Abstract
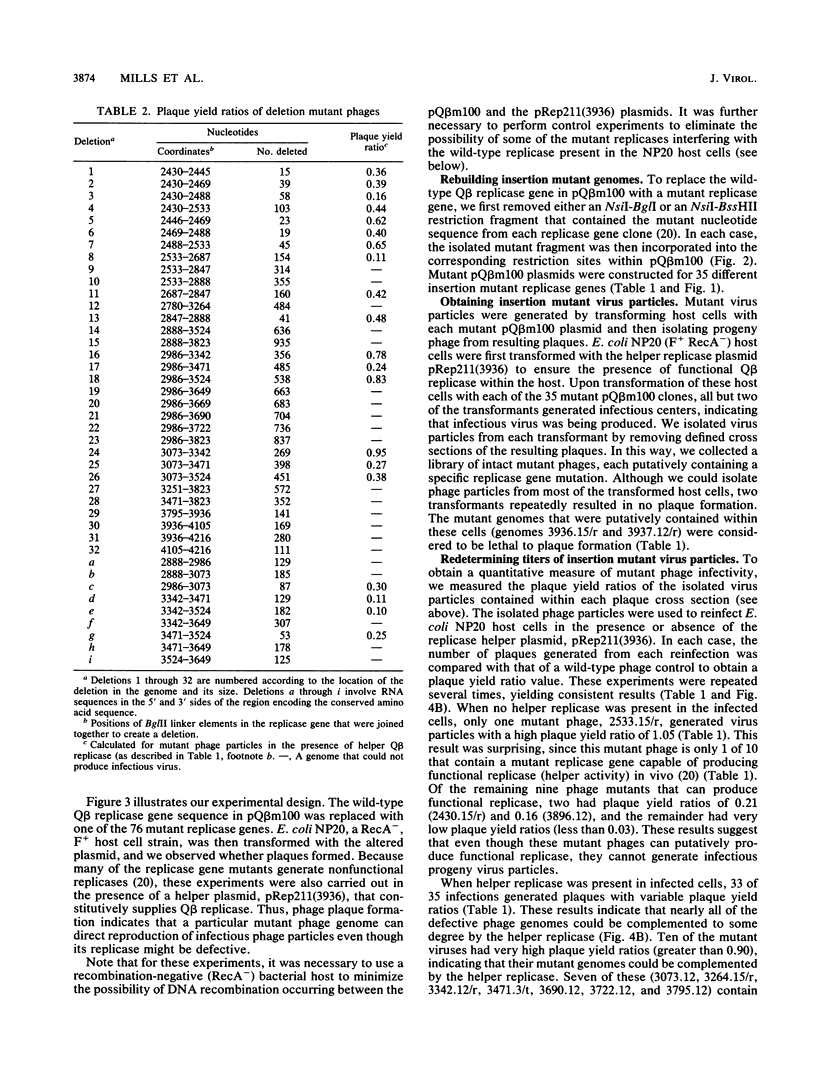
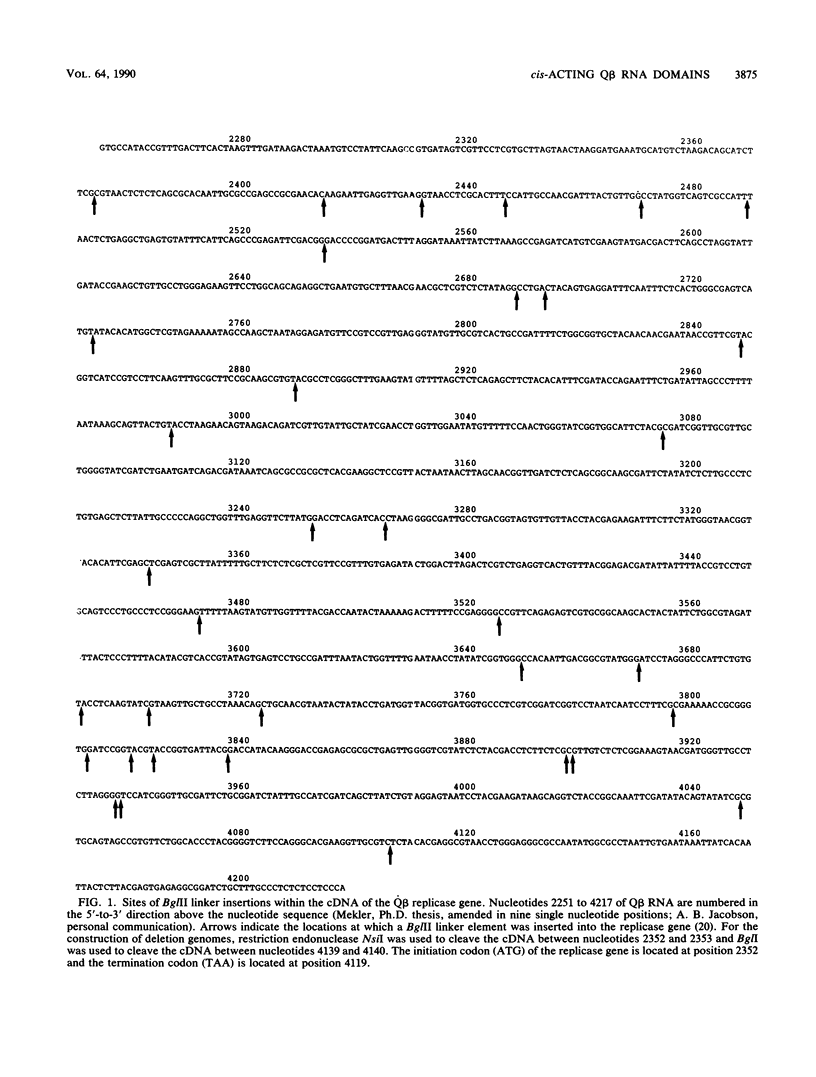
We have identified, for the first time, regions of cis-acting RNA elements within the bacteriophage Q beta replicase cistron by analyzing the infectivities of 76 replicase gene mutant phages in the presence of a helper replicase. Two separate classes of mutant Q beta phage genomes (35 different insertion mutants, each containing an insertion of 3 to 15 nucleotides within the replicase gene, and 41 deletion genomes, each having from 15 to 935 nucleotides deleted from different regions of the gene) were constructed, and their corresponding RNAs were tested for the ability to direct the formation of progeny virus particles. Each mutant phage was tested for plaque formation in an Escherichia coli (F+) host strain that supplied helper Q beta replicase in trans from a plasmid DNA. Of the 76 mutant genomes, 34% were able to direct virus production at or close to wild-type levels (with plaque yield ratios of greater than 0.5), another 36% also produced virus particles, but at much lower levels than those of wild-type virus (with plaque yield ratios of less than 0.05), and the remaining 30% produced no virus at all. From these data, we have been able to define regions within the Q beta replicase gene that contain functional cis-acting RNA elements and further correlate them with regions of RNA that are solely required to code for functional RNA polymerase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins J. F., Steitz J. A., Anderson C. W., Model P. Binding of mammalian ribosomes to MS2 phage RNA reveals an overlapping gene encoding a lysis function. Cell. 1979 Oct;18(2):247–256. doi: 10.1016/0092-8674(79)90044-8. [DOI] [PubMed] [Google Scholar]
