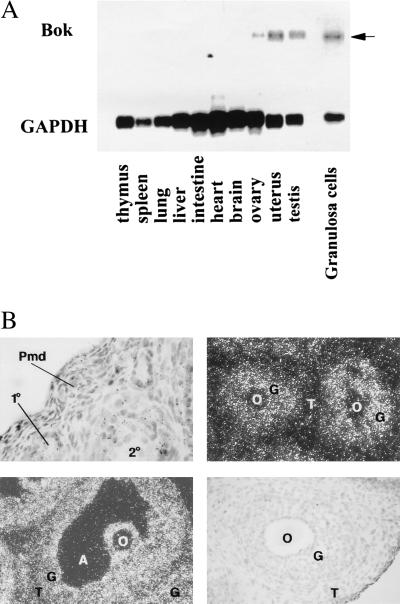
The expression of Bok mRNA in diverse rat tissues was examined. As shown in Fig. 5A, high levels of Bok transcript of ≈1.5 kb were abundant in the ovary, testis, and uterus but negligible in other tissues examined. Further analysis of Bok mRNA in isolated granulosa cells demonstrate high levels of expression in these cells that undergo apoptosis during follicle degeneration. In situ hybridization analyses further confirmed high levels of the Bok transcript in the granulosa cells of antral and preantral follicles, with minimal signals in theca and interstitial cells (Fig. 5B).
Figure 5.
Expression of Bok mRNA transcripts in rat tissues. (A) For Northern blot analysis, poly(A)+-selected RNA from different tissues of rats at 27 days of age or from isolated granulosa cells of estrogen-treated rats was hybridized with a 32P-labeled Bok cRNA probe. After washing, the blots were exposed to x-ray films at −70°C for five days. Subsequent hybridization with a glyceraldehyde-3-phosphate dehydrogenase cRNA probe was performed to estimate nucleic acid loading (8 h exposure). Specific Bok transcripts are indicated by an arrow. (B) In situ hybridization analysis of Bok expression in the ovary. Ovaries from immature equine chorionic gonadotropin-treated rats were probed with the antisense Bok cRNA. Upper, Left: hybridization signals in representative primary (1°) and secondary (2°) but not primordial (Pmd) follicles (magnification, ×100). Upper, Right (magnification, ×200) and Lower, Left (magnification, ×100): positive signals in granulosa cells of preantral follicles and an antral follicle, respectively. Lower, Right: no signal was found in a section hybridized with the sense Bok probe. O, oocyte; G, granulosa cells; T, theca cells; A, antrum.
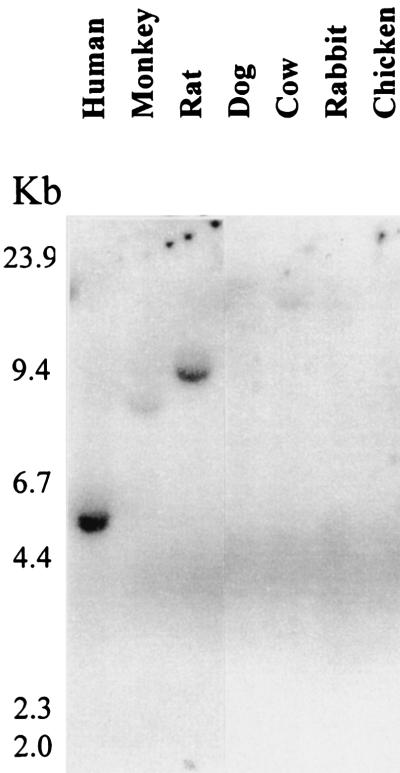
Conservation of the Bok gene in diverse vertebrates was tested using Southern blot hybridization of genomic DNA from different species. Under high stringency washing conditions, the rat cDNA hybridized strongly with rat and human genomic DNA, weakly with monkey DNA and negligibly with DNA from other species (Fig. 6).
Figure 6.
Conservation of Bok in diverse vertebrate species. Southern blot analysis of genomic DNA from different vertebrate species was performed. DNA was digested with the EcoRI enzyme and probed with a Bok cDNA probe. Following hybridization at 68°C, the membrane was washed under high stringency conditions (1% SDS/0.1 × SSC at 65°C) before exposure.
