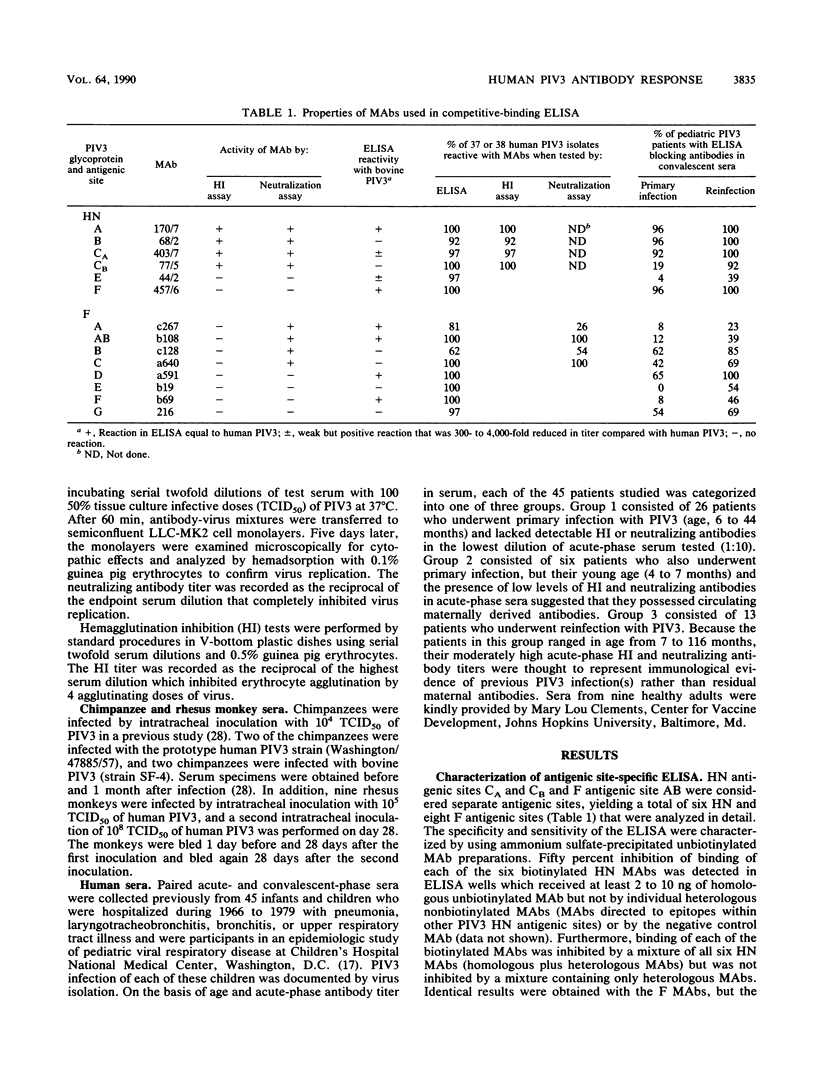
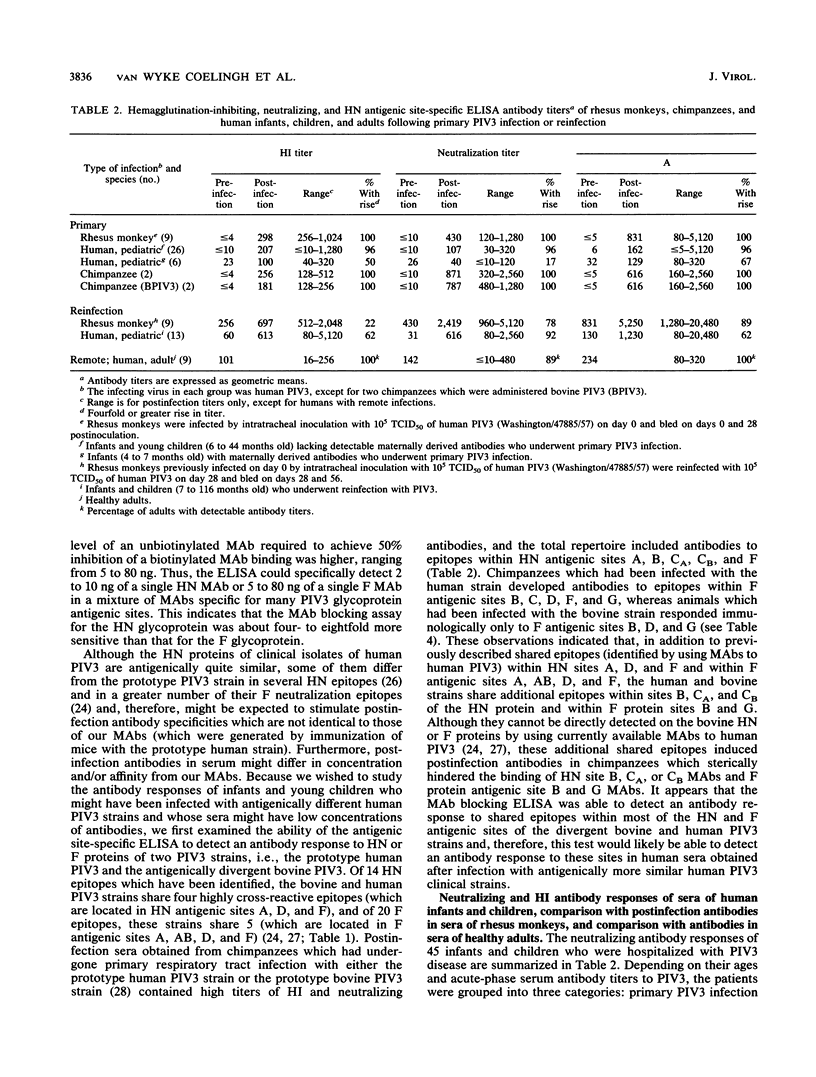
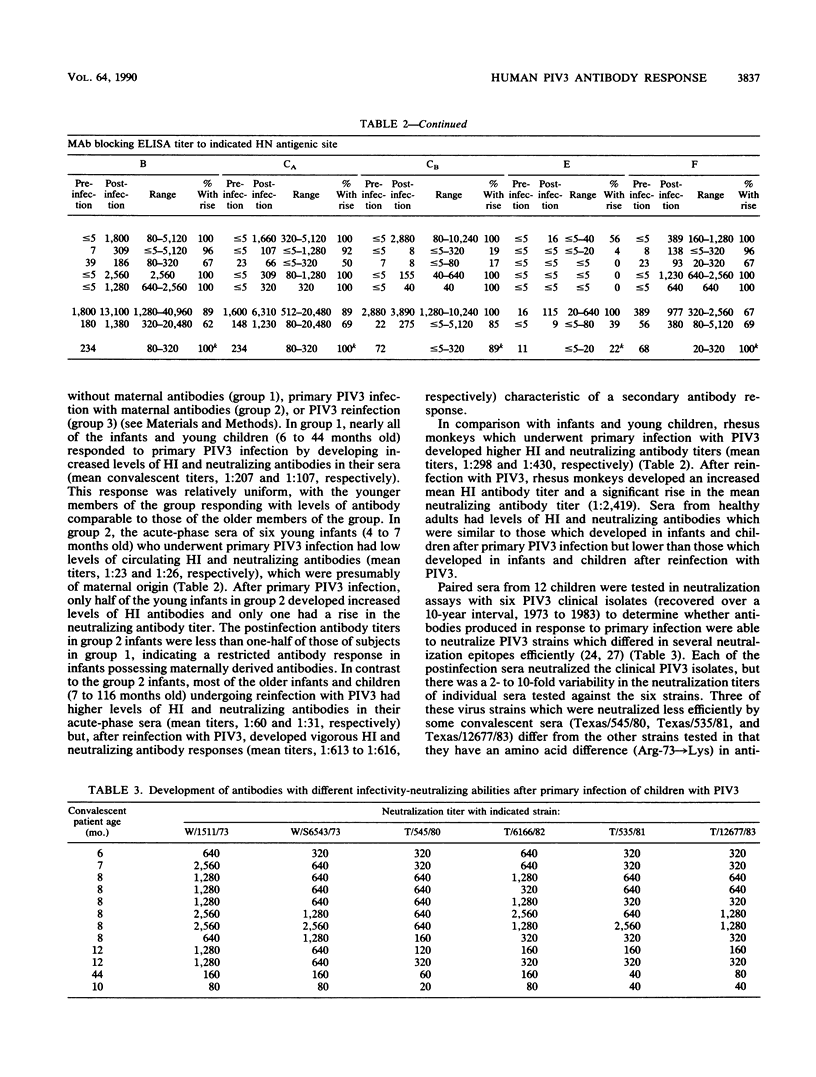
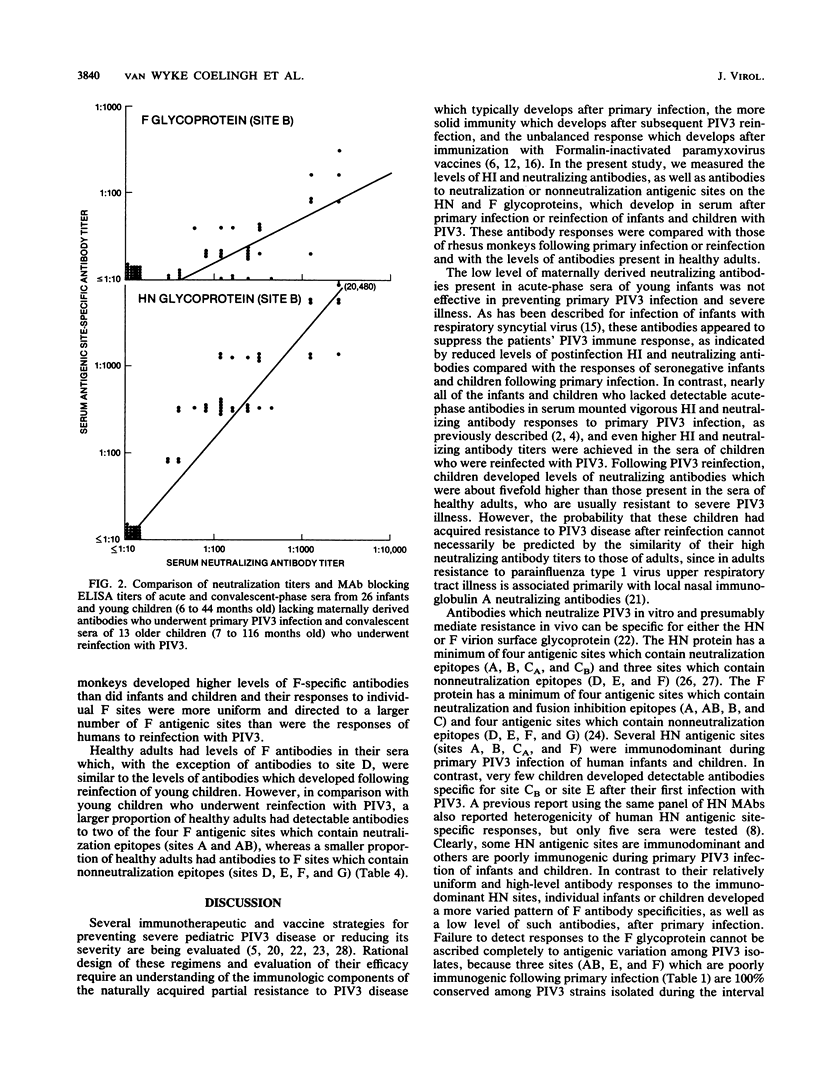
Abstract
An unusual feature of human parainfluenza virus type 3 (PIV3) is ita ability to cause reinfection with high efficiency. The antibody responses of 45 humans and 9 rhesus monkeys to primary infection or subsequent reinfection with PIV3 were examined to identify deficiencies in host immunologic responses that might contribute to the ability of the virus to cause reinfection with high frequency. Antibody responses in serum were tested by using neutralization and hemagglutination inhibition (HI) assays and a monoclonal antibody blocking immunoassay able to detect antibodies to epitopes within six antigenic sites on the PIV3 hemagglutinin-neuraminidase (HN) glycoprotein and eight antigenic sites on the fusion (F) protein. Primary infection of seronegative infants or children with PIV3 stimulated strong and rather uniform HI and neutralizing antibody responses. More than 90% of the individuals developed antibodies to four of the six HN antigenic sites (including three of the four neutralization sites), but the responses to F antigenic sites were of lesser magnitude and varied considerably from person to person. Young infants who possessed maternally derived antibodies in their sera developed lower levels and less frequent HI, neutralizing, and antigenic site-specific responses to the HN and F glycoproteins than did seronegative infants and children. In contrast, children reinfected with PIV3 developed even higher HI and neutralizing antibody responses than those observed during primary infection. Reinfection broadened the HN and F antigenic site-specific responses, but the latter remained relatively restricted. Adults possessed lower levels of HI, neutralizing, and antigenic site-specific antibodies in their sera than did children who had been reinfected, suggesting that these antibodies decay with time. Rhesus monkeys developed more vigorous primary and secondary antibody responses than did humans, but even in these highly responsive animals, response to the F glycoprotein