Abstract
The cholinergic system has been implicated in learning and memory. The nucleus basalis (NB) provides acetylcholine (ACh) to the cerebral cortex. Pairing a tone with NB stimulation (NBstm) to alter cortical state induces both associative specific tuning plasticity in the primary auditory cortex (A1) and associative specific auditory behavioral memory. NB-induced memory has major features of natural memory that is induced by pairing a tone with motivational reinforcers, e.g., food or shock, suggesting that the cholinergic system may be a “final common pathway” whose activation promotes memory storage. Alternatively, NB-stimulation might itself be motivationally significant, either rewarding or punishing. To investigate these alternatives, adult male rats (n = 7) first formed a specific NB-induced memory (CS = 8.0 kHz, 2.0 s paired with NBstm, ISI = 1.8 s, 200 trials), validated by post-training (24 h) frequency generalization gradients (1–15 kHz) of respiration interruption that were specific to the CS frequency. Thereafter, they received the same level of NBstm that had induced memory, while confined to one quadrant of an arena, and later tested for place preference, i.e., avoidance or seeking of the quadrant of NBstm. This NBstm group exhibited neither preference for nor against the stimulated quadrant, compared to sham-operated subjects (n = 7). The findings indicate that specific associative memory can be induced by direct activation of the NB without detectable motivational effects of NB stimulation. These results are concordant with a memory-promoting role for the nucleus basalis that places it “downstream” of motivational systems, which activate it to initiate the storage of the current state of its cholinergic targets.
Keywords: Auditory cortex, Acetylcholine, Association, Learning
INTRODUCTION
The cholinergic system has long been implicated in learning and memory. For example, pharmacological blockade of the cholinergic system impairs many forms of memory (Deutsch, 1971; Flood, Landry, & Jarvik, 1981; Power, Vazdarjanova, & McGaugh, 2003; Rudy, 1996). Cholinergic agonists and cholinesterase antagonists can facilitate memory (Introini-Collison & McGaugh, 1988; Stratton & Petrinovich, 1963), promote recovery of memory from brain damage (Russell, Escobar, Booth, & Bermudez-Rattoni, 1994) and achieve rescue from memory deficits in transgenic mice (Fisher, Brandeis, Chapman, Pittel, & Michaelson, 1998). Also, several non-cholinergic treatments that facilitate memory, such as adrenergic agents and stress hormones, affect memory via actions on the cholinergic system (Salinas, Introini-Collison, Dalmaz, & McGaugh, 1997).
The nucleus basalis of the basal forebrain (NB) is the major source of extrinsic acetylcholine (ACh) to the cerebral cortex (Bigl, Woolf, & Butcher, 1982; Johnston, McKinney, & Coyle, 1979; Luiten, Gaykema, Traber, & Spencer, 1987; Mesulam, Mufson, Wainer, & Levey, 1983; Rye, Wainer, Mesulam, Mufson, & Saper, 1984). Activation of the NB to release ACh in the cerebral cortex has a profound effect on cortical state. It shifts the spectrum of the electroencephalogram (EEG) from higher voltage slower waves, indicative of drowsiness or sleep, to lower voltage fast waves (cortical “desynchronization” or “activation”) that are characteristic of an alert waking state and also rapid-eye-movement (REM) sleep. Both states are thought to promote information processing in the cerebral cortex. Cortical activation depends upon the release of ACh (Casamenti, Deffenu, Abbamondi, & Pepeu, 1986; Celesia & Jasper, 1966; Detari, Juhasz, & Kukorelli, 1983, 1984, 1987; Jimenez-Capdeville, Dykes, & Myasnikov, 1997; Juhasz, Detari, & Kukorelli, 1985; Kukorelli, Feuer, Juhasz, & Detari, 1986; Rasmusson, Clow, & Szerb, 1992, 1994; Rasmusson, Szerb, & Jordan, 1996; Szymusiak & McGinty, 1986) that engages cortical muscarinic receptors (Phillis & York, 1968; Szerb, 1964).