DISCUSSION
We have identified a new pro-apoptotic Bcl-2-related protein Bok, based on its binding to an ovarian anti-apoptosis protein Mcl-1. In addition to its restricted expression in several reproductive tissues, Bok also shows a selective heterodimerization property by interacting with some (Mcl-1, BHRF1, and Bfl-1) but not other (Bcl-2, Bcl-xL, and Bcl-w) anti-apoptotic proteins. Coupled with findings showing that Bok-induced apoptosis could only be antagonized by selective anti-apoptotic proteins, the present data suggest that different pro- and anti-apoptotic Bcl-2 protein pairs may play tissue-specific roles in the regulation of apoptosis. Because of the restricted expression of Bok to ovarian granulosa cells and several reproductive tissues characterized by hormonally regulated cyclic cell turnover, further analyses of Bok action in the gonads and uterus could provide unique models to study the hormonal regulation of apoptosis. Because most of the Bcl-2-related proteins have been identified in the lymphoid system, the present yeast two-hybrid screen provides an experimental paradigm to isolate novel Bcl-2 homologs essential for apoptosis regulation in other tissues.
Although the mechanism by which the Bcl-2 proteins participates in the “decision” step of apoptosis is not clear, the ratio of anti- and pro-apoptotic Bcl-2 members and their hetero- and homodimerization are believed to determine whether a cell will respond to an apoptotic signal (11–13). Among the Bcl-2 family of proteins, several homology domains have been found to be essential for their function. Bok contains conserved BH1, -2, and -3 domains but lacks the BH4 domain found in most anti-apoptotic members. In addition, the conserved NH1 region important for the survival function of several anti-apoptotic Bcl-2 proteins (24, 25) is also absent in Bok. Consistent with its structural features, overexpression of Bok in CHO cells induces apoptosis based on observed cell morphology and internucleosomal DNA fragmentation. Bok-induced cell killing, like that induced by Bax and BAD (26, 27), is mediated by caspases as demonstrated by the suppressive actions of the baculoviral P35 protein.
Among the pro-apoptotic Bcl-2 proteins, Bok is most similar to Bax and Bak in having the BH1, -2, and -3 domains plus the C-terminal transmembrane sequence. Studies on Bax and Bak with truncation in different BH domains suggested that these pro-apoptotic proteins might exert their effects by heterodimerizing with Bcl-2 or Bcl-xL (12, 28). The remaining pro-apoptotic members (Bik, Hrk, and BID) do not have BH1 and BH2 domains but retain the BH3 domain (29–32). Among them, the soluble BID has been proposed to serve as a death ligand for the membrane-bound receptor Bax through interactions between its amphipathic helical BH3 domain and the BH1 domain of Bax (31).
Competitive dimerization between selective pairs of anti- and pro-apoptotic Bcl-2 proteins is believed to be involved in the “decision” step of apoptosis (3, 13, 28). Furthermore, interactions among the Bcl-2 family of proteins appear to exhibit a defined selectivity and hierarchy (30, 32, 33–36). For example, the anti-apoptotic E1B protein shows preferential binding to pro-apoptotic Bcl-2 proteins, whereas the pro-apoptotic Hrk binds only to the anti-apoptotic family member. Thus, the pro-apoptotic protein Bok may regulate apoptosis through similar mechanisms by forming heterodimers with selective anti-apoptotic proteins.
Analysis of the relatedness of amino acid sequences of different Bcl-2 proteins indicated that Bok is not closely related to any particular Bcl-2 member and probably diverged early during evolution. The less conserved BH1 domain of Bok may determine its unique heterodimerization property. In both yeast and mammalian cells, Bok interacts with some but not other anti-apoptotic proteins, suggesting the possible evolution of selective pairs of death agonists and antagonists with restricted heterodimerization properties to confer specificity of the death program. Apoptosis induced by Bok in transfected CHO cells could be mediated through inhibition of the protection afforded by Mcl-1 or other Bok partners. It is likely that Bok may interact with its dimerization partner(s) including Mcl-1 and Bfl-1 in reproductive tissues to regulate apoptosis. Of interest, our recent data indicated that Mcl-1, but not Bcl-2, is highly expressed in ovarian cells (data not shown). Based on the suppression of Bok-induced apoptosis by BHRF1, it is possible that reproductive tissues expressing Bok are potential targets for this anti-apoptotic protein encoded by the Epstein–Barr virus (37). Recent studies have suggested that anti-apoptotic proteins may bind to ced-4/Apaf-1 homologs, which, in turn, activate downstream caspases (7, 8, 38, 39). Elucidation of the heterodimerization partner(s) for Bok in gonads and uterus would allow characterization of the putative ced-4 homologs in these tissues.
