Abstract
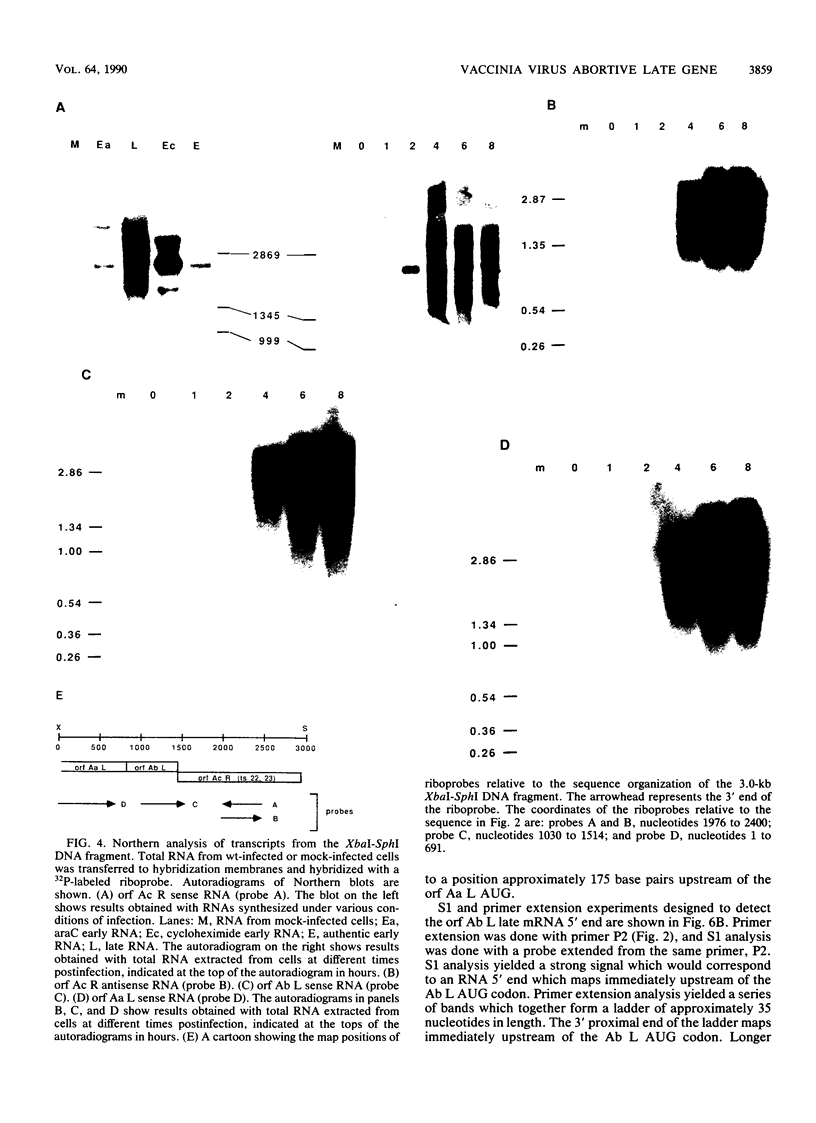
Three noncomplementing vaccinia virus temperature-sensitive mutants, ts4, ts22, and ts23, exhibit an abortive late phenotype characterized by the simultaneous cessation of protein synthesis, the breakdown of rRNA and viral mRNA, and an increase in intracellular concentrations of 2'-5'-linked oligoadenylates late during infection at the nonpermissive temperature (R.F. Pacha and R.C. Condit, J. Virol. 56:395-403, 1985; R.J. Cohrs, R.C. Condit, R.F. Pacha, C.L. Thompson, and O.K. Sharma, J. Virol. 63:948-951, 1989). We have identified the virus gene affected by the abortive late mutants, determined its DNA sequence, and analyzed its transcription. The gene resides in the HindIII A DNA fragment, it has a predicted coding capacity of 57 kilodaltons, and it is transcribed both early and late during infection. The early transcript of the abortive late gene is unusual; it contains a 426-nucleotide 5' untranslated region, and it must be synthesized by transcription through an early transcription termination signal which is located in the middle of the gene in a hairpin loop structure. DNA sequence and transcription analysis of two flanking genes is also presented.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertholet C., Van Meir E., ten Heggeler-Bordier B., Wittek R. Vaccinia virus produces late mRNAs by discontinuous synthesis. Cell. 1987 Jul 17;50(2):153–162. doi: 10.1016/0092-8674(87)90211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyles S. S., Moss B. Homology between RNA polymerases of poxviruses, prokaryotes, and eukaryotes: nucleotide sequence and transcriptional analysis of vaccinia virus genes encoding 147-kDa and 22-kDa subunits. Proc Natl Acad Sci U S A. 1986 May;83(10):3141–3145. doi: 10.1073/pnas.83.10.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohrs R. J., Condit R. C., Pacha R. F., Thompson C. L., Sharma O. K. Modulation of ppp(A2'p)nA-dependent RNase by a temperature-sensitive mutant of vaccinia virus. J Virol. 1989 Feb;63(2):948–951. doi: 10.1128/jvi.63.2.948-951.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit R. C., Motyczka A. Isolation and preliminary characterization of temperature-sensitive mutants of vaccinia virus. Virology. 1981 Aug;113(1):224–241. doi: 10.1016/0042-6822(81)90150-1. [DOI] [PubMed] [Google Scholar]
- Condit R. C., Motyczka A., Spizz G. Isolation, characterization, and physical mapping of temperature-sensitive mutants of vaccinia virus. Virology. 1983 Jul 30;128(2):429–443. doi: 10.1016/0042-6822(83)90268-4. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Wittek R., Moss B. Extension of the transcriptional and translational map of the left end of the vaccinia virus genome to 21 kilobase pairs. J Virol. 1981 Sep;39(3):733–745. doi: 10.1128/jvi.39.3.733-745.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P. L., Jones E. V., Moss B. Homology between DNA polymerases of poxviruses, herpesviruses, and adenoviruses: nucleotide sequence of the vaccinia virus DNA polymerase gene. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3659–3663. doi: 10.1073/pnas.83.11.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami B. B., Sharma O. K. Degradation of rRNA in interferon-treated vaccinia virus-infected cells. J Biol Chem. 1984 Feb 10;259(3):1371–1374. [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hirt P., Hiller G., Wittek R. Localization and fine structure of a vaccinia virus gene encoding an envelope antigen. J Virol. 1986 Jun;58(3):757–764. doi: 10.1128/jvi.58.3.757-764.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ink B. S., Pickup D. J. Vaccinia virus directs the synthesis of early mRNAs containing 5' poly(A) sequences. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1536–1540. doi: 10.1073/pnas.87.4.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Chen G. J., Bourgeois N., Davidson K., Condit R. C., Niles E. G. Structure of the transcription initiation and termination sequences of seven early genes in the vaccinia virus HindIII D fragment. Virology. 1988 Mar;163(1):64–79. doi: 10.1016/0042-6822(88)90234-6. [DOI] [PubMed] [Google Scholar]
