Abstract
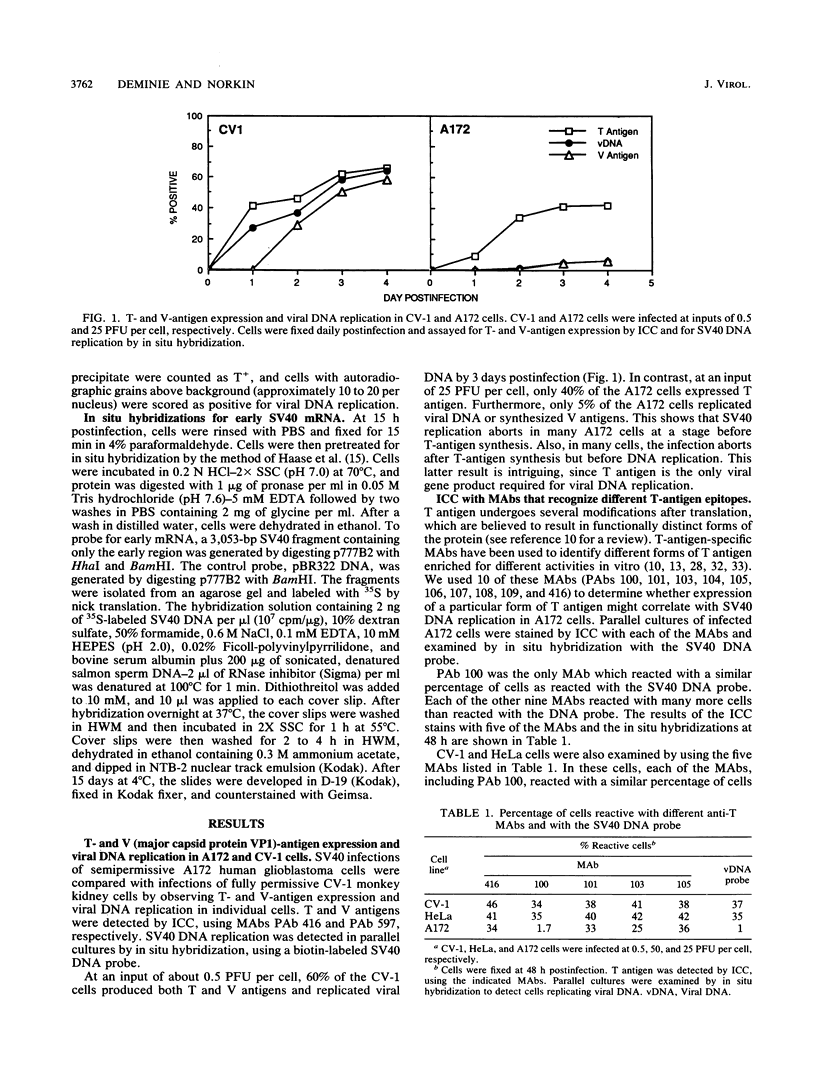
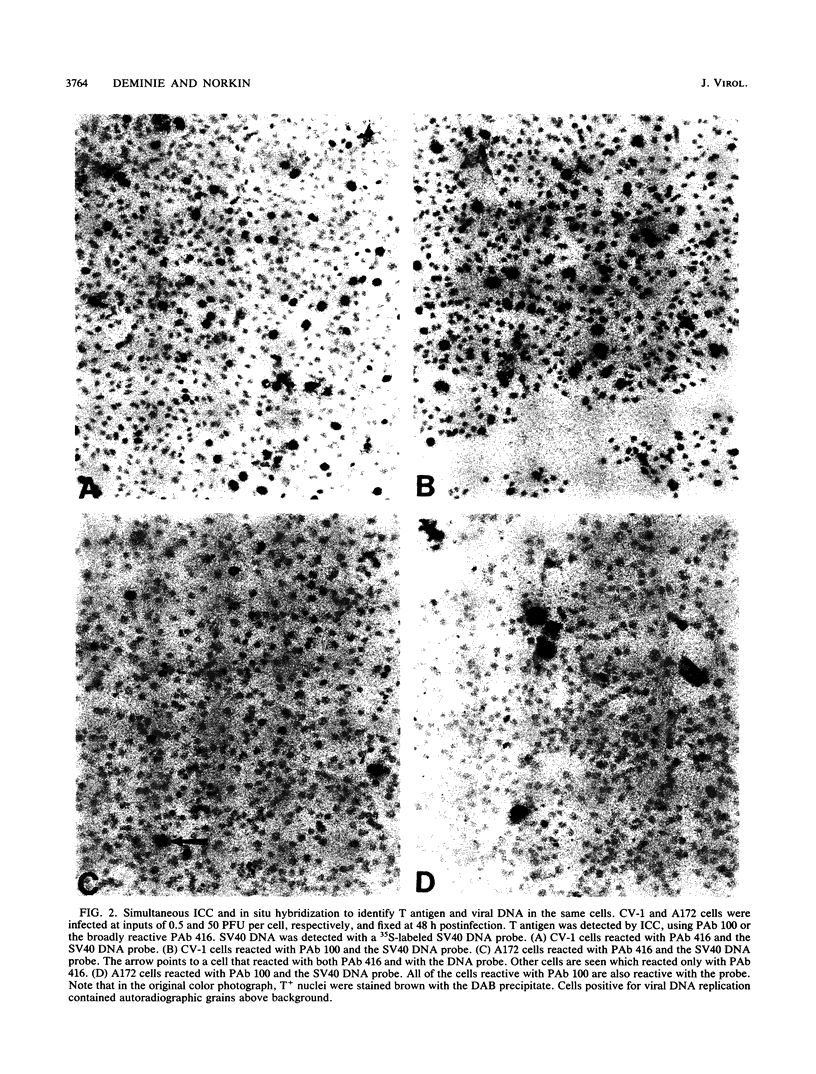
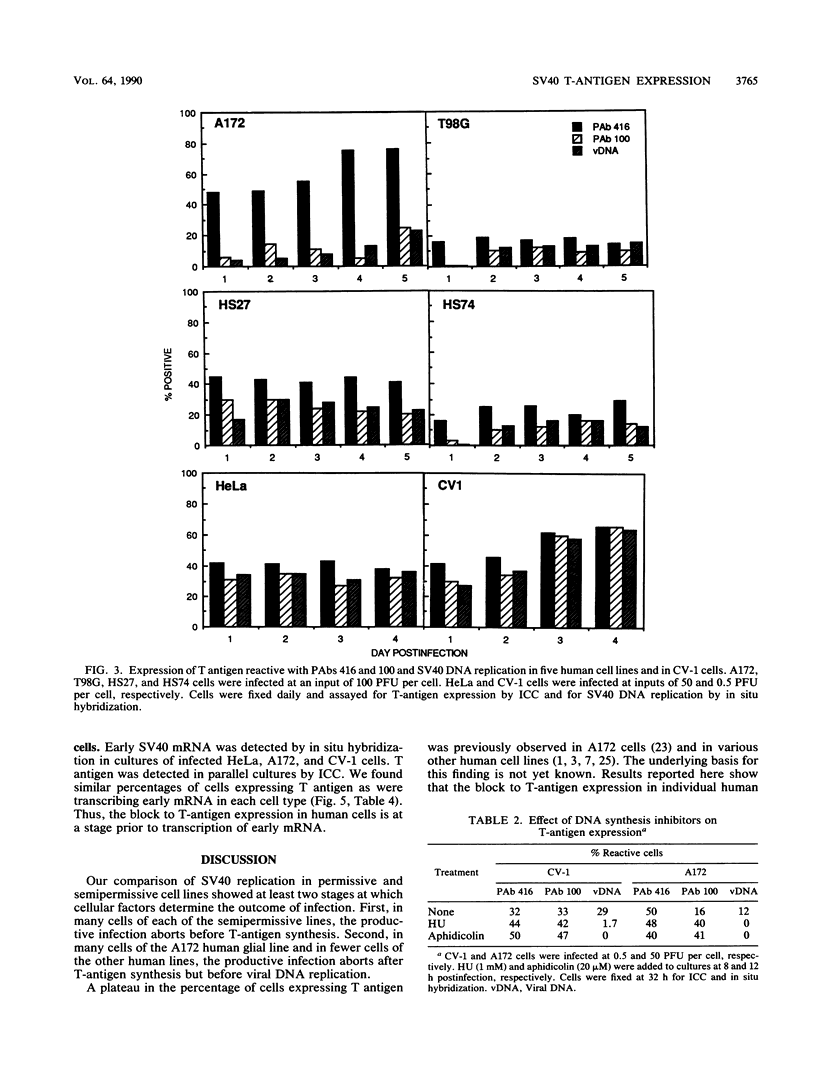
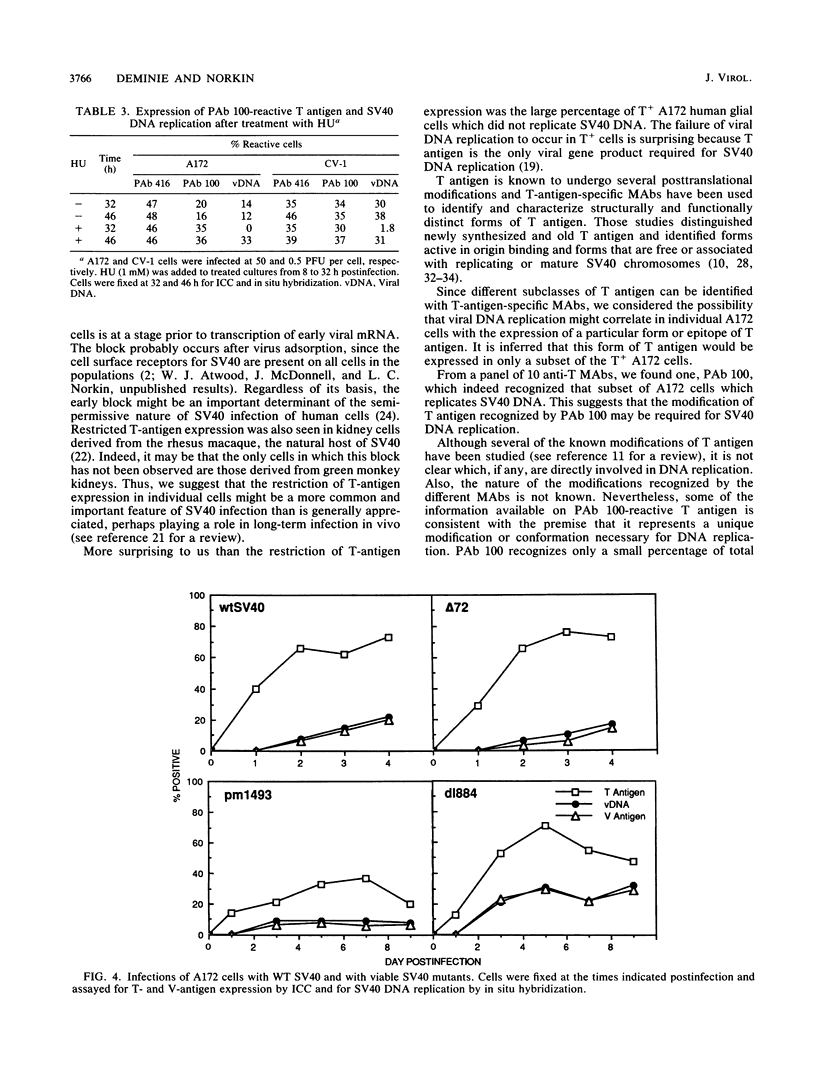
Immunocytochemistry and in situ hybridization were used to identify simian virus 40 (SV40) large T-antigen expression and viral DNA replication in individual cells of infected semipermissive human cell lines. SV40 infection aborts before T-antigen expression in many cells of each of the human cell lines examined. In all but one of the human cell lines, most of the T-antigen-producing cells replicated viral DNA. However, in the A172 line of human glial cells only a small percentage of the T-antigen-expressing cells replicated viral DNA. Since different structural and functional classes of T antigen can be recognized with anti-T monoclonal antibodies, we examined infected A172 cells with a panel of 10 anti-T monoclonal antibodies to determine whether viral DNA replication might correlate with the expression of a particular epitope of T antigen. One anti-T monoclonal antibody, PAb 100, did specifically recognize that subset of A172 cells which replicated SV40 DNA. The percentage of PAb 100-reactive A172 cells was dramatically increased by the DNA synthesis inhibitors hydroxyurea and aphidicolin. Removal of the hydroxyurea was followed by an increase in the percentage of cells replicating viral DNA corresponding to the increased percentage reactive with PAb 100. The pattern of SV40 infection in A172 cells was not altered by infection with viable viral mutants containing lesions in the small t protein, the agnoprotein, or the enhancer region. Finally, in situ hybridization was used to show that the percentage of human cells expressing T antigen was similar to the percentage transcribing early SV40 mRNA. Thus, the block to T-antigen expression in human cells is at a stage prior to transcription of early SV40 mRNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. SV40 T antigen induction and transformation in human fibroblast cell strains. Virology. 1968 Oct;36(2):254–261. doi: 10.1016/0042-6822(68)90142-6. [DOI] [PubMed] [Google Scholar]
