Abstract
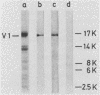
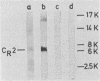
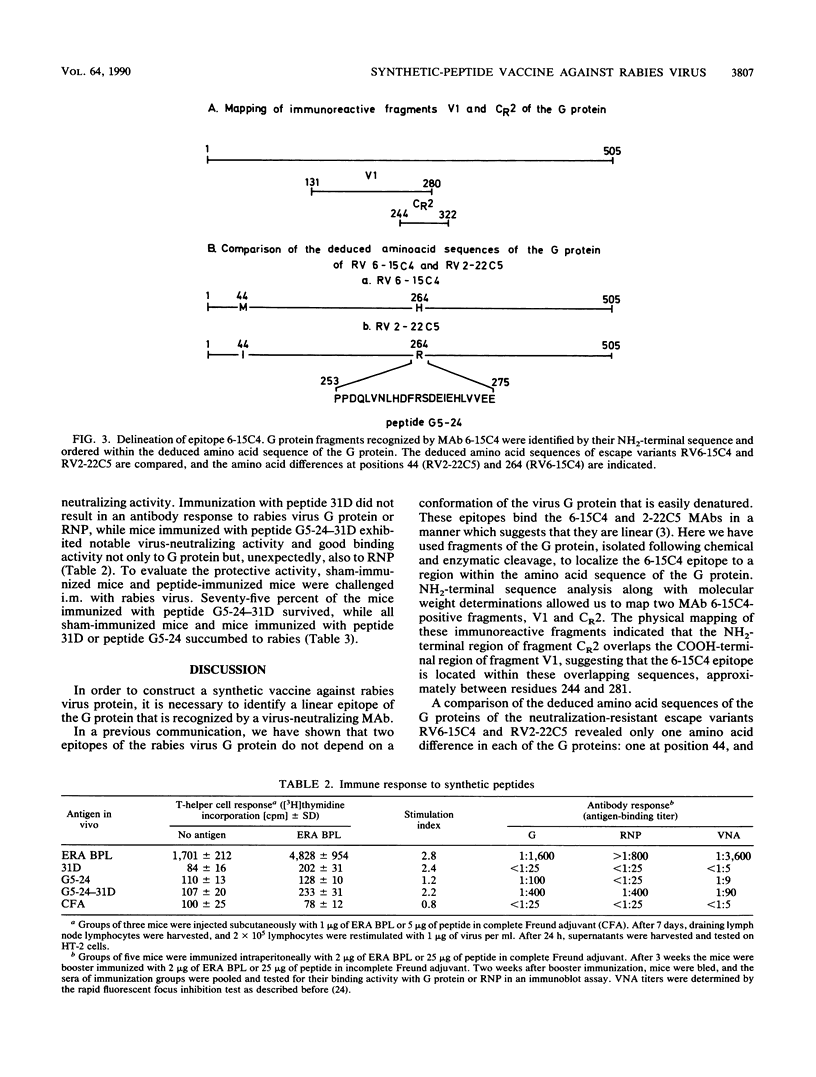
We have mapped a linear epitope recognized by the virus-neutralizing monoclonal antibody 6-15C4 within the primary sequence of the G protein from the Evelyn-Rokitnicki-Abelseth strain of rabies virus. This was accomplished by using fragments of the rabies virus G protein and deduced amino acid sequences of neutralization-resistant variant rabies viruses. The monoclonal antibody 6-15C4 specifically recognized a synthetic peptide (peptide G5-24) which resembles the 6-15C4 epitope in structure. In addition, a tandem peptide constructed from the G5-24 peptide and a dominant TH cell epitope of the rabies virus N protein induced protective immunity against lethal rabies virus challenge infection in mice.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borras-Cuesta F., Petit-Camurdan A., Fedon Y. Engineering of immunogenic peptides by co-linear synthesis of determinants recognized by B and T cells. Eur J Immunol. 1987 Aug;17(8):1213–1215. doi: 10.1002/eji.1830170820. [DOI] [PubMed] [Google Scholar]
- Bunschoten H., Gore M., Claassen I. J., Uytdehaag F. G., Dietzschold B., Wunner W. H., Osterhaus A. D. Characterization of a new virus-neutralizing epitope that denotes a sequential determinant on the rabies virus glycoprotein. J Gen Virol. 1989 Feb;70(Pt 2):291–298. doi: 10.1099/0022-1317-70-2-291. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Cox J. H., Dietzschold B., Schneider L. G. Rabies virus glycoprotein. II. Biological and serological characterization. Infect Immun. 1977 Jun;16(3):754–759. doi: 10.1128/iai.16.3.754-759.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. H., Dietzschold B., Weiland F., Schneider L. G. Preparation and characterization of rabies virus hemagglutinin. Infect Immun. 1980 Nov;30(2):572–577. doi: 10.1128/iai.30.2.572-577.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzschold B., Cox J. H., Schneider L. G., Wiktor T. J., Koprowski H. Isolation and purification of a polymeric form of the glycoprotein of rabies virus. J Gen Virol. 1978 Jul;40(1):131–139. doi: 10.1099/0022-1317-40-1-131. [DOI] [PubMed] [Google Scholar]
- Dietzschold B., Lafon M., Wang H., Otvos L., Jr, Celis E., Wunner W. H., Koprowski H. Localization and immunological characterization of antigenic domains of the rabies virus internal N and NS proteins. Virus Res. 1987 Aug;8(2):103–125. doi: 10.1016/0168-1702(87)90023-2. [DOI] [PubMed] [Google Scholar]
- Dietzschold B., Wiktor T. J., Macfarlan R., Varrichio A. Antigenic structure of rabies virus glycoprotein: ordering and immunological characterization of the large CNBr cleavage fragments. J Virol. 1982 Nov;44(2):595–602. doi: 10.1128/jvi.44.2.595-602.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertl H. C., Dietzschold B., Gore M., Otvos L., Jr, Larson J. K., Wunner W. H., Koprowski H. Induction of rabies virus-specific T-helper cells by synthetic peptides that carry dominant T-helper cell epitopes of the viral ribonucleoprotein. J Virol. 1989 Jul;63(7):2885–2892. doi: 10.1128/jvi.63.7.2885-2892.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HJERTEN S. Chromatographic separation according to size of macromolecules and cell particles on columns of agarose suspensions. Arch Biochem Biophys. 1962 Dec;99:466–475. doi: 10.1016/0003-9861(62)90295-3. [DOI] [PubMed] [Google Scholar]