- Beckett D., Uhlenbeck O. C. Ribonucleoprotein complexes of R17 coat protein and a translational operator analog. J Mol Biol. 1988 Dec 20;204(4):927–938. doi: 10.1016/0022-2836(88)90052-6. [DOI] [PubMed] [Google Scholar]
- Bernardi A., Spahr P. F. Nucleotide sequence at the binding site for coat protein on RNA of bacteriophage R17. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3033–3037. doi: 10.1073/pnas.69.10.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biebricher C. K., Diekmann S., Luce R. Structural analysis of self-replicating RNA synthesized by Qbeta replicase. J Mol Biol. 1982 Feb 5;154(4):629–648. doi: 10.1016/s0022-2836(82)80019-3. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Carmichael G. G. RNA replication: function and structure of Qbeta-replicase. Annu Rev Biochem. 1979;48:525–548. doi: 10.1146/annurev.bi.48.070179.002521. [DOI] [PubMed] [Google Scholar]
- Boni I. V., Isaeva D. M., Budovskii E. I. Ribosomnyi belok S1 v sostave kompleksa 30S ribosomnoi subchastitsy E. coli s RNK faga MS2 vzaimodeistvuet s vnutrennim raionom gena replikazy. Bioorg Khim. 1986 Feb;12(2):293–296. [PubMed] [Google Scholar]
- Fiers W., Contreras R., Duerinck F., Haegeman G., Iserentant D., Merregaert J., Min Jou W., Molemans F., Raeymaekers A., Van den Berghe A. Complete nucleotide sequence of bacteriophage MS2 RNA: primary and secondary structure of the replicase gene. Nature. 1976 Apr 8;260(5551):500–507. doi: 10.1038/260500a0. [DOI] [PubMed] [Google Scholar]