- Atwood W. J., Norkin L. C. Class I major histocompatibility proteins as cell surface receptors for simian virus 40. J Virol. 1989 Oct;63(10):4474–4477. doi: 10.1128/jvi.63.10.4474-4477.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner W. A., Lubiniecki A. S., Mulvihill J. J., Lalley P., Fraumeni J. F., Jr Genetics of SV40 T-antigen expression: studies of twins, heritable syndromes and cancer families. Int J Cancer. 1978 Sep 15;22(3):231–238. doi: 10.1002/ijc.2910220303. [DOI] [PubMed] [Google Scholar]
- Bradley M. K., Smith T. F., Lathrop R. H., Livingston D. M., Webster T. A. Consensus topography in the ATP binding site of the simian virus 40 and polyomavirus large tumor antigens. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4026–4030. doi: 10.1073/pnas.84.12.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahic M., Haase A. T., Cash E. Simultaneous in situ detection of viral RNA and antigens. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5445–5448. doi: 10.1073/pnas.81.17.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigati D. J., Myerson D., Leary J. J., Spalholz B., Travis S. Z., Fong C. K., Hsiung G. D., Ward D. C. Detection of viral genomes in cultured cells and paraffin-embedded tissue sections using biotin-labeled hybridization probes. Virology. 1983 Apr 15;126(1):32–50. doi: 10.1016/0042-6822(83)90460-9. [DOI] [PubMed] [Google Scholar]
- Carp R. I., Gilden R. V. A comparison of the replication cycles of simian virus 40 in human diploid and African green monkey kidney cells. Virology. 1966 Jan;28(1):150–162. doi: 10.1016/0042-6822(66)90316-3. [DOI] [PubMed] [Google Scholar]
