Abstract
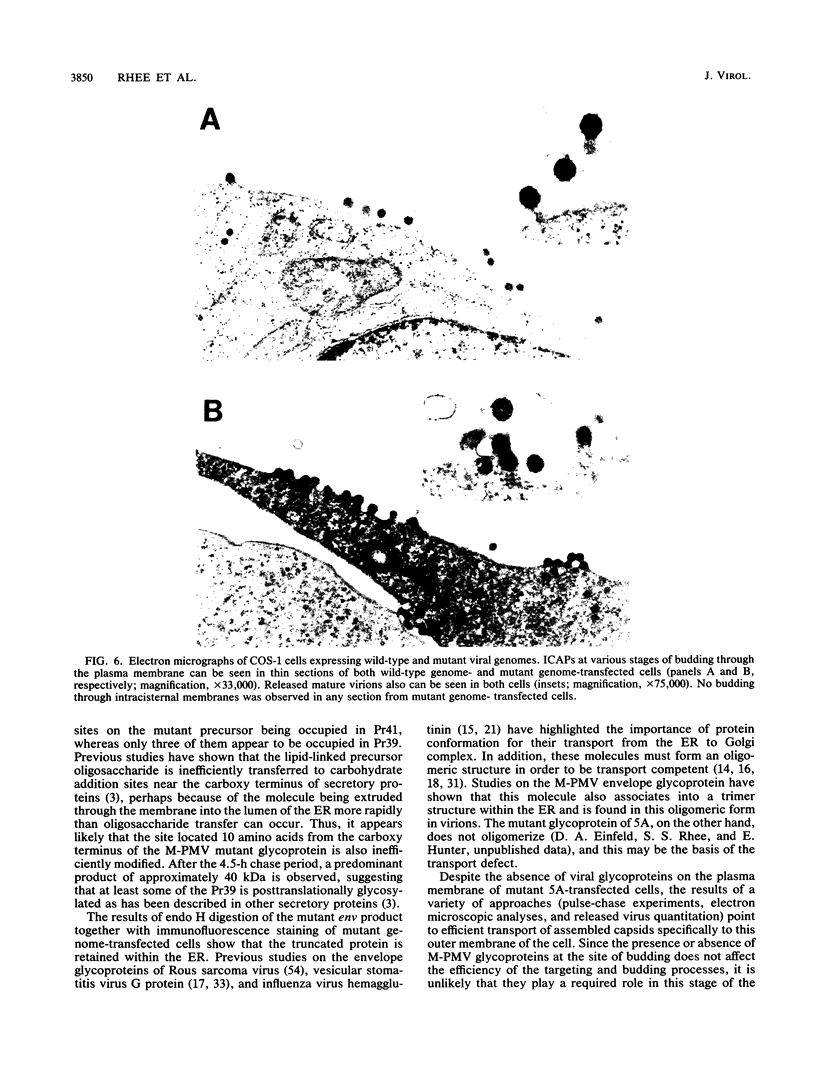
The capsids of Mason-Pfizer monkey virus (M-PMV), an immunosuppressive type D retrovirus, are preassembled in the infected cell cytoplasm and are then transported to the plasma membrane, where they are enveloped in a virus glycoprotein-containing lipid bilayer. The role of viral glycoprotein in intracellular transport of M-PMV capsids was investigated with a spontaneous mutant (5A) of M-PMV, which we show here to be defective in envelope glycoprotein biosynthesis. DNA sequence analysis of the env gene of mutant 5A reveals a single nucleotide deletion in the middle of the gene, which results in the synthesis of a truncated form of the envelope glycoprotein. Evidence is presented showing that the mutant glycoprotein is not expressed at the cell surface but is retained in the endoplasmic reticulum. Normal levels of gag-pro-pol precursor polyproteins are made and processed in mutant genome-transfected cells, and high levels of noninfectious particles lacking viral glycoprotein are released with normal kinetics into the culture medium. No intracisternal budding of capsids is observed. We conclude that viral glycoprotein is required neither for targeting preassembled capsids of M-PMV to the plasma membrane for final maturation nor for the budding process. Since the presence or absence of M-PMV glycoprotein at the site of budding does not affect the efficiency or kinetics of the targeting process, the preassembled capsid of M-PMV, in contrast to those of intracisternal type A particles, appears to have an intrinsic signal for intracellular transport to the plasma membrane.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken A., Cohen P., Santikarn S., Williams D. H., Calder A. G., Smith A., Klee C. B. Identification of the NH2-terminal blocking group of calcineurin B as myristic acid. FEBS Lett. 1982 Dec 27;150(2):314–318. doi: 10.1016/0014-5793(82)80759-x. [DOI] [PubMed] [Google Scholar]
- Barker C. S., Pickel J., Tainsky M., Hunter E. Molecular cloning of the Mason-Pfizer monkey virus genome: biological characterization of genome length clones and molecular comparisons to other retroviruses. Virology. 1986 Sep;153(2):201–214. doi: 10.1016/0042-6822(86)90023-1. [DOI] [PubMed] [Google Scholar]
- Bergman L. W., Kuehl W. M. Temporal relationship of translation and glycosylation of immunoglobulin heavy and light chains. Biochemistry. 1978 Nov 28;17(24):5174–5180. doi: 10.1021/bi00617a017. [DOI] [PubMed] [Google Scholar]
- Bradac J. A., Hunter E. Polypeptides of Mason-Pfizer monkey virus. III. Translational order of proteins on the gag and env gene specified precursor polypeptides. Virology. 1986 Apr 30;150(2):503–508. doi: 10.1016/0042-6822(86)90314-4. [DOI] [PubMed] [Google Scholar]
- Bradac J., Hunter E. Polypeptides of Mason-Pfizer monkey virus. I. Synthesis and processing of the gag-gene products. Virology. 1984 Oct 30;138(2):260–275. doi: 10.1016/0042-6822(84)90350-7. [DOI] [PubMed] [Google Scholar]
- Bradac J., Hunter E. Polypeptides of Mason-Pfizer monkey virus. II. Synthesis and processing of the env gene products. Virology. 1986 Apr 30;150(2):491–502. doi: 10.1016/0042-6822(86)90313-2. [DOI] [PubMed] [Google Scholar]