In addition to its role in activating the cortex, the NB can induce cortical plasticity. For example, stimulation of the nucleus basalis (NBstm) paired with sensory stimulation produces enduring facilitation of responses in the somatosensory cortex (Tremblay, Warren, & Dykes, 1990), atropine-sensitive persistent modification of evoked responses (Metherate & Ashe, 1992, 1993) and facilitation of responses to tone (Edeline, Hars, Maho, & Hennevin, 1994; Edeline, Maho, Hars, & Hennevin, 1994) in the primary auditory cortex (A1). Pairing a tone with NBstm produces associative, frequency-specific shifts of neuronal tuning (Bakin & Weinberger, 1996; Bjordahl, Dimyan, & Weinberger, 1998; Dimyan & Weinberger, 1999; Ma & Suga, 2003) and enlargement of auditory cortical representation of the paired tone frequency (Kilgard & Merzenich, 1998a, 1998b; Kilgard, Pandya, Engineer, & Moucha, 2002). NB-induced associative receptive field tuning shifts are dependent upon the engagement of muscarinic receptors in the auditory cortex (Miasnikov, McLin, & Weinberger, 2001).
The nucleus basalis not only induces specific cortical plasticity, but it also induces behavioral memory. (We use the term “behavioral memory” to distinguish actual memory from learning-related neural plasticity, which unfortunately is often called “memory”.) Thus, pairing a tone with NBstm produces conditioned stimulus (CS)-specific behavioral memory as indexed by training (i.e., pairing) with one frequency but later testing with many frequencies to obtain behavioral generalization gradients. (Preferential responses to the CS frequency would indicate that subjects learned about the specific frequency, while responses to most frequencies would indicate that learning was not specific to the CS frequency but rather learned about tones in general (Mackintosh, 1974; Mostofsky, 1965; Pavlov, 1927).)
We found previously that pairing a tone with stimulation of the nucleus basalis induces memory that is both associative and contains detail about the absolute frequency of the conditioned stimulus. Rats that received extensive pairing of a single tone with NBstm (3,000 trials over 15 days) later exhibited behavioral frequency response profiles (for both the interruption of ongoing respiration and changes in heart rate) in the absence of NBstm that were maximal at the CS frequency. In contrast, rats receiving unpaired stimulation failed to develop such behavioral CS-specificity (McLin, Miasnikov, & Weinberger, 2002a, 2003). Thereafter, we found that such extensive training is not necessary. Rather, specific associative memory can be induced rapidly, with a single training session of 200 trials (Miasnikov, Chen, & Weinberger, 2006). Additionally, the NB can control the amount of detail that is learned. Thus, pairing an 8.0 kHz tone with weak (~45 µA) stimulation of the NB that produces minimal EEG activation induces associative memory that is equal across the frequency spectrum (i.e., a “flat” generalization gradient). In contrast, moderate stimulation (~65 µA), that causes stronger EEG activation, induces associative auditory memory that is specific to the frequency band of the conditioned stimulus (Weinberger, Miasnikov, & Chen, 2006).
Despite the extensive documentation of the involvement of the cholinergic system in learning and memory, and of the role of the nucleus basalis in modulating cortical state, mediating specific cortical plasticity and inducing behavioral memory, the actual role of the nucleus basalis is unknown. Two major functional explanations are readily apparent. First engagement of the NB/ACh system could itself serve as a positive or negative reinforcer, i.e., be rewarding or punishing. Second, the NB/ACh system could be “downstream” of motivational systems but be engaged by them (both positive and negative in valence) to promote the storage of the information currently being processed, perhaps throughout the cortex and at any other cholinergic targets. To investigate these two alternatives, we used a place-preference test in an arena divided into four quadrants (Bardo, Rowlett, & Harris, 1995; Hasenohrl, Oitzl, & Huston, 1989; see also Panos, Rademacher, Renner, & Steinpreis, 1999; Sahraei, Pirzadeh-Jahromi, Noorbakhshnia, Asgari, Haeri-Rohani, Khoshbaten, Poorheidari, Sepehri, Ghoshooni, & Zarrindast, 2004). We first induced specific auditory memory in rats and later confined them to a quadrant of an arena while they received the same NBstm that had been used to induce memory. Subsequently they were allowed to move freely at which time we determined whether or not they exhibited a preference either for or against the stimulated location.