was relatively restricted following primary infection. Bovine PIV3 induced a broader response to human PIV3 in monkeys than was anticipated on the basis of their known relatedness as defined by using monoclonal antibodies to human PIV3. These observations suggest that the restricted antibody responses to multiple antigenic sites on the F glycoprotein in young seronegative infants and children and the decreased responses to both the F and HN glycoproteins in young infants and children with maternally derived antibodies may play a role in the susceptibility of human infants and young children to reinfection with PIV3.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANOCK R. M., PARROTT R. H., COOK K., ANDREWS B. E., BELL J. A., REICHELDERFER T., KAPIKIAN A. Z., MASTROTA F. M., HUEBNER R. J. Newly recognized myxoviruses from children with respiratory disease. N Engl J Med. 1958 Jan 30;258(5):207–213. doi: 10.1056/NEJM195801302580502. [DOI] [PubMed] [Google Scholar]
- CHANOCK R. M., PARROTT R. H., JOHNSON K. M., KAPIKIAN A. Z., BELL J. A. MYXOVIRUSES: PARAINFLUENZA. Am Rev Respir Dis. 1963 Sep;88:SUPPL–166. doi: 10.1164/arrd.1963.88.3P2.152. [DOI] [PubMed] [Google Scholar]
- Coelingh K. J., Winter C. C., Murphy B. R., Rice J. M., Kimball P. C., Olmsted R. A., Collins P. L. Conserved epitopes on the hemagglutinin-neuraminidase proteins of human and bovine parainfluenza type 3 viruses: nucleotide sequence analysis of variants selected with monoclonal antibodies. J Virol. 1986 Oct;60(1):90–96. doi: 10.1128/jvi.60.1.90-96.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelingh K. V., Winter C. C. Naturally occurring human parainfluenza type 3 viruses exhibit divergence in amino acid sequence of their fusion protein neutralization epitopes and cleavage sites. J Virol. 1990 Mar;64(3):1329–1334. doi: 10.1128/jvi.64.3.1329-1334.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crookshanks-Newman F. K., Belshe R. B. Protection of weanling hamsters from experimental infection with wild-type parainfluenza virus type 3 (para 3) by cold-adapted mutants of para 3. J Med Virol. 1986 Feb;18(2):131–137. doi: 10.1002/jmv.1890180205. [DOI] [PubMed] [Google Scholar]
