Abstract
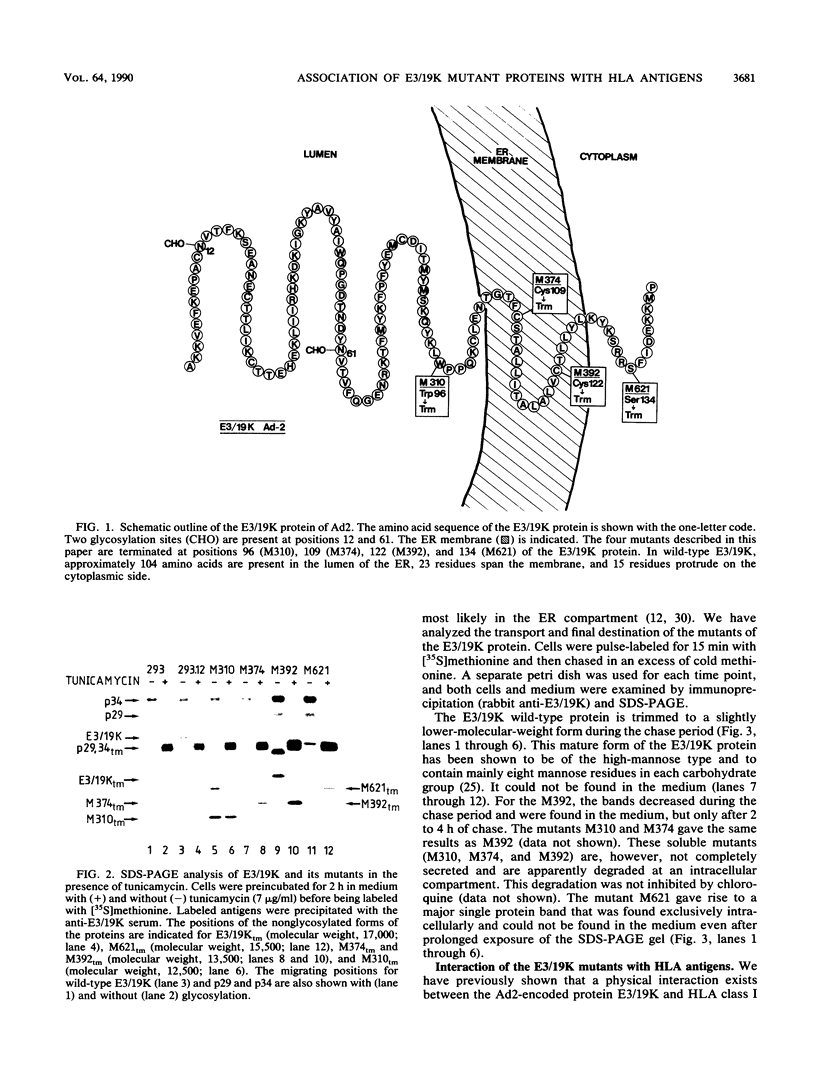
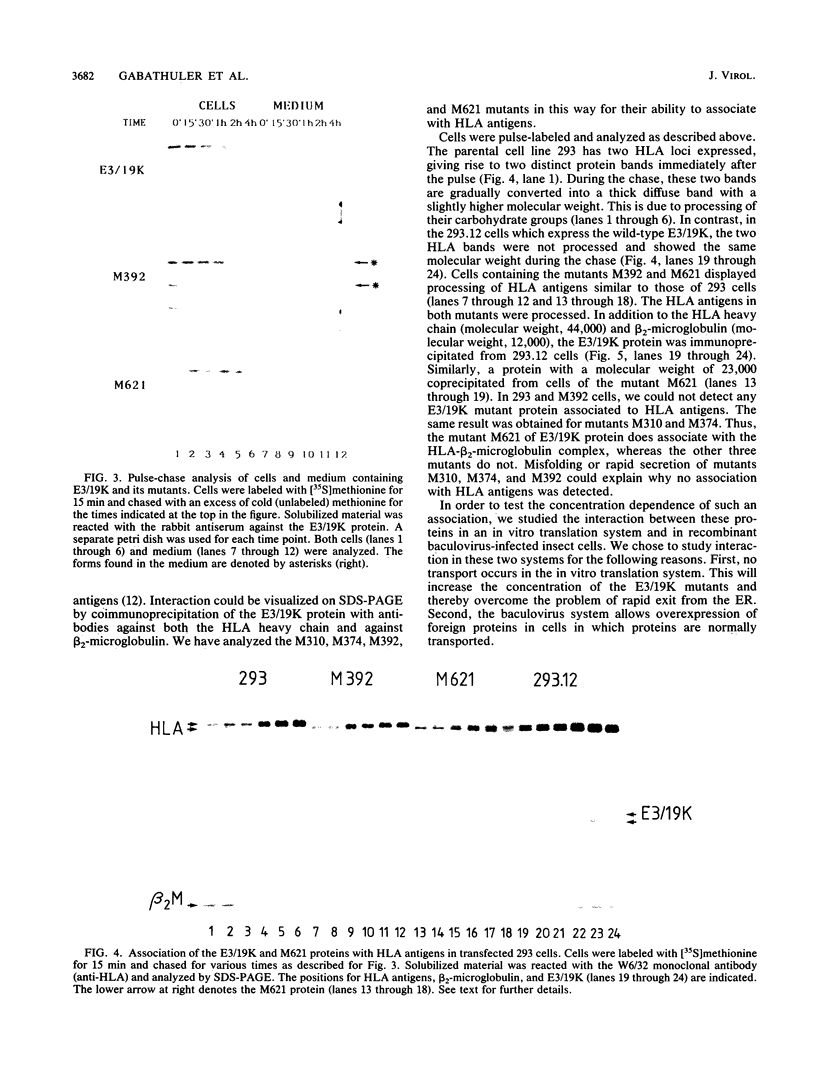
The E3/19K protein of human adenovirus type 2 is a resident of the endoplasmic reticulum (ER). Immediately after synthesis, it associates with major histocompatibility complex class I antigens and prevents their intracellular transport and cell surface expression. We have generated several C-terminal deletion mutants of the E3/19K protein that are preterminated at various positions on both sides of the membrane-spanning segment of the protein. One of these mutants is terminated at the luminal side of the membrane (M310), and two are terminated in the hydrophobic segment (M374 and M392), whereas mutant M621 is terminated on the cytoplasmic side of the ER membrane. The M310, M374, and M392 mutants are soluble proteins. They do not associate with HLA antigens in transfected 293 cells, and they are, to some extent, secreted into the medium. The M621 mutant protein is integrated in the ER membrane, associates immediately after its synthesis with HLA antigens, and exits from the ER. By using either an in vitro translation system supplemented with microsomes or overexpression in insect cells, we showed that M374 and E3/19K are able to associate with HLA antigens. These results indicate that the conformation of the luminal part of the E3/19K protein is not grossly altered by the mutations. Rapid transport of the M374 mutant out of the ER and partial degradation of this protein may prevent the interaction with HLA class I antigens in transfected 293 cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed C. M., Chanda R. S., Stow N. D., Zain B. S. The nucleotide sequence of mRNA for the Mr 19 000 glycoprotein from early gene block III of adenovirus 2. Gene. 1982 Dec;20(3):339–346. doi: 10.1016/0378-1119(82)90202-5. [DOI] [PubMed] [Google Scholar]
- Andersson M., Päbo S., Nilsson T., Peterson P. A. Impaired intracellular transport of class I MHC antigens as a possible means for adenoviruses to evade immune surveillance. Cell. 1985 Nov;43(1):215–222. doi: 10.1016/0092-8674(85)90026-1. [DOI] [PubMed] [Google Scholar]
- Arnold B., Burgert H. G., Hamann U., Hämmerling G., Kees U., Kvist S. Cytolytic T cells recognize the two amino-terminal domains of H-2 K antigens in tandem in influenza A infected cells. Cell. 1984 Aug;38(1):79–87. doi: 10.1016/0092-8674(84)90528-2. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Structure of the adenovirus 2 early mRNAs. Cell. 1978 Jul;14(3):695–711. doi: 10.1016/0092-8674(78)90252-0. [DOI] [PubMed] [Google Scholar]