MATERIALS AND METHODS
Most materials and methods are identical to those previously reported, so they will be described only briefly. All procedures were performed in accordance with the University of California Irvine Animal Research Committee and the NIH Animal Welfare guidelines. During training and testing, subjects were continuously video monitored.
Subjects and Surgery
The subjects were 14 adult male Sprague–Dawley rats (433 ± 69 g), housed individually with ad libitum food and water, on a 12/12h light–dark cycle (lights on at 7:15 AM). Following several days of adaptation to the vivarium, they were handled and learned to sit calmly during attachment of a thermistor assembly and a cable to their skull pedestal. They were divided into two groups: NB-induction of memory (NB-Mem) and sham-operated controls (Cont). While both groups underwent place-preference testing (below), only the NB-Mem group underwent the induction of memory by NBstm.
Under general anesthesia (sodium pentobarbital, 35 mg/kg, i.p., initial dose supplemented as necessary to maintain areflexia), they were placed in a stereotaxic frame while body temperature was maintained. After clearing the calvaria, a dental acrylic pedestal was built containing two aluminum hex threaded standoffs. Two screws over the frontal sinus served as reference electrodes. For the NB-Mem group, a recording screw to monitor the electroencephalogram (EEG) was threaded into a burr hole overlying the auditory cortex at a locus of short-latency, high-amplitude local field potentials (EFP) evoked by a burst of band-passed white noise (1–15 kHz) (Miasnikov et al., 2006). A concentric bipolar stainless-steel stimulating electrode was lowered through the contralateral hemisphere at a 45° angle to vertical in the frontal plane at AP –2.2, ML 3.2 (Paxinos & Watson, 1997) while stimulation was applied (100 Hz, 200–300 ms trains, 0.2 ms bipolar) until it reached the ipsilateral (right) NB; the final locus was determined physiologically by obtaining 1–5 s of consistent auditory cortical EEG activation. All leads were connected to a miniature socket that could be led to a commutator via a multi-conductor cable. Subjects were allowed 1–2 weeks to recover from surgery.
Induction of Memory: Stimuli, Recording and Data Analysis
In the NB-Mem group, training and testing for memory induction took place with each subject in a box (20 × 20 × 28.5 cm) containing fresh bedding and lined with acoustical tile, contained in a double-walled acoustic room (122 × 119 × 199 cm). Acoustic stimuli were calibrated pure tones (1.0–15.0 kHz, 2 s, cosine, 10 ms rise/fall time, 70 dB SPL) delivered to a loudspeaker 44 cm above the floor of the box. NBstm current (66 ± 6 µA, 100 Hz, 0.2 ms bipolar, 200 ms train) was much lower than during surgery because anesthesia increases the threshold for NB-induced EEG activation. This “moderate” level of stimulation was below threshold for the elicitation of any behavior and was previously used to induce CS-specific associative memory (Weinberger et al., 2006).
As previously, we assessed the induction of behavioral memory by measuring disruption of ongoing respiration to the CS tone (8.0 kHz) and to other non-CS tones (1.0–15.0 kHz, see below). Respiration was detected by a glass-encapsulated thermistor attached to a lightweight pedestal-mounted assembly of custom design and fabrication, located 1–2 mm lateral to a naris (McLin et al., 2002a, 2003). The thermistor, sensitive to temperature fluctuations during breathing, served as one arm of a pre-balanced resistor bridge circuit. The output signal from the bridge was fed to a differential band-pass amplifier (1–100 Hz), digitized by two A–D modules, one for on-line calculation of the autocorrelation function (ACF) (100 samples/s), the other for off-line analysis (2,000 samples/s). The ACF was used to present tones only when the subject was in a state of quiet waking as determined by regular respiration; the ACF criterion was 80% of a perfect sinusoid for a period of 4 s. Trials meeting this criterion were presented if the scheduled intertrial interval period had passed (30–180 s). This state control was employed to avoid giving stimuli when very high levels of ACh were being released in the cortex, as during exploration or REM sleep (Giovannini, Rakovska, Benton, Pazzagli, Bianchi, & Pepeu, 2001; Jasper & Tessier, 1971; Kametani & Kawamura, 1990; Marrosu, Portas, Mascia, Casu, Fa, Giagheddu, Imperato, & Gessa, 1995) to prevent a ceiling effect, thus promoting a physiologically-effective release of ACh by NBstm.