- Haruna I., Spiegelman S. Autocatalytic synthesis of a viral RNA in vitro. Science. 1965 Nov 12;150(3698):884–886. doi: 10.1126/science.150.3698.884. [DOI] [PubMed] [Google Scholar]
- Heffron F., So M., McCarthy B. J. In vitro mutagenesis of a circular DNA molecule by using synthetic restriction sites. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6012–6016. doi: 10.1073/pnas.75.12.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi K., Matsuhashi S. Three cistrons in bacteriophage Q beta. Virology. 1970 Sep;42(1):49–60. doi: 10.1016/0042-6822(70)90237-0. [DOI] [PubMed] [Google Scholar]
- Inokuchi Y., Jacobson A. B., Hirose T., Inayama S., Hirashima A. Analysis of the complete nucleotide sequence of the group IV RNA coliphage SP. Nucleic Acids Res. 1988 Jul 11;16(13):6205–6221. doi: 10.1093/nar/16.13.6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi Y., Takahashi R., Hirose T., Inayama S., Jacobson A. B., Hirashima A. The complete nucleotide sequence of the group II RNA coliphage GA. J Biochem. 1986 Apr;99(4):1169–1180. doi: 10.1093/oxfordjournals.jbchem.a135580. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D., Weissmann C. Q replicase as repressor of Q RNA-directed protein synthesis. Biochim Biophys Acta. 1971 Sep 24;246(3):596–599. doi: 10.1016/0005-2787(71)90799-4. [DOI] [PubMed] [Google Scholar]
- Meyer F., Weber H., Weissmann C. Interactions of Q beta replicase with Q beta RNA. J Mol Biol. 1981 Dec 15;153(3):631–660. doi: 10.1016/0022-2836(81)90411-3. [DOI] [PubMed] [Google Scholar]
- Mills D. R., Kramer F. R., Spiegelman S. Complete nucleotide sequence of a replicating RNA molecule. Science. 1973 Jun 1;180(4089):916–927. doi: 10.1126/science.180.4089.916. [DOI] [PubMed] [Google Scholar]