- Fulginiti V. A., Eller J. J., Sieber O. F., Joyner J. W., Minamitani M., Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol. 1969 Apr;89(4):435–448. doi: 10.1093/oxfordjournals.aje.a120956. [DOI] [PubMed] [Google Scholar]
- Glezen W. P., Frank A. L., Taber L. H., Kasel J. A. Parainfluenza virus type 3: seasonality and risk of infection and reinfection in young children. J Infect Dis. 1984 Dec;150(6):851–857. doi: 10.1093/infdis/150.6.851. [DOI] [PubMed] [Google Scholar]
- Green K. Y., Taniguchi K., Mackow E. R., Kapikian A. Z. Homotypic and heterotypic epitope-specific antibody responses in adult and infant rotavirus vaccinees: implications for vaccine development. J Infect Dis. 1990 Apr;161(4):667–679. doi: 10.1093/infdis/161.4.667. [DOI] [PubMed] [Google Scholar]
- Henrickson K. J., Portner A. Antibody response in children to antigen sites on human PIV-3 HN: correlation with known epitopes mapped by monoclonal antibodies. Vaccine. 1990 Feb;8(1):75–80. doi: 10.1016/0264-410x(90)90182-l. [DOI] [PubMed] [Google Scholar]
- Icenogle J. P., Minor P. D., Ferguson M., Hogle J. M. Modulation of humoral response to a 12-amino-acid site on the poliovirus virion. J Virol. 1986 Oct;60(1):297–301. doi: 10.1128/jvi.60.1.297-301.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B. E., Bucher D. J., Kilbourne E. D. Purified influenza virus hemagglutinin and neuraminidase are equivalent in stimulation of antibody response but induce contrasting types of immunity to infection. J Virol. 1989 Mar;63(3):1239–1246. doi: 10.1128/jvi.63.3.1239-1246.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasel J. A., Frank A. L., Keitel W. A., Taber L. H., Glezen W. P. Acquisition of serum antibodies to specific viral glycoproteins of parainfluenza virus 3 in children. J Virol. 1984 Dec;52(3):828–832. doi: 10.1128/jvi.52.3.828-832.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. W., Canchola J. G., Brandt C. D., Pyles G., Chanock R. M., Jensen K., Parrott R. H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969 Apr;89(4):422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- Merz D. C., Scheid A., Choppin P. W. Immunological studies of the functions of paramyxovirus glycoproteins. Virology. 1981 Feb;109(1):94–105. doi: 10.1016/0042-6822(81)90474-8. [DOI] [PubMed] [Google Scholar]