- Lafon M., Ideler J., Wunner W. H. Investigation of the antigenic structure of rabies virus glycoprotein by monoclonal antibodies. Dev Biol Stand. 1984;57:219–225. [PubMed] [Google Scholar]
- Lafon M., Wiktor T. J., Macfarlan R. I. Antigenic sites on the CVS rabies virus glycoprotein: analysis with monoclonal antibodies. J Gen Virol. 1983 Apr;64(Pt 4):843–851. doi: 10.1099/0022-1317-64-4-843. [DOI] [PubMed] [Google Scholar]
- Macfarlan R. I., Dietzschold B., Wiktor T. J., Kiel M., Houghten R., Lerner R. A., Sutcliffe J. G., Koprowski H. T cell responses to cleaved rabies virus glycoprotein and to synthetic peptides. J Immunol. 1984 Nov;133(5):2748–2752. [PubMed] [Google Scholar]
- Prehaud C., Coulon P., LaFay F., Thiers C., Flamand A. Antigenic site II of the rabies virus glycoprotein: structure and role in viral virulence. J Virol. 1988 Jan;62(1):1–7. doi: 10.1128/jvi.62.1.1-7.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Seif I., Coulon P., Rollin P. E., Flamand A. Rabies virulence: effect on pathogenicity and sequence characterization of rabies virus mutations affecting antigenic site III of the glycoprotein. J Virol. 1985 Mar;53(3):926–934. doi: 10.1128/jvi.53.3.926-934.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikes R. K., Cleary W. F., Koprowski H., Wiktor T. J., Kaplan M. M. Effective protection of monkeys against death from street virus by post-exposure administration of tissue-culture rabies vaccine. Bull World Health Organ. 1971;45(1):1–11. [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., Dietzschold B., Leamnson R. N., Koprowski H. Induction and biological properties of defective interfering particles of rabies virus. J Virol. 1977 Feb;21(2):626–635. doi: 10.1128/jvi.21.2.626-635.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., Macfarlan R. I., Foggin C. M., Koprowski H. Antigenic analysis of rabies and Mokola virus from Zimbabwe using monoclonal antibodies. Dev Biol Stand. 1984;57:199–211. [PubMed] [Google Scholar]
- Wiktor T. J., Macfarlan R. I., Reagan K. J., Dietzschold B., Curtis P. J., Wunner W. H., Kieny M. P., Lathe R., Lecocq J. P., Mackett M. Protection from rabies by a vaccinia virus recombinant containing the rabies virus glycoprotein gene. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7194–7198. doi: 10.1073/pnas.81.22.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunner W. H., Dietzschold B., Smith C. L., Lafon M., Golub E. Antigenic variants of CVS rabies virus with altered glycosylation sites. Virology. 1985 Jan 15;140(1):1–12. doi: 10.1016/0042-6822(85)90440-4. [DOI] [PubMed] [Google Scholar]