- Clark R., Peden K., Pipas J. M., Nathans D., Tjian R. Biochemical activities of T-antigen proteins encoded by simian virus 40 A gene deletion mutants. Mol Cell Biol. 1983 Feb;3(2):220–228. doi: 10.1128/mcb.3.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C. N., Tornow J., Clark R., Tjian R. Properties of the simian virus 40 (SV40) large T antigens encoded by SV40 mutants with deletions in gene A. J Virol. 1986 Feb;57(2):539–546. doi: 10.1128/jvi.57.2.539-546.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppert W., Gurney E. G., Harrison R. O. Monoclonal antibodies against simian virus 40 tumor antigens: analysis of antigenic binding sites, using adenovirus type 2-simian virus 40 hybrid viruses. J Virol. 1981 Jan;37(1):478–482. doi: 10.1128/jvi.37.1.478-482.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney E. G., Harrison R. O., Fenno J. Monoclonal antibodies against simian virus 40 T antigens: evidence for distinct sublcasses of large T antigen and for similarities among nonviral T antigens. J Virol. 1980 Jun;34(3):752–763. doi: 10.1128/jvi.34.3.752-763.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney E. G., Tamowski S., Deppert W. Antigenic binding sites of monoclonal antibodies specific for simian virus 40 large T antigen. J Virol. 1986 Mar;57(3):1168–1172. doi: 10.1128/jvi.57.3.1168-1172.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebkowski J. S., Clancy S., Calos M. P. Simian virus 40 replication in adenovirus-transformed human cells antagonizes gene expression. Nature. 1985 Sep 12;317(6033):169–171. doi: 10.1038/317169a0. [DOI] [PubMed] [Google Scholar]
- Lewis E. D., Manley J. L. Repression of simian virus 40 early transcription by viral DNA replication in human 293 cells. Nature. 1985 Sep 12;317(6033):172–175. doi: 10.1038/317172a0. [DOI] [PubMed] [Google Scholar]
- Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6973–6977. doi: 10.1073/pnas.81.22.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manos M. M., Gluzman Y. Genetic and biochemical analysis of transformation-competent, replication-defective simian virus 40 large T antigen mutants. J Virol. 