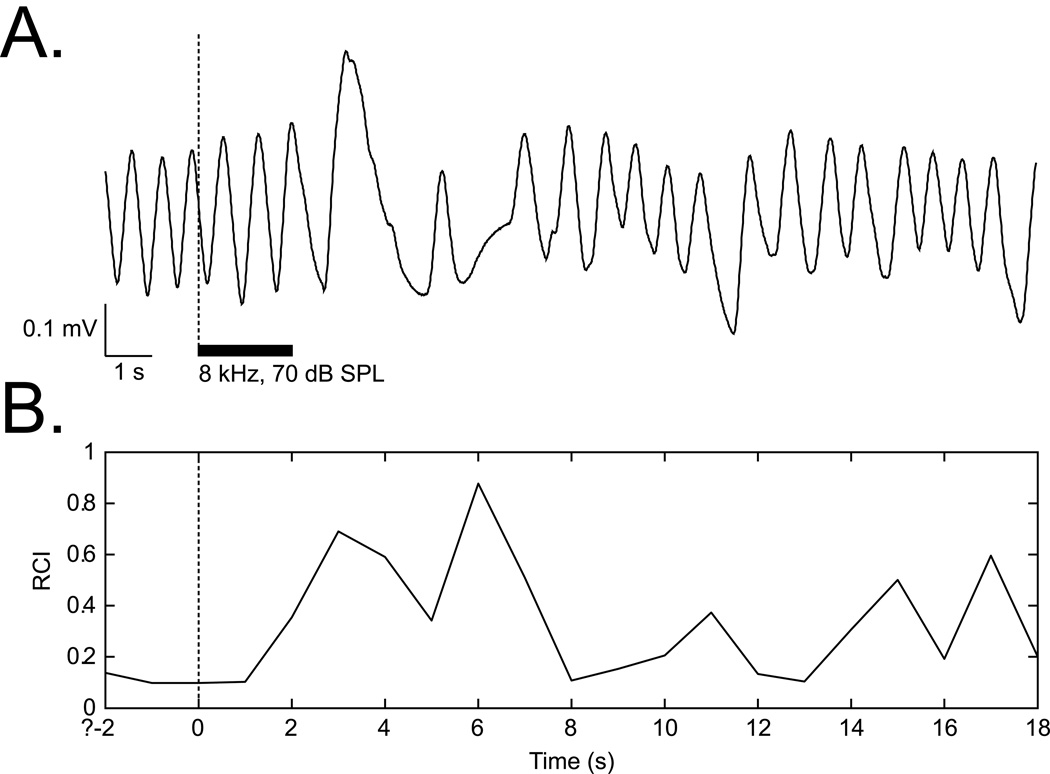
Offline analysis quantified the effect of tones on respiration. Analysis consisted of the calculation of Fast Fourier Transform (FFT) functions for a period of 2 s preceding a trial (Pre), 2 s during a CS tone (Dur) and 24 s after the tone (Post). Major changes in respiration occurred within 0.5–12.5 s after tone onset. The respiration signal was almost completely contained within the bandwidth of 0.975–2.925 Hz. The FFT data were used to calculate a “Respiration Change Index” (RCI), on a second-by-second basis. The index was sensitive to increases and decreases of both frequency and amplitude. RCIs were calculated as: RCIi = (|Posti – Pre|) / (Posti + Pre). A value of zero would indicate no change and a value of 1.0 would indicate complete cessation of respiration (Figure 1).
Figure 1.
Respiration signal and its quantification. (A) An example of a regular sinusoidal baseline respiration record disrupted by tone presentation. (B) Quantification of the respiration record shown in (A). The “Respiration Change Index” (RCI, see Methods) is sensitive to both increases and decreases in signal amplitude and frequency.
Induction of Memory: Experimental Design
To induce behavioral memory, we paired a tone with stimulation of the NB, and to determine the degree of frequency specificity, we obtained post-training behavioral frequency generalization gradients (McLin et al., 2002a; Miasnikov & Weinberger, 2003; Weinberger et al., 2006). Stimulus-specific memory would be revealed by a non-flat gradient with peaks at or near the CS frequency. As single tone conditioning was employed, rather than two-tone discrimination training, any increased responses were not expected to be confined to the CS frequency but rather to stimuli at and near the CS frequency (Mackintosh, 1974). Because change in respiration is a highly sensitive measure, we were also able to obtain pre-training (baseline) frequency response functions that could be used as the basis for comparison to post-training frequency responses.