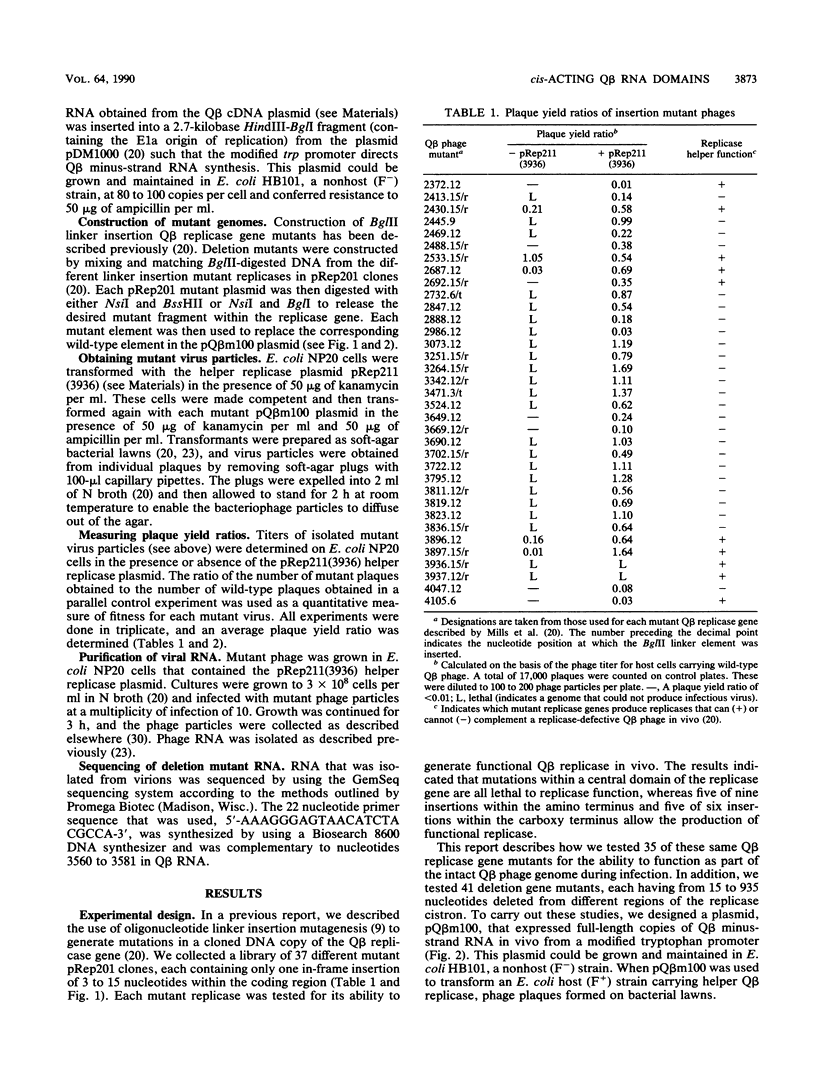
- Mills D. R., Priano C., DiMauro P., Binderow B. D. Q beta replicase: mapping the functional domains of an RNA-dependent RNA polymerase. J Mol Biol. 1989 Feb 20;205(4):751–764. doi: 10.1016/0022-2836(89)90319-7. [DOI] [PubMed] [Google Scholar]
- Nishihara T., Mills D. R., Kramer F. R. Localization of the Q beta replicase recognition site in MDV-1 RNA. J Biochem. 1983 Mar;93(3):669–674. doi: 10.1093/jb/93.3.669. [DOI] [PubMed] [Google Scholar]
- Pace N. R., Spiegelman S. In vitro synthesis of an infectious mutant RNA with a normal RNA replicase. Science. 1966 Jul 1;153(3731):64–67. doi: 10.1126/science.153.3731.64. [DOI] [PubMed] [Google Scholar]
- Priano C., Kramer F. R., Mills D. R. Evolution of the RNA coliphages: the role of secondary structures during RNA replication. Cold Spring Harb Symp Quant Biol. 1987;52:321–330. doi: 10.1101/sqb.1987.052.01.037. [DOI] [PubMed] [Google Scholar]
- Romaniuk P. J., Lowary P., Wu H. N., Stormo G., Uhlenbeck O. C. RNA binding site of R17 coat protein. Biochemistry. 1987 Mar 24;26(6):1563–1568. doi: 10.1021/bi00380a011. [DOI] [PubMed] [Google Scholar]
- Weber H. The binding site for coat protein on bacteriophage Qbeta RNA. Biochim Biophys Acta. 1976 Jan 19;418(2):175–183. doi: 10.1016/0005-2787(76)90067-8. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Weber K. Natural read-through at the UGA termination signal of Q-beta coat protein cistron. Nat New Biol. 1971 Sep 15;234(50):206–209. doi: 10.1038/newbio234206a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]