Abstract
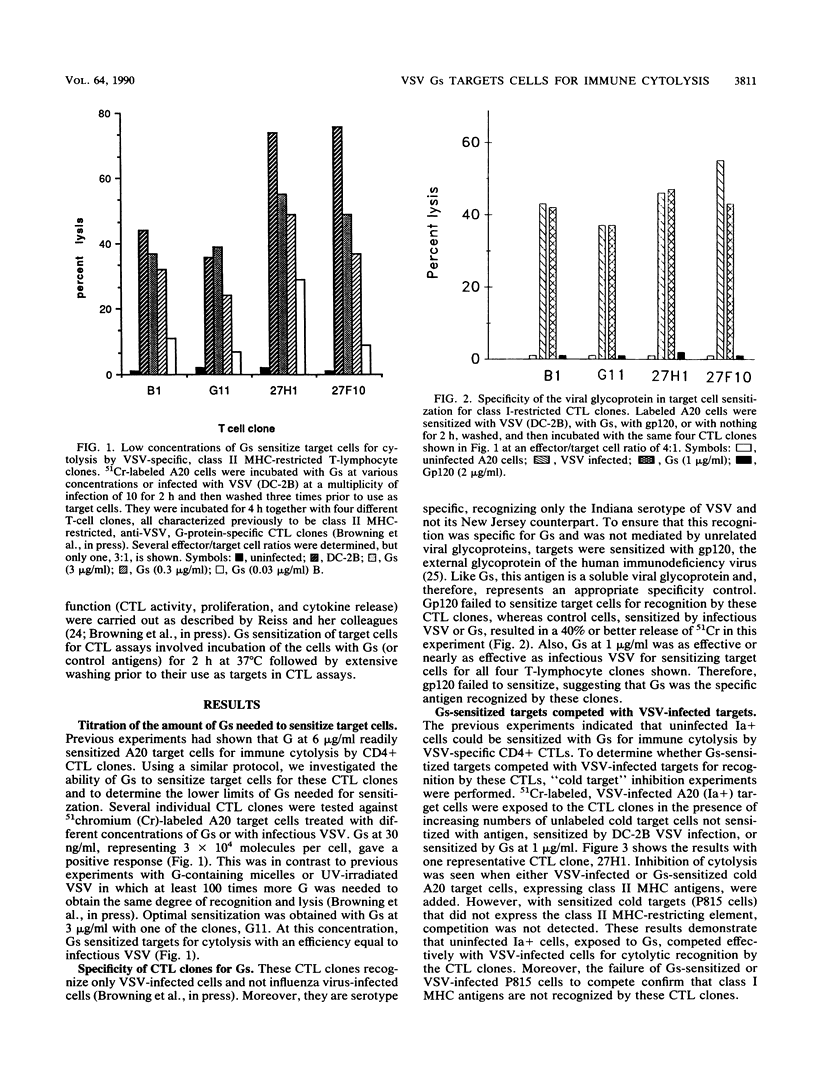
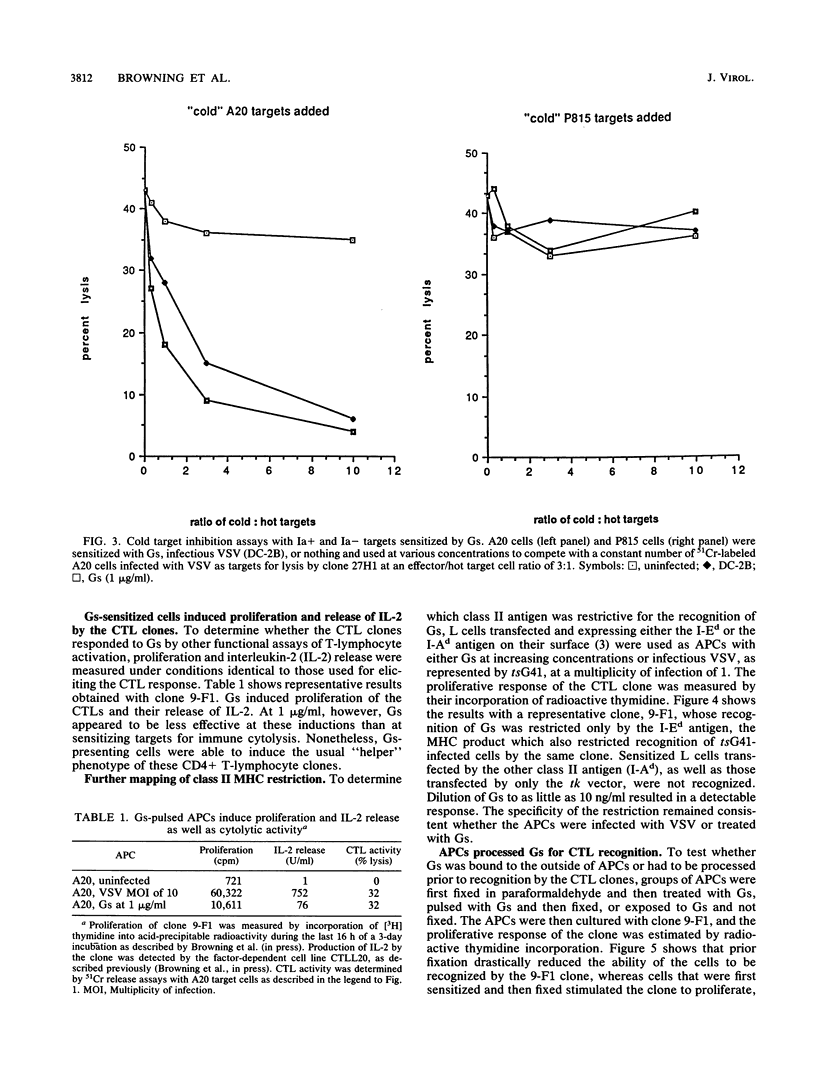
The soluble glycoprotein Gs of vesicular stomatitis virus (VSV), at approximately 10(4) molecules per cell, sensitized target cells for lysis by clones of CD4+ cytolytic T lymphocytes (CTL). In addition to lysis, the clones responded by proliferation and interleukin-2 release. Targets sensitized by Gs competed effectively with VSV-infected cells for recognition. Immune cytolysis by these CD4+ CTLs was restricted by class II major histocompatibility complex (MHC) antigens and was specific to VSV. The specific class II MHC antigen which was restricting for each clone remained the same whether the targets were sensitized by infection with VSV or by exogenously added soluble antigen. Sensitization by Gs appeared to require prior processing because the antigen-presenting cells that were fixed prior to exposure to Gs failed to be recognized by the CTL clones. The high efficiency of this uptake and processing was suggested by the inability of Gs at concentrations up to 10(7) per cell to block superinfection by VSV or to effect the RNA-synthetic machinery of uninfected cells. Also, Gs failed to hemolyze sheep erythrocytes when there was hemolysis by virions or an amino-terminal peptide of the VSV glycoprotein. Extrapolation of these results to viral diseases was possible because soluble viral glycoproteins were naturally synthesized during many viral infections and class II MHC antigens were inducible in cells of nonlymphoid origin. Therefore, CD4+ CTLs may be important participants in increasing virus-induced pathology, especially among adjacent uninfected cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolognesi D. P., Langlois A. J., Schäfer W. Polypeptides of mammalian oncornaviruses. IV. Structural components of murine leukemia virus released as soluble antigens in cell culture. Virology. 1975 Dec;68(2):550–555. doi: 10.1016/0042-6822(75)90297-4. [DOI] [PubMed] [Google Scholar]
- Braunstein N. S., Germain R. N. Allele-specific control of Ia molecule surface expression and conformation: implications for a general model of Ia structure-function relationships. Proc Natl Acad Sci U S A. 1987 May;84(9):2921–2925. doi: 10.1073/pnas.84.9.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. S., Ariel N., Huang A. S. Membrane anchors of vesicular stomatitis virus: characterization and incorporation into virions. J Virol. 1988 Aug;62(8):2552–2556. doi: 10.1128/jvi.62.8.2552-2556.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. S., Doherty R., O'Rourke E. J., Ariel N., Huang A. S. Effects of transport inhibitors on the generation and transport of a soluble viral glycoprotein. Virology. 1987 Oct;160(2):482–484. doi: 10.1016/0042-6822(87)90021-3. [DOI] [PubMed] [Google Scholar]
- Chen S. S., Huang A. S. Further characterization of the vesicular stomatitis virus temperature-sensitive O45 mutant: intracellular conversion of the glycoprotein to a soluble form. J Virol. 1986 Aug;59(2):210–215. doi: 10.1128/jvi.59.2.210-215.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzschold B., Wiktor T. J., Wunner W. H., Varrichio A. Chemical and immunological analysis of the rabies soluble glycoprotein. Virology. 1983 Jan 30;124(2):330–337. doi: 10.1016/0042-6822(83)90349-5. [DOI] [PubMed] [Google Scholar]
- Garreis-Wabnitz C., Kruppa J. Intracellular appearance of a glycoprotein in VSV-infected BHK cells lacking the membrane-anchoring oligopeptide of the viral G-protein. EMBO J. 1984 Jul;3(7):1469–1476. doi: 10.1002/j.1460-2075.1984.tb01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromkowski S. H., Hepler K. M., Janeway C. A., Jr Low doses of interleukin 2 induce bystander cell lysis by antigen-specific CD4+ inflammatory T cell clones in short-term assay. Eur J Immunol. 1988 Sep;18(9):1385–1389. doi: 10.1002/eji.1830180913. [DOI] [PubMed] [Google Scholar]
- Hendricks D. A., McIntosh K., Patterson J. L. Further characterization of the soluble form of the G glycoprotein of respiratory syncytial virus. J Virol. 1988 Jul;62(7):2228–2233. doi: 10.1128/jvi.62.7.2228-2233.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S., Richert J. R., Biddison W. E., Satinsky A., Hartzman R. J., McFarland H. F. Measles virus-specific T4+ human cytotoxic T cell clones are restricted by class II HLA antigens. J Immunol. 1984 Aug;133(2):754–757. [PubMed] [Google Scholar]
- Ju S. T., Ruddle N. H., Strack P., Dorf M. E., DeKruyff R. H. Expression of two distinct cytolytic mechanisms among murine CD4 subsets. J Immunol. 1990 Jan 1;144(1):23–31. [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. 3. Intracellular synthesis and extracellular appearance of virus-specific proteins. Virology. 1971 Dec;46(3):678–690. doi: 10.1016/0042-6822(71)90070-5. [DOI] [PubMed] [Google Scholar]
- Kaplan A. S., Erickson J. S., Ben-Porat T. Synthesis of proteins in cells infected with herpesvirus. X. Proteins excreted by cells infected with herpes simplex virus, types 1 and 2. Virology. 1975 Mar;64(1):132–143. doi: 10.1016/0042-6822(75)90085-9. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Griffith R., Braciale V. L., Braciale T. J. Influenza virus-specific human cytotoxic T cell clones: heterogeneity in antigenic specificity and restriction by class II MHC products. Cell Immunol. 1984 Oct 1;88(1):193–206. doi: 10.1016/0008-8749(84)90064-9. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S. P., Huang A. S. Shedding of the glycoprotein from vesicular stomatitis virus-infected cells. J Virol. 1978 Aug;27(2):330–339. doi: 10.1128/jvi.27.2.330-339.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S. P., Huang A. S. Synthesis and distribution of vesicular stomatitis virus-specific polypeptides in the absence of progeny production. Virology. 1977 Aug;81(1):37–47. doi: 10.1016/0042-6822(77)90056-3. [DOI] [PubMed] [Google Scholar]
- Londei M., Lamb J. R., Bottazzo G. F., Feldmann M. Epithelial cells expressing aberrant MHC class II determinants can present antigen to cloned human T cells. Nature. 1984 Dec 13;312(5995):639–641. doi: 10.1038/312639a0. [DOI] [PubMed] [Google Scholar]
- Metsikkö K., Simons K. The budding mechanism of spikeless vesicular stomatitis virus particles. EMBO J. 1986 Aug;5(8):1913–1920. doi: 10.1002/j.1460-2075.1986.tb04444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison L. A., Lukacher A. E., Braciale V. L., Fan D. P., Braciale T. J. Differences in antigen presentation to MHC class I-and class II-restricted influenza virus-specific cytolytic T lymphocyte clones. J Exp Med. 1986 Apr 1;163(4):903–921. doi: 10.1084/jem.163.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Collins T., Gimbrone M. A., Jr, Cotran R. S., Gitlin J. D., Fiers W., Clayberger C., Krensky A. M., Burakoff S. J., Reiss C. S. Lymphocytes recognize human vascular endothelial and dermal fibroblast Ia antigens induced by recombinant immune interferon. Nature. 1983 Oct 20;305(5936):726–729. doi: 10.1038/305726a0. [DOI] [PubMed] [Google Scholar]
- Reiss C. S., Chen S. S., Huang A. S., Doherty R. VSV G protein induces murine cytolytic T lymphocytes. Microb Pathog. 1986 Jun;1(3):261–267. doi: 10.1016/0882-4010(86)90050-1. [DOI] [PubMed] [Google Scholar]
- Robey W. G., Safai B., Oroszlan S., Arthur L. O., Gonda M. A., Gallo R. C., Fischinger P. J. Characterization of envelope and core structural gene products of HTLV-III with sera from AIDS patients. Science. 1985 May 3;228(4699):593–595. doi: 10.1126/science.2984774. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R., Wade M. Biologically active peptides of the vesicular stomatitis virus glycoprotein. J Virol. 1985 Jan;53(1):319–323. doi: 10.1128/jvi.53.1.319-323.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier R. D., Gnann J. W., Jr, Landes R., Lockshin C., Richman D., McCutchan A., Kennedy C., Oldstone M. B., Nelson J. A. T cell recognition of HIV synthetic peptides in a natural infection. J Immunol. 1989 Feb 15;142(4):1166–1176. [PubMed] [Google Scholar]
- Siliciano R. F., Lawton T., Knall C., Karr R. W., Berman P., Gregory T., Reinherz E. L. Analysis of host-virus interactions in AIDS with anti-gp120 T cell clones: effect of HIV sequence variation and a mechanism for CD4+ cell depletion. Cell. 1988 Aug 12;54(4):561–575. doi: 10.1016/0092-8674(88)90078-5. [DOI] [PubMed] [Google Scholar]
- Yasukawa M., Zarling J. M. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. I. Lysis restricted by HLA class II MB and DR antigens. J Immunol. 1984 Jul;133(1):422–427. [PubMed] [Google Scholar]