The protocol was run over four days: Days 1 and 2, pre-training baseline behavior to test tones; Day 3, training which consisted of 200 trials of CS (8.0 kHz, 2 s) paired with co-terminating NBstm (0.2 s), interstimulus interval (ISI) = 1.8 s, intertrial interval (ITI) ~ 80 s (30–180 s); Day 4, post-training generalization testing. The first session (Day 1) was used to acclimatize subjects to the testing environment and thus data from this session were not analyzed. Contextual transfer between training and frequency testing sessions was reduced by delivering animals to the lab via different circuitous routes and training them in the dark (red light) but testing them (pre- and post-training) in the light. During pre- and post-training assessment of responses to tones, nine frequencies (1.00, 2.75, 4.50, 6.25, 8.00, 9.75, 11.50, 13.25 and 15.00 kHz, all 70 dB, mean interval = 94 s) were presented in pseudo-random order with the restriction of not more than two consecutive tones of the same frequency.
Several days after completion of the place-preference test (below), subjects were returned to the training box where they received a single session of 200 presentations each of the CS tone and NBstm. The current level of NBstm was identical to that which each subject had received during training and the place-preference test. Tones and NBstm were presented in random order at the same mean density as during training (average of two stimuli per 80 s), with the restrictions that no more than three of the same type could occur consecutively and that NBstm could not occur during a 15 s period either following or preceding presentation of the CS. The purpose of this assessment was to determine if NBstm had maintained its physiological effectiveness after its use in the training and place-preference phases of the experiment; it also allowed verification that tone conditioned EEG activation had developed during prior tone-NBstm pairing.
The physiological effectiveness of NBstm was determined by quantitative analysis of the EEG using fast-fourier transforms (FFT) to determine the power in EEG bands both preceding (“pre”) and following (“post”) each presentation of stimulation, and then computing a Power Change Index (PCI): [(post − pre)/(post + pre)], as previously documented (McLin, Miasnikov and Weinberger, 2003). The major EEG changes during activation consist of a decrease of power in the alpha band (8.8 – 14.6 Hz) and an increase of power in the gamma band (33.0 – 59.0 Hz). To provide for a direct statistical comparison of the effectiveness of NBstm during tone-NBstm paired trials and after the place-preference test, an “EEG Activation Index” (EAI) was computed: for both phases of the experiment: EAI = [(Gamma PCI) + (Alpha PCI × −1.0)]. This yielded generally positive values that gave equal weighting to changes in both alpha and gamma power.
Place-Preference Test
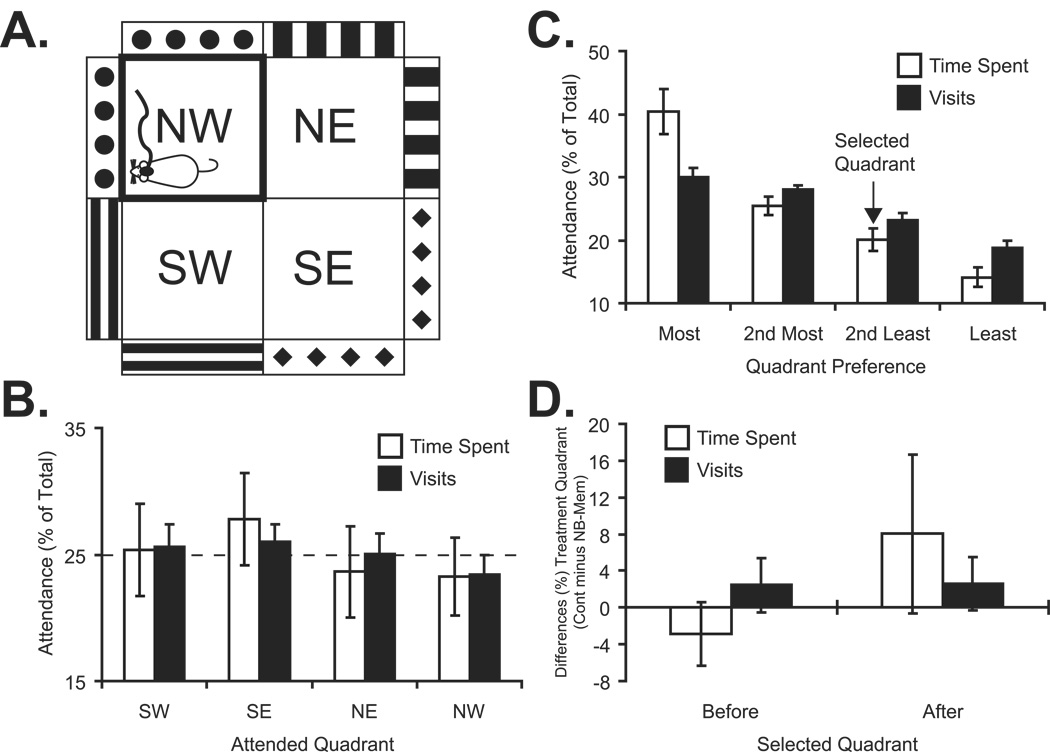