- Bhat B. M., Wold W. S. Genetic analysis of mRNA synthesis in adenovirus region E3 at different stages of productive infection by RNA-processing mutants. J Virol. 1986 Oct;60(1):54–63. doi: 10.1128/jvi.60.1.54-63.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Braciale T. J., Morrison L. A., Sweetser M. T., Sambrook J., Gething M. J., Braciale V. L. Antigen presentation pathways to class I and class II MHC-restricted T lymphocytes. Immunol Rev. 1987 Aug;98:95–114. doi: 10.1111/j.1600-065x.1987.tb00521.x. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Bodmer W. F., Parham P. Characterization of a monoclonal anti-beta 2-microglobulin antibody and its use in the genetic and biochemical analysis of major histocompatibility antigens. Eur J Immunol. 1979 Jul;9(7):536–545. doi: 10.1002/eji.1830090709. [DOI] [PubMed] [Google Scholar]
- Burgert H. G., Kvist S. An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Cell. 1985 Jul;41(3):987–997. doi: 10.1016/s0092-8674(85)80079-9. [DOI] [PubMed] [Google Scholar]
- Burgert H. G., Kvist S. The E3/19K protein of adenovirus type 2 binds to the domains of histocompatibility antigens required for CTL recognition. EMBO J. 1987 Jul;6(7):2019–2026. doi: 10.1002/j.1460-2075.1987.tb02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgert H. G., Maryanski J. L., Kvist S. "E3/19K" protein of adenovirus type 2 inhibits lysis of cytolytic T lymphocytes by blocking cell-surface expression of histocompatibility class I antigens. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1356–1360. doi: 10.1073/pnas.84.5.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin W. W., Maizel J. V., Jr The polypeptides of adenovirus. VII. Further studies of early polypeptides in vivo and localization of E2 and E2A to the cell plasma membrane. Virology. 1976 Jun;71(2):518–530. doi: 10.1016/0042-6822(76)90378-0. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Broker T. R., Lewis J. B. Complex splicing patterns of RNAs from the early regions of adenovirus-2. J Mol Biol. 1979 Oct 25;134(2):265–303. doi: 10.1016/0022-2836(79)90036-6. [DOI] [PubMed] [Google Scholar]
- EVANS A. S. Latent adenovirus infections of the human respiratory tract. Am J Hyg. 1958 May;67(3):256–266. doi: 10.1093/oxfordjournals.aje.a119932. [DOI] [PubMed] [Google Scholar]