Recent crystallographic analyses of complexes formed between the anti-apoptotic protein Bcl-xL and the BH3 domain of the pro-apoptotic Bak indicated that the α-helix in the BH3 domain of different Bcl-2 proteins plays a central role in defining the binding specificity to Bcl-xL (40). Because Bok does not interact with Bcl-xL in the yeast two-hybrid system, further studies on the BH3 region of Bok and related proteins could define the specificity of heterodimerization among different pro- and anti-apoptotic protein pairs and their role in apoptosis regulation.
Although overexpression of Bax and Bak induces yeast cell death (26, 41), the present Bok fusion protein did not affect yeast cell survival. In addition, lack of interactions between Bok and different pro-apoptotic Bcl-2 proteins in the two-hybrid assay are not due to detrimental effects of these death agonists on yeast cells because cotransformation of these apoptosis agonists with Bcl-xL led to activation of the reporter genes. It is likely that moderate expression of these death agonists using the present expression vector may not significantly affect yeast cell survival, thus allowing studies on interactions between different Bcl-2 proteins.
The majority of ovarian follicles and ≈50% of testicular germ cells undergo apoptosis under normal physiological conditions (15), whereas the menstruation involves monthly apoptosis of uterine endometrial cells (42). The restricted expression of Bok in the gonads and uterus suggests its potential role in the regulation of apoptosis in these tissues. It is likely that selective pairs of Bcl-2 agonists/antagonists may play tissue-specific roles in the regulation of apoptosis. Indeed, mutant mice deficient in Bcl-2 or Bax showed abnormality in apoptosis regulation only in distinct cell lineage (43–45). Although Bax-deficient mice showed an accumulation of granulosa cells in atretic follicles, these cells were still apoptotic (43), suggesting the involvement of additional pro-apoptotic factors during ovarian follicle atresia. Because the pro-apoptotic protein Bax has been suggested to function as a tumor suppressor gene in colon adenocarcinomas (46–48) and because inactivation of Bax in transgenic mice leads to enhanced tumorigenesis (47), it would also be interesting to investigate changes in Bok function during gonadal and uterine tumorigenesis. Because cyclic variations in reproductive hormones are essential in the regulation of apoptosis in gonadal and uterine tissues, future investigations on the hormonal regulation of Bok and its dimerization partner(s) in these reproductive tissues would allow the design of novel strategies to modulate reproductive functions. These studies could also provide understanding of the role of Bok in gonadal and uterine diseases associated with aberrant regulation of apoptosis.
Acknowledgments
We thank C. Spencer for editorial assistance. We also acknowledge the following investigators for the provision of cDNAs for Bcl-2-related proteins: M. Cleary (Stanford, CA; Bcl-2), S. Cory (Victoria, Australia; Bcl-w), G. Chinnadurai (St. Louis; Bik and Bfl-1/A1), A. Rickinson (Birmingham, England; BHRF1), T. Chittenden (Cambridge, MA; Bak), and C. Thompson (Chicago; Bclx-L). This work was supported by the National Institutes of Health Grant HD 31566. S.Y.H. is a recipient of National Institutes of Health Training Grant HD 7493.
ABBREVIATIONS
- BH
Bcl-2 homology
- AD
activation domain
- Bok
Bcl-2-related ovarian killer
- β-gal
β-galactosidase
- CHO
Chinese hamster ovary
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF027954).