The apparatus consisted of a custom-made square transparent Lucite arena (60 × 60 × 40 cm) divided into equal quadrants demarcated by thin black tape affixed to the underside of the floor. The arena was enclosed in a non-transparent, brown cardboard box placed over a vibration attenuating Styrofoam plate on a table in a closed room. Subjects could be confined to a single quadrant by insertion of a cross-shaped Lucite 40 cm high transparent divider. Quadrants were visually distinct from each other by different black–white patterns covering their outside walls and floor. The arena quadrants were equally illuminated from above by two 15 W “soft white” lamps. Between individual sessions, the arena (and where appropriate the divider) was cleaned twice with 70% alcohol to remove olfactory cues. All sessions were video-recorded by an overhead camera. A pilot study of exploration in the open arena by nine naïve rats showed no bias in time spent (Kruskal–Wallis, Chi-square (3) = 5.17, p > 0.05) or number of entries (Chi-square (3) = 2.21, p > 0.05) for or against any quadrant.
The place-preference test was conducted at least three days after completion of NB-induction of memory for the NB-Mem group, and after a number of days following surgery for the Cont group that was approximately equal to the total time since surgery for the NB-Mem group. The protocol required three days: Day 1, pre-confinement preference test; Day 2, confinement (with NBstm for the NB-Mem group); Day 3, post-confinement test for quadrant preference. All sessions were conducted at the same time of day. The pre- and post-confinement sessions began with subjects placed on the center of the open arena facing away from the experimenter and lasted for 10 minutes beginning with the time that the animal moved into a quadrant. A rat was considered to enter (and occupy) a quadrant when all four of its paws were within that quadrant. The confinement quadrant was determined for each rat to be the second-least preferred quadrant, to avoid using the area in which the rat spent the most or the least time.
On Day 2, the barrier was inserted into the arena and the subjects placed in the appropriate quadrant. The NB-Mem animals were connected to a cable and overhead commutator and received 200 trials of NBstm alone at random inter-trial intervals (mean = 45 s, range = 30–60 s). The stimulation parameters were identical to those used for prior memory induction for each subject. The Cont group was also connected to a dummy plug in their pedestals and remained confined for the same period as the NB-Mem group.
Histology
Following termination of the experiments, an electrolytic lesion (4 ms pulses at 100 Hz, 500 µA for 60 s) was made with bipolar current through the stimulating electrode of the NB-Mem group while the animal was under sodium pentobarbital anesthesia. It was then given an overdose of sodium pentobarbital and perfused through the heart with saline followed with 3.7% paraformaldehyde in 0.1 M phosphate buffer (pH 7.3). The brain was removed and coordinates of the recording electrode on the skull were measured from Bregma. Following several days of post-fixation in paraformaldehyde solution with 0.8 M sucrose added for subsequent cryoprotection, the brain was sectioned at 50 µm with a freezing microtome, sections mounted onto gelatin-coated slides, dried and stained for Nissl substance to recover the electrolytic lesion sites and determine the actual loci of stimulation. Auditory cortex recording sites, which had been determined by click-induced local field potentials, were verified by post-mortem precise measurement from the interior of the calvaria of the A–P and M–L distances from Bregma and midline, respectively, and plotted on a stereotaxic map of the auditory and surrounding areas of cortex derived from the Paxinos and Watson (1997) atlas.