- Lee-Chen G. J., Niles E. G. Map positions of the 5' ends of eight mRNAs synthesized from the late genes in the vaccinia virus HindIII D fragment. Virology. 1988 Mar;163(1):80–92. doi: 10.1016/0042-6822(88)90235-8. [DOI] [PubMed] [Google Scholar]
- Lengyel P. Biochemistry of interferons and their actions. Annu Rev Biochem. 1982;51:251–282. doi: 10.1146/annurev.bi.51.070182.001343. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Mahr A., Roberts B. E. Arrangement of late RNAs transcribed from a 7.1-kilobase EcoRI vaccinia virus DNA fragment. J Virol. 1984 Feb;49(2):510–520. doi: 10.1128/jvi.49.2.510-520.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Pacha R. F., Condit R. C. Characterization of a temperature-sensitive mutant of vaccinia virus reveals a novel function that prevents virus-induced breakdown of RNA. J Virol. 1985 Nov;56(2):395–403. doi: 10.1128/jvi.56.2.395-403.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. D., Pickup D. J. The second-largest subunit of the poxvirus RNA polymerase is similar to the corresponding subunits of procaryotic and eucaryotic RNA polymerases. J Virol. 1989 Mar;63(3):1076–1086. doi: 10.1128/jvi.63.3.1076-1086.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988 Jul 28;334(6180):320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- Plucienniczak A., Schroeder E., Zettlmeissl G., Streeck R. E. Nucleotide sequence of a cluster of early and late genes in a conserved segment of the vaccinia virus genome. Nucleic Acids Res. 1985 Feb 11;13(3):985–998. doi: 10.1093/nar/13.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrmann G., Yuen L., Moss B. Transcription of vaccinia virus early genes by enzymes isolated from vaccinia virions terminates downstream of a regulatory sequence. Cell. 1986 Sep 26;46(7):1029–1035. doi: 10.1016/0092-8674(86)90702-6. [DOI] [PubMed] [Google Scholar]
- Rosel J. L., Earl P. L., Weir J. P., Moss B. Conserved TAAATG sequence at the transcriptional and translational initiation sites of vaccinia virus late genes deduced by structural and functional analysis of the HindIII H genome fragment. J Virol. 1986 Nov;60(2):436–449. doi: 10.1128/jvi.60.2.436-449.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt J. F., Stunnenberg H. G. Sequence and transcriptional analysis of the vaccinia virus HindIII I fragment. J Virol. 1988 Jun;62(6):1889–1897. doi: 10.1128/jvi.62.6.1889-1897.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwer B., Visca P., Vos J. C., Stunnenberg H. G. Discontinuous transcription or RNA processing of vaccinia virus late messengers results in a 5' poly(A) leader. Cell. 1987 Jul 17;50(2):163–169. doi: 10.1016/0092-8674(87)90212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman S., Moss B. Factor-dependent transcription termination by vaccinia virus RNA polymerase. Evidence that the cis-acting termination signal is in nascent RNA. J Biol Chem. 1988 May 5;263(13):6220–6225. [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. L., Condit R. C. Marker rescue mapping of vaccinia virus temperature-sensitive mutants using overlapping cosmid clones representing the entire virus genome. Virology. 1986 Apr 15;150(1):10–20. doi: 10.1016/0042-6822(86)90261-8. [DOI] [PubMed] [Google Scholar]
- Wittek R., Richner B., Hiller G. Mapping of the genes coding for the two major vaccinia virus core polypeptides. Nucleic Acids Res. 1984 Jun 25;12(12):4835–4848. doi: 10.1093/nar/12.12.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen L., Moss B. Oligonucleotide sequence signaling transcriptional termination of vaccinia virus early genes. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6417–6421. doi: 10.1073/pnas.84.18.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Magistris L., Stunnenberg H. G. Cis-acting sequences affecting the length of the poly(A) head of vaccinia virus late transcripts. Nucleic Acids Res. 1988 Apr 25;16(8):3141–3156. doi: 10.1093/nar/16.8.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]