1985 Jan;53(1):120–127. doi: 10.1128/jvi.53.1.120-127.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkin L. C., Ouellette J. Cell killing by simian virus 40: variation in the pattern of lysosomal enzyme release, cellular enzyme release, and cell death during productive infection of normal and simian virus 40-transformed simian cell lines. J Virol. 1976 Apr;18(1):48–57. doi: 10.1128/jvi.18.1.48-57.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkin L. C. Papovaviral persistent infections. Microbiol Rev. 1982 Dec;46(4):384–425. doi: 10.1128/mr.46.4.384-425.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkin L. C., Steinberg V. I., Kosz-Vnenchak M. Human glioblastoma cells persistently infected with simian virus 40 carry nondefective episomal viral DNA and acquire the transformed phenotype and numerous chromosomal abnormalities. J Virol. 1985 Feb;53(2):658–666. doi: 10.1128/jvi.53.2.658-666.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer H. L., Slater M. L., Dermody J. J., Mandel M. Replication of simian virus 40 DNA in normal human fibroblasts and in fibroblasts from xeroderma pigmentosum. J Virol. 1981 Aug;39(2):481–489. doi: 10.1128/jvi.39.2.481-489.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter C. W., Potter A. M., Oxford J. S. Comparison of transformation and T antigen induction in human cell lines. J Virol. 1970 Mar;5(3):293–298. doi: 10.1128/jvi.5.3.293-298.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick J., Shenk T. Simian virus 40 agnoprotein facilitates normal nuclear location of the major capsid polypeptide and cell-to-cell spread of virus. J Virol. 1986 Dec;60(3):1098–1106. doi: 10.1128/jvi.60.3.1098-1106.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller A., Covey L., Barnet B., Prives C. A small subclass of SV40 T antigen binds to the viral origin of replication. Cell. 1982 Jun;29(2):375–383. doi: 10.1016/0092-8674(82)90154-4. [DOI] [PubMed] [Google Scholar]