RESULTS
Effectiveness of NB Stimulation
All of the stimulation sites were located within the basal forebrain in structures containing corticopetal cholinergic cells, including those that project to the ipsilateral auditory cortex (Bigl et al., 1982; Johnston et al., 1979; Luiten et al., 1987; Mesulam et al., 1983; Rye et al., 1984) (Figure 2C).
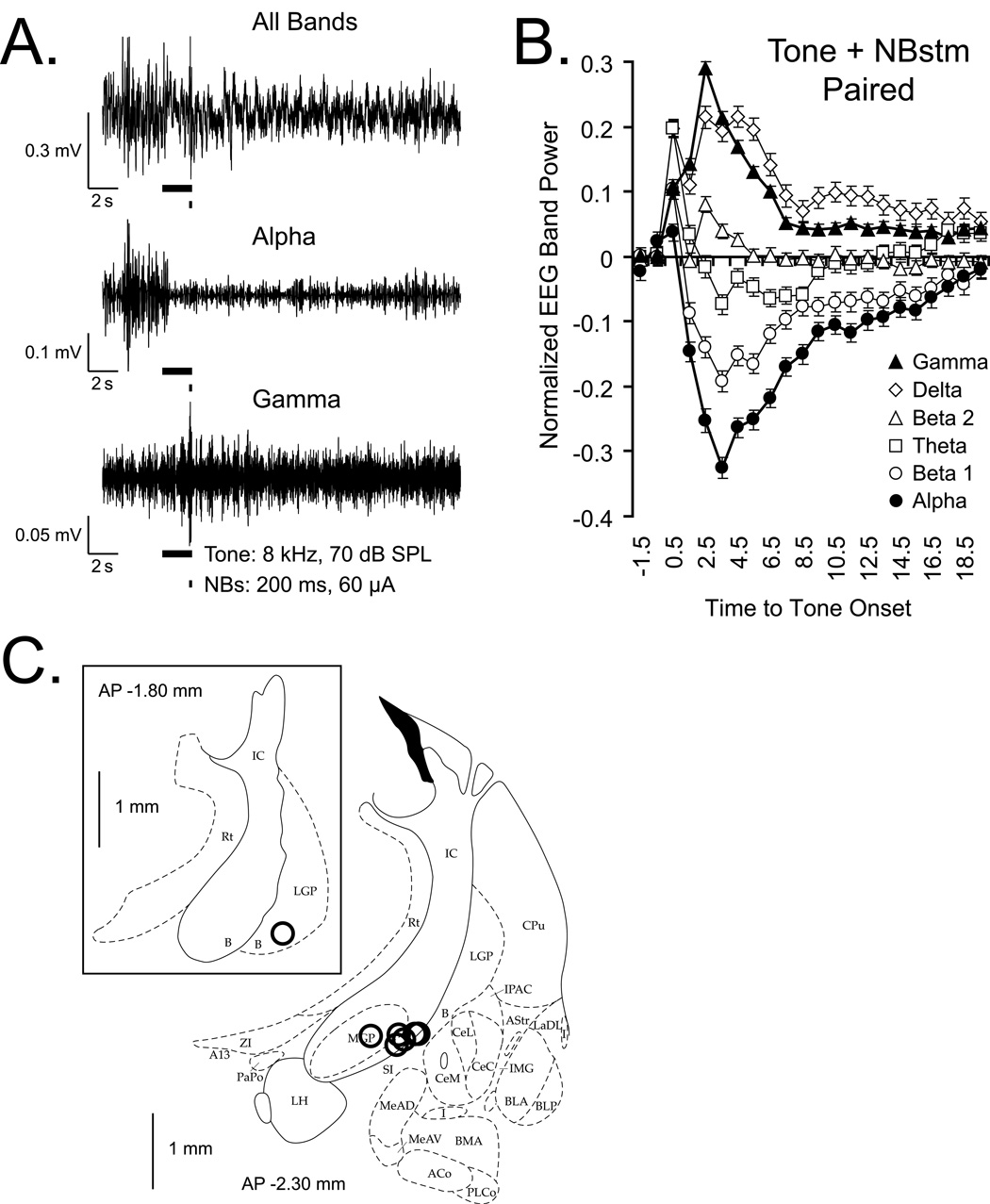
Figure 2.