- Garoff H., Simons K., Dobberstein B. Assembly of the Semliki Forest virus membrane glycoproteins in the membrane of the endoplasmic reticulum in vitro. J Mol Biol. 1978 Oct 5;124(4):587–600. doi: 10.1016/0022-2836(78)90173-0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Klein J. The major histocompatibility complex of the mouse. Science. 1979 Feb 9;203(4380):516–521. doi: 10.1126/science.104386. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Wold W. S. Structures of the oligosaccharides of the glycoprotein coded by early region E3 of adenovirus 2. J Virol. 1981 Nov;40(2):440–449. doi: 10.1128/jvi.40.2.440-449.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvist S., Ostberg L., Persson H., Philipson L., Peterson P. A. Molecular association between transplantation antigens and cell surface antigen in adenovirus-transformed cell line. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5674–5678. doi: 10.1073/pnas.75.11.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvist S., Ostberg L., Peterson P. A. Reactions and crossreactions of a rabbit anti-H2 antigen serum. Scand J Immunol. 1978 Apr;7(4):265–276. doi: 10.1111/j.1365-3083.1978.tb00454.x. [DOI] [PubMed] [Google Scholar]
- Kvist S., Wiman K., Claesson L., Peterson P. A., Dobberstein B. Membrane insertion and oligomeric assembly of HLA-DR histocompatibility antigens. Cell. 1982 May;29(1):61–69. doi: 10.1016/0092-8674(82)90090-3. [DOI] [PubMed] [Google Scholar]
- Kämpe O., Bellgrau D., Hammerling U., Lind P., Päbo S., Severinsson L., Peterson P. A. Complex formation of class I transplantation antigens and a viral glycoprotein. J Biol Chem. 1983 Sep 10;258(17):10594–10598. [PubMed] [Google Scholar]
- Leff T., Elkaim R., Goding C. R., Jalinot P., Sassone-Corsi P., Perricaudet M., Kédinger C., Chambon P. Individual products of the adenovirus 12S and 13S EIa mRNAs stimulate viral EIIa and EIII expression at the transcriptional level. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4381–4385. doi: 10.1073/pnas.81.14.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Persson H., Jansson M., Philipson L. Synthesis and genomic site for an adenovirus type 2 early glycoprotein. J Mol Biol. 1980 Feb 5;136(4):375–394. doi: 10.1016/0022-2836(80)90396-4. [DOI] [PubMed] [Google Scholar]
- Persson H., Jörnvall H., Zabielski J. Multiple mRNA species for the precursor to an adenovirus-encoded glycoprotein: identification and structure of the signal sequence. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6349–6353. doi: 10.1073/pnas.77.11.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson H., Signäs C., Philipson L. Purification and characterization of an early glycoprotein from adenovirus type 2-infected cells. J Virol. 1979 Mar;29(3):938–948. doi: 10.1128/jvi.29.3.938-948.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploegh H. L., Orr H. T., Strominger J. L. Major histocompatibility antigens: the human (HLA-A, -B, -C) and murine (H-2K, H-2D) class I molecules. Cell. 1981 May;24(2):287–299. doi: 10.1016/0092-8674(81)90318-4. [DOI] [PubMed] [Google Scholar]