- Buss J. E., Kamps M. P., Gould K., Sefton B. M. The absence of myristic acid decreases membrane binding of p60src but does not affect tyrosine protein kinase activity. J Virol. 1986 May;58(2):468–474. doi: 10.1128/jvi.58.2.468-474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr S. A., Biemann K., Shoji S., Parmelee D. C., Titani K. n-Tetradecanoyl is the NH2-terminal blocking group of the catalytic subunit of cyclic AMP-dependent protein kinase from bovine cardiac muscle. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6128–6131. doi: 10.1073/pnas.79.20.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S., Bradac J. A., Hunter E. Effect of monensin on Mason-Pfizer monkey virus glycoprotein synthesis. J Virol. 1982 Dec;44(3):1003–1012. doi: 10.1128/jvi.44.3.1003-1012.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S., Hunter E., Whitley R. Effect of cloned human interferons on protein synthesis and morphogenesis of herpes simplex virus. J Virol. 1985 Nov;56(2):419–425. doi: 10.1128/jvi.56.2.419-425.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu I. M., Callahan R., Tronick S. R., Schlom J., Aaronson S. A. Major pol gene progenitors in the evolution of oncoviruses. Science. 1984 Jan 27;223(4634):364–370. doi: 10.1126/science.6197754. [DOI] [PubMed] [Google Scholar]
- Chopra H. C., Mason M. M. A new virus in a spontaneous mammary tumor of a rhesus monkey. Cancer Res. 1970 Aug;30(8):2081–2086. [PubMed] [Google Scholar]
- Copeland C. S., Doms R. W., Bolzau E. M., Webster R. G., Helenius A. Assembly of influenza hemagglutinin trimers and its role in intracellular transport. J Cell Biol. 1986 Oct;103(4):1179–1191. doi: 10.1083/jcb.103.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland C. S., Zimmer K. P., Wagner K. R., Healey G. A., Mellman I., Helenius A. Folding, trimerization, and transport are sequential events in the biogenesis of influenza virus hemagglutinin. Cell. 1988 Apr 22;53(2):197–209. doi: 10.1016/0092-8674(88)90381-9. [DOI] [PubMed] [Google Scholar]
- Doms R. W., Keller D. S., Helenius A., Balch W. E. Role for adenosine triphosphate in regulating the assembly and transport of vesicular stomatitis virus G protein trimers. J Cell Biol. 1987 Nov;105(5):1957–1969. doi: 10.1083/jcb.105.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms R. W., Ruusala A., Machamer C., Helenius J., Helenius A., Rose J. K. Differential effects of mutations in three domains on folding, quaternary structure, and intracellular transport of vesicular stomatitis virus G protein. J Cell Biol. 1988 Jul;107(1):89–99. doi: 10.1083/jcb.107.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einfeld D., Hunter E. Oligomeric structure of a prototype retrovirus glycoprotein. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8688–8692. doi: 10.1073/pnas.85.22.8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine D. L., Landon J. C., Pienta R. J., Kubicek M. T., Valerio M. G., Loeb W. F., Chopra H. C. Responses of infant rhesus monkeys to inoculation with Mason-Pfizer monkey virus materials. J Natl Cancer Inst. 1975 Mar;54(3):651–658. [PubMed] [Google Scholar]