References
- 1.Thompson C B. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 2.Steller H. Science. 1995;267:1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- 3.Kroemer G. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 4.Miura M, Zhu H, Rotello R, Hartwieg E A, Yuan J. Cell. 1993;75:653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- 5.Yuan J, Shaham S, Ledoux S, Ellis H M, Horvitz H R. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- 6.Hengartner M O, Horvitz H R. Cell. 1994;76:665–676. doi: 10.1016/0092-8674(94)90506-1. [DOI] [PubMed] [Google Scholar]
- 7.Chinnaiyan A M, O’Rourke K, Lane B R, Dixit V M. Science. 1997;275:1122–1126. doi: 10.1126/science.275.5303.1122. [DOI] [PubMed] [Google Scholar]
- 8.Zou H, Henzel W J, Liu X, Lutschg A, Wang X. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 9.Cleary M L, Smith S D, Sklar J. Cell. 1986;47:19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- 10.Nunez G, London L, Hockenbery D, Alexander M, McKearn J P, Korsmeyer S J. Immunology. 1990;144:3602–3610. [PubMed] [Google Scholar]
- 11.Yin X, Oltvai Z N, Korsmeyer S J. Nature (London) 1994;369:321–323. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]
- 12.Chittenden T, Flemington C, Houghton A B, Ebb R G, Gallo G J, Elangovan B, Chinnadurai G, Lutz R J. EMBO J. 1995;14:5589–5596. doi: 10.1002/j.1460-2075.1995.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White E. Genes Dev. 1996;10:1–15. doi: 10.1101/gad.10.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Cory S, Harris A W, Strasser A. Philos Trans R Soc London B. 1994;345:289–295. doi: 10.1098/rstb.1994.0108. [DOI] [PubMed] [Google Scholar]
- 15.Hsueh A J W, Eisenhauer K, Chun S-Y, Hsu S-Y, Billig H. Recent Prog Horm Res. 1996;51:433–455. [PubMed] [Google Scholar]
- 16.Fields S, Song O. Nature (London) 1989;340:245–247. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 17.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 18.Bartel P L, Chien C T, Sternglanz R, Field S. In: Cellular Interaction in Development: A Practical Approach. Hartley D A, editor. Oxford: Oxford Univ. Press; 1993. pp. 153–179. [Google Scholar]
- 19.Kumar S, Kinoshita M, Noda M, Copeland N G, Jenkins N A. Genes Dev. 1994;8:1613–1626. doi: 10.1101/gad.8.14.1613. [DOI] [PubMed] [Google Scholar]
- 20.Hsu S-Y, Lai R J-M, Finegold M, Hsueh A J W. Endocrinology. 1996;137:4837–4843. doi: 10.1210/endo.137.11.8895354. [DOI] [PubMed] [Google Scholar]
- 21.Bicsak T A, Tucker E M, Cappel S, Vaughan J, Rivier J, Vale W, Hsueh A J W. Endocrinology. 1986;119:2711–2719. doi: 10.1210/endo-119-6-2711. [DOI] [PubMed] [Google Scholar]
- 22.Hsu S-Y, Kubo M, Chun S-Y, Haluska F G, Housman D E, Hsueh A J W. Mol Endocrinol. 1995;9:1356–1366. doi: 10.1210/mend.9.10.8544844. [DOI] [PubMed] [Google Scholar]
- 23.Bump N J, Hackett M, Hugunin M, Seshagiri S, Brady K, Chen P, Ferenz C, Franklin S, Ghayur T, Li P, Licari P, Mankovich J, Shi L, Greenberg A, Miller L, Wong W. Science. 1995;269:1885–1888. doi: 10.1126/science.7569933. [DOI] [PubMed] [Google Scholar]
- 24.Gibson L, Holmgreen S P, Huang D C, Bernard O, Copeland N G, Jenkins N A, Sutherland G R, Baker E, Adams J M, Cory S. Oncogene. 1996;13:665–675. [PubMed] [Google Scholar]
- 25.Subramanian T, Tarodi B, Chinnadurai G. Cell Growth Differ. 1995;6:131–137. [PubMed] [Google Scholar]