- Milich D. R., Leroux-Roels G. G., Louie R. E., Chisari F. V. Genetic regulation of the immune response to hepatitis B surface antigen (HBsAg). IV. Distinct H-2-linked Ir genes control antibody responses to different HBsAg determinants on the same molecule and map to the I-A and I-C subregions. J Exp Med. 1984 Jan 1;159(1):41–56. doi: 10.1084/jem.159.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Alling D. W., Snyder M. H., Walsh E. E., Prince G. A., Chanock R. M., Hemming V. G., Rodriguez W. J., Kim H. W., Graham B. S. Effect of age and preexisting antibody on serum antibody response of infants and children to the F and G glycoproteins during respiratory syncytial virus infection. J Clin Microbiol. 1986 Nov;24(5):894–898. doi: 10.1128/jcm.24.5.894-898.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby E., Enders-Ruckle G., Meulen V. Differences in the appearance of antibodies to structural components of measles virus after immunization with inactivated and live virus. J Infect Dis. 1975 Sep;132(3):262–269. doi: 10.1093/infdis/132.3.262. [DOI] [PubMed] [Google Scholar]
- PARROTT R. H., VARGOSKO A. J., KIMHW, BELL J. A., CHANOCK R. M. Acute respiratory diseases of viral etiology. III. parainfluenza. Myxoviruses. Am J Public Health Nations Health. 1962 Jun;52:907–917. doi: 10.2105/ajph.52.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R. G., Lamb R. A., Moss B., Murphy B. R. Comparison of the relative roles of the F and HN surface glycoproteins of the paramyxovirus simian virus 5 in inducing protective immunity. J Virol. 1987 Jun;61(6):1972–1977. doi: 10.1128/jvi.61.6.1972-1977.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R., Glaze B. J., Compans R. W. Role of individual glycoproteins of human parainfluenza virus type 3 in the induction of a protective immune response. J Virol. 1988 Mar;62(3):783–787. doi: 10.1128/jvi.62.3.783-787.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R., Glaze B. J., Moldoveanu Z., Compans R. W. Intranasal immunization of hamsters with envelope glycoproteins of human parainfluenza virus type 3. J Infect Dis. 1988 Apr;157(4):648–654. doi: 10.1093/infdis/157.4.648. [DOI] [PubMed] [Google Scholar]