- Fitting T., Kabat D. Evidence for a glycoprotein "signal" involved in transport between subcellular organelles. Two membrane glycoproteins encoded by murine leukemia virus reach the cell surface at different rates. J Biol Chem. 1982 Dec 10;257(23):14011–14017. [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gumbiner B., Kelly R. B. Two distinct intracellular pathways transport secretory and membrane glycoproteins to the surface of pituitary tumor cells. Cell. 1982 Jan;28(1):51–59. doi: 10.1016/0092-8674(82)90374-9. [DOI] [PubMed] [Google Scholar]
- Haeuptle M. T., Flint N., Gough N. M., Dobberstein B. A tripartite structure of the signals that determine protein insertion into the endoplasmic reticulum membrane. J Cell Biol. 1989 Apr;108(4):1227–1236. doi: 10.1083/jcb.108.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Sowder R., Smythers G., Benveniste R. E., Oroszlan S. Purification and N-terminal amino acid sequence comparisons of structural proteins from retrovirus-D/Washington and Mason-Pfizer monkey virus. J Virol. 1985 Sep;55(3):778–787. doi: 10.1128/jvi.55.3.778-787.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Isolation of defective mutant of avian sarcoma virus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3493–3497. doi: 10.1073/pnas.70.12.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kreis T. E., Lodish H. F. Oligomerization is essential for transport of vesicular stomatitis viral glycoprotein to the cell surface. Cell. 1986 Sep 12;46(6):929–937. doi: 10.1016/0092-8674(86)90075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M., Fenno J., Burnette W. N., Rohrschneider L. Synthesis and processing of viral glycoproteins in two nonconditional mutants of Rous sarcoma virus. J Virol. 1980 Oct;36(1):280–290. doi: 10.1128/jvi.36.1.280-290.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Kong N. Reversible block in intracellular transport and budding of mutant vesicular stomatitis virus glycoproteins. Virology. 1983 Mar;125(2):335–348. doi: 10.1016/0042-6822(83)90206-4. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. Effects of glucosamine, 2-deoxyglucose, and tunicamycin on glycosylation, sulfation, and assembly of influenza viral proteins. Virology. 1978 Feb;84(2):303–319. doi: 10.1016/0042-6822(78)90250-7. [DOI] [PubMed] [Google Scholar]
- Ono M., Toh H., Miyata T., Awaya T. Nucleotide sequence of the Syrian hamster intracisternal A-particle gene: close evolutionary relationship of type A particle gene to types B and D oncovirus genes. J Virol. 1985 Aug;55(2):387–394. doi: 10.1128/jvi.55.2.387-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Ramsay G., Hayman M. J. Analysis of cells transformed by defective leukemia virus OK10: production of noninfectious particles and synthesis of Pr76gag and an additional 200,000-dalton protein. Virology. 1980 Oct 15;106(1):71–81. doi: 10.1016/0042-6822(80)90222-6. [DOI] [PubMed] [Google Scholar]