- 26.Jurgensmeier J M, Krajewski S, Armstrong R C, Wilson G M, Oltersdorf T, Fritz L C, Reed J C, Ottilie S. Mol Cell Biol. 1997;8:325–339. doi: 10.1091/mbc.8.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu S Y, Kaipia A, Zhu L, Hsueh A J W. Mol Endocrinol. 1997;11:1858–1867. doi: 10.1210/mend.11.12.0023. [DOI] [PubMed] [Google Scholar]
- 28.Reed J C. Nature (London) 1997;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 29.Boyd J M, Gallo G J, Elangovan B, Houghton A B, Malstrom S, Avery B J, Ebb R G, Subramanian T, Chittenden T, Lutz R J, Chinnadurai G. Oncogene. 1996;11:1921–1928. [PubMed] [Google Scholar]
- 30.Han J, Sabbatini P, White E. Mol Cell Biol. 1996;16:5857–5864. doi: 10.1128/mcb.16.10.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang K, Yin X M, Chao D T, Milliman C L, Korsmeyer S J. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 32.Inohara N, Ding L, Chen S, Nunez G. EMBO J. 1997;16:1686–1694. doi: 10.1093/emboj/16.7.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato T, Hanada M, Bodrug S, Irie S, Iwama N, Boise L H, Thompson C B, Golemis E, Fong L, Wang H G, Reed J C. Proc Natl Acad Sci USA. 1994;91:9238–9242. doi: 10.1073/pnas.91.20.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sedlak T W, Oltvai Z N, Yang E, Wang K, Boise L H, Thompson C B, Korsmeyer S J. Proc Natl Acad Sci USA. 1995;92:7834–7838. doi: 10.1073/pnas.92.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen G, Branton P E, Yang E, Korsmeyer S J, Shore G C. J Biol Chem. 1996;171:24221–24225. doi: 10.1074/jbc.271.39.24221. [DOI] [PubMed] [Google Scholar]
- 36.Cheng E H, Nicholas J, Bellows D S, Hayward G S, Guo H G, Reitz M S, Hardwick J M. Proc Natl Acad Sci USA. 1997;94:690–694. doi: 10.1073/pnas.94.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marchini A, Tomkinson B, Cohen J I, Kieff E. J Virol. 1991;65:5991–6000. doi: 10.1128/jvi.65.11.5991-6000.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu D, Wallen H D, Nunez G. Science. 1997;275:1126–1129. doi: 10.1126/science.275.5303.1126. [DOI] [PubMed] [Google Scholar]
- 39.Spector M S, Desnoyers S, Hoeppner D J, Hengartner M O. Nature (London) 1997;385:653–656. doi: 10.1038/385653a0. [DOI] [PubMed] [Google Scholar]
- 40.Sattler M, Liang H, Nettesheim D, Meadows R P, Harlan J E, Eberstadt M, Yoon H S, Shuker S B, Chang B S, Minn A J, Thompson C B, Fesik S W. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 41.Tao W K, Kurschner C, Morgan J I. J Biol Chem. 1997;272:15547–15552. doi: 10.1074/jbc.272.24.15547. [DOI] [PubMed] [Google Scholar]
- 42.Kokawa K, Shikone T, Nakano R. J Clin Endocrinol Metab. 1996;81:4144–4147. doi: 10.1210/jcem.81.11.8923873. [DOI] [PubMed] [Google Scholar]
- 43.Knudson C M, Tung K S K, Tourtellotte W G, Brown G A J, Korsmeyer S J. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 44.Veis D J, Sorenson C M, Shutter J R, Korsmeyer S J. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 45.Kamada S, Shimono A, Shinto Y, Tsujimura T, Takahashi T, Noda T, Kitamura Y, Kondoh H, Tsujimoto Y. Cancer Res. 1995;55:354–359. [PubMed] [Google Scholar]
- 46.McCurrach M E, Connor T M, Knudson C M, Korsmeyer S J, Lowe S W. Proc Natl Acad Sci USA. 1997;94:2345–2349. doi: 10.1073/pnas.94.6.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin C, Knudson C M, Korsmeyer S J, Van Dyke T. Nature (London) 1997;385:637–640. doi: 10.1038/385637a0. [DOI] [PubMed] [Google Scholar]
- 48.Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, Reed J C, Perucho M. Science. 1997;275:967–969. doi: 10.1126/science.275.5302.967. [DOI] [PubMed] [Google Scholar]