- Päbo S., Bhat B. M., Wold W. S., Peterson P. A. A short sequence in the COOH-terminus makes an adenovirus membrane glycoprotein a resident of the endoplasmic reticulum. Cell. 1987 Jul 17;50(2):311–317. doi: 10.1016/0092-8674(87)90226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Päbo S., Weber F., Kämpe O., Schaffner W., Peterson P. A. Association between transplantation antigens and a viral membrane protein synthesized from a mammalian expression vector. Cell. 1983 Jun;33(2):445–453. doi: 10.1016/0092-8674(83)90426-9. [DOI] [PubMed] [Google Scholar]
- Päbo S., Weber F., Nilsson T., Schaffner W., Peterson P. A. Structural and functional dissection of an MHC class I antigen-binding adenovirus glycoprotein. EMBO J. 1986 Aug;5(8):1921–1927. doi: 10.1002/j.1460-2075.1986.tb04445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S., Levine A. J. The genomic map position of the adenovirus type 2 glycoprotein. Virology. 1979 Dec;99(2):427–430. doi: 10.1016/0042-6822(79)90023-0. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Lodish H. F. Synchronised transmembrane insertion and glycosylation of a nascent membrane protein. Nature. 1977 Oct 27;269(5631):775–780. doi: 10.1038/269775a0. [DOI] [PubMed] [Google Scholar]
- Scheele G., Dobberstein B., Blobel G. Transfer of proteins across membranes, Biosynthesis in vitro of pretrypsinogen and trypsinogen by cell fractions of canine pancreas. Eur J Biochem. 1978 Jan 16;82(2):593–599. doi: 10.1111/j.1432-1033.1978.tb12055.x. [DOI] [PubMed] [Google Scholar]
- Shenk T., Williams J. Genetic analysis of adenoviruses. Curr Top Microbiol Immunol. 1984;111:1–39. doi: 10.1007/978-3-642-69549-0_1. [DOI] [PubMed] [Google Scholar]
- Signäs C., Katze M. G., Persson H., Philipson L. An adenovirus glycoprotein binds heavy chains of class I transplantation antigens from man and mouse. Nature. 1982 Sep 9;299(5879):175–178. doi: 10.1038/299175a0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Wold W. S., Cladaras C., Deutscher S. L., Kapoor Q. S. The 19-kDa glycoprotein coded by region E3 of adenovirus. Purification, characterization, and structural analysis. J Biol Chem. 1985 Feb 25;260(4):2424–2431. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]