- Shortle D. R., Margolskee R. F., Nathans D. Mutational analysis of the simian virus 40 replicon: pseudorevertants of mutants with a defective replication origin. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6128–6131. doi: 10.1073/pnas.76.12.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B., Gerard R. D., Guggenheimer R. A., Gluzman Y. T antigen and template requirements for SV40 DNA replication in vitro. EMBO J. 1985 Nov;4(11):2933–2939. doi: 10.1002/j.1460-2075.1985.tb04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack L. C., Wright J. H., Deb S. P., Tegtmeyer P. The p53 complex from monkey cells modulates the biochemical activities of simian virus 40 large T antigen. J Virol. 1989 Mar;63(3):1310–1317. doi: 10.1128/jvi.63.3.1310-1317.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack L. C., Wright J. H., Gurney E. G. Alterations in the structure of new and old forms of simian virus 40 large T antigen (T) defined by age-dependent epitope changes: new T is the same as ATPase-active T. J Virol. 1989 May;63(5):2352–2356. doi: 10.1128/jvi.63.5.2352-2356.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack L. C., Wright J. H., Gurney E. G. Characterization of simian virus 40 large T antigen by using different monoclonal antibodies: T-p53 complexes are preferentially ATPase active and adenylylated. J Virol. 1988 Mar;62(3):1028–1037. doi: 10.1128/jvi.62.3.1028-1037.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack L. C., Wright J. H., Gurney E. G. Free and viral chromosome-bound simian virus 40 T antigen: changes in reactivity of specific antigenic determinants during lytic infection. J Virol. 1986 May;58(2):635–646. doi: 10.1128/jvi.58.2.635-646.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmappaya B., Shenk T. Nucleotide sequence analysis of viable deletion mutants lacking segments of the simian virus 40 genome coding for small t antigen. J Virol. 1979 Jun;30(3):668–673. doi: 10.1128/jvi.30.3.668-673.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]