- Rein A., McClure M. R., Rice N. R., Luftig R. B., Schultz A. M. Myristylation site in Pr65gag is essential for virus particle formation by Moloney murine leukemia virus. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7246–7250. doi: 10.1073/pnas.83.19.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. S., Hunter E. Myristylation is required for intracellular transport but not for assembly of D-type retrovirus capsids. J Virol. 1987 Apr;61(4):1045–1053. doi: 10.1128/jvi.61.4.1045-1053.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin D., Compans R. W. Identification of the spike proteins of Rous sarcoma virus. Virology. 1971 Nov;46(2):485–489. doi: 10.1016/0042-6822(71)90049-3. [DOI] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M., Müller H., Schmidt M. F., Rott R. Myristoylation of budgerigar fledgling disease virus capsid protein VP2. J Virol. 1989 Jan;63(1):429–431. doi: 10.1128/jvi.63.1.429-431.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. M., Rein A. Unmyristylated Moloney murine leukemia virus Pr65gag is excluded from virus assembly and maturation events. J Virol. 1989 May;63(5):2370–2373. doi: 10.1128/jvi.63.5.2370-2373.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R. T., Rohrschneider J. M., Schmidt M. F. Suppression of glycoprotein formation of Semliki Forest, influenza, and avian sarcoma virus by tunicamycin. J Virol. 1976 Sep;19(3):782–791. doi: 10.1128/jvi.19.3.782-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Garoff H. The budding mechanisms of enveloped animal viruses. J Gen Virol. 1980 Sep;50(1):1–21. doi: 10.1099/0022-1317-50-1-1. [DOI] [PubMed] [Google Scholar]
- Sonigo P., Barker C., Hunter E., Wain-Hobson S. Nucleotide sequence of Mason-Pfizer monkey virus: an immunosuppressive D-type retrovirus. Cell. 1986 May 9;45(3):375–385. doi: 10.1016/0092-8674(86)90323-5. [DOI] [PubMed] [Google Scholar]
- Stohrer R., Hunter E. Inhibition of Rous sarcoma virus replication by 2-deoxyglucose and tunicamycin: identification of an unglycosylated env gene product. J Virol. 1979 Nov;32(2):412–419. doi: 10.1128/jvi.32.2.412-419.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow N. D., Wilkie N. M. An improved technique for obtaining enhanced infectivity with herpes simplex virus type 1 DNA. J Gen Virol. 1976 Dec;33(3):447–458. doi: 10.1099/0022-1317-33-3-447. [DOI] [PubMed] [Google Scholar]
- Streuli C. H., Griffin B. E. Myristic acid is coupled to a structural protein of polyoma virus and SV40. Nature. 1987 Apr 9;326(6113):619–622. doi: 10.1038/326619a0. [DOI] [PubMed] [Google Scholar]
- Takami N., Misumi Y., Kuroki M., Matsuoka Y., Ikehara Y. Evidence for carboxyl-terminal processing and glycolipid-anchoring of human carcinoembryonic antigen. J Biol Chem. 1988 Sep 5;263(25):12716–12720. [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Towler D. A., Gordon J. I., Adams S. P., Glaser L. The biology and enzymology of eukaryotic protein acylation. Annu Rev Biochem. 1988;57:69–99. doi: 10.1146/annurev.bi.57.070188.000441. [DOI] [PubMed] [Google Scholar]
- Wills J. W., Srinivas R. V., Hunter E. Mutations of the Rous sarcoma virus env gene that affect the transport and subcellular location of the glycoprotein products. J Cell Biol. 1984 Dec;99(6):2011–2023. doi: 10.1083/jcb.99.6.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]