Changes in the auditory cortical EEG during tone-NBstm pairing, and NB stimulation sites. (A) Example of changes in the EEG frequency bands that exhibited the largest changes in power. “All Bands”: original records obtained with band-pass filters set at 1–1000 Hz. “Alpha” and “Gamma”: corresponding records band-passed with digital filters set at 8.8–14.6 Hz to emphasize alpha and 33–59 Hz to emphasize gamma bands, respectively. This example is for a pairing trial (tone = 8.0 kHz, 70 db, 2s, with 200 ms overlapping train, 100 Hz, 60 µA bipolar stimulation of the NB) in unanesthetized rat. Black bars show tone and NBstim. Note the EEG activation, including a distinct decrease in higher voltage, slower waves (“Alpha”) and increase in lower voltage faster waves (“Gamma”). During pairing, EEG activation reflects a combination of effects of the NBstm and its preceding tone. (B) Group mean EEG spectral changes relative to pre-NBstm, computed as the EEG Power Change Index: EEG PCIi = (Posti − Pre) / (Posti + Pre) where the “Pre” period was the mean of the first 2 s out of four immediately preceding tone onset and post measures were calculated for consecutive periods of 1 s. Note major effects are an increase in gamma (closed triangles) and a decrease in alpha (closed circles) power. (C) Stimulating loci reconstructed for the NB-Mem animals. Sites of nucleus basalis stimulation projected onto outlines of frontal section at closest relevant sections anterior to posterior (AP) distance caudal from Bregma in millimeters (Paxinos & Watson, 1997). In all animals, stimulation was within the caudal nucleus basalis (ventrolateral internal capsule, ventromedial lateral globus pallidus and nucleus basalis of Meynart) which projects preferentially to the auditory cortex. The stimulation sites in 6 animals were found at AP −2.3, and in one animal — at AP −1.8 (insert). Abbreviations: B, basal nucleus of Meynert; CeM, amygdala central nucleus medial; CeL, amygdala central nucleus lateral; CPu, caudate–putamen; IC, internal capsule; IPAC, interstitial nucleus of posterior limb of anterior commissure; LGP, lateral globus pallidus; LH, lateral hypothalamus; SI, substantia innominata; SIB, substantia innominata, basal; SIV, substantia innominata, ventral.
To determine the outcome of NB activation on the induction of specific memory, it was necessary to provide a direct measure of its physiological effectiveness. Therefore, we analyzed the effects of NBstm on the EEG spectrum for the 200 training trials. However, a pure measure of its effectiveness during training is not possible because NBstm necessarily was preceded by the CS tone, and we have found that pairing a tone with NBstm produces conditioned EEG activation (McLin, Miasnikov, & Weinberger, 2003). Therefore, changes in the EEG during training trials reflect both tone conditioned and NBstm unconditioned effects. Examples of raw EEG (“All Bands”) and digitally band-passed Alpha and Gamma activity are presented in Figure 2A. The group data are shown in Figure 2B. Note the marked activation of several seconds duration of the “All Bands” EEG, involving large decreased activity in the alpha band (8.8 – 14.6 Hz) and increased activity in the gamma band (33.0 – 59.0 Hz) (McLin, Miasnikov, & Weinberger, 2002b; Miasnikov et al., 2006; Rasmusson et al., 1992; Weinberger et al., 2006). It might be argued that EEG activation during tone-NBstm training sessions was caused by the CS tone rather than by NBstm. However, tone alone (intermixed randomly with NBstm) does not induce EEG activation (McLin, Miasnikov, & Weinberger, 2003). Thus, any tone-induced EEG activation observed in this study must have been caused by its pairing with NBstm. Thus, we conclude that NBstm during training trials was physiologically effective at the auditory cortex.