- Smith C. B., Purcell R. H., Bellanti J. A., Chanock R. M. Protective effect of antibody to parainfluenza type 1 virus. N Engl J Med. 1966 Nov 24;275(21):1145–1152. doi: 10.1056/NEJM196611242752101. [DOI] [PubMed] [Google Scholar]
- Wang M. L., Skehel J. J., Wiley D. C. Comparative analyses of the specificities of anti-influenza hemagglutinin antibodies in human sera. J Virol. 1986 Jan;57(1):124–128. doi: 10.1128/jvi.57.1.124-128.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P. F., Murphy B. R., Kervina M., Lawrence E. M., Phelan M. A., Karzon D. T. Secretory immunological response after intranasal inactivated influenza A virus vaccinations: evidence for immunoglobulin A memory. Infect Immun. 1983 Jun;40(3):1092–1095. doi: 10.1128/iai.40.3.1092-1095.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Murphy B. R., Collins P. L., Lebacq-Verheyden A. M., Battey J. F. Expression of biologically active and antigenically authentic parainfluenza type 3 virus hemagglutinin-neuraminidase glycoprotein by a recombinant baculovirus. Virology. 1987 Oct;160(2):465–472. doi: 10.1016/0042-6822(87)90018-3. [DOI] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Winter C. C., Tierney E. L., London W. T., Murphy B. R. Attenuation of bovine parainfluenza virus type 3 in nonhuman primates and its ability to confer immunity to human parainfluenza virus type 3 challenge. J Infect Dis. 1988 Apr;157(4):655–662. doi: 10.1093/infdis/157.4.655. [DOI] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Winter C., Murphy B. R. Antigenic variation in the hemagglutinin-neuraminidase protein of human parainfluenza type 3 virus. Virology. 1985 Jun;143(2):569–582. doi: 10.1016/0042-6822(85)90395-2. [DOI] [PubMed] [Google Scholar]
- van Wyke Coelingh K., Tierney E. L. Antigenic and functional organization of human parainfluenza virus type 3 fusion glycoprotein. J Virol. 1989 Jan;63(1):375–382. doi: 10.1128/jvi.63.1.375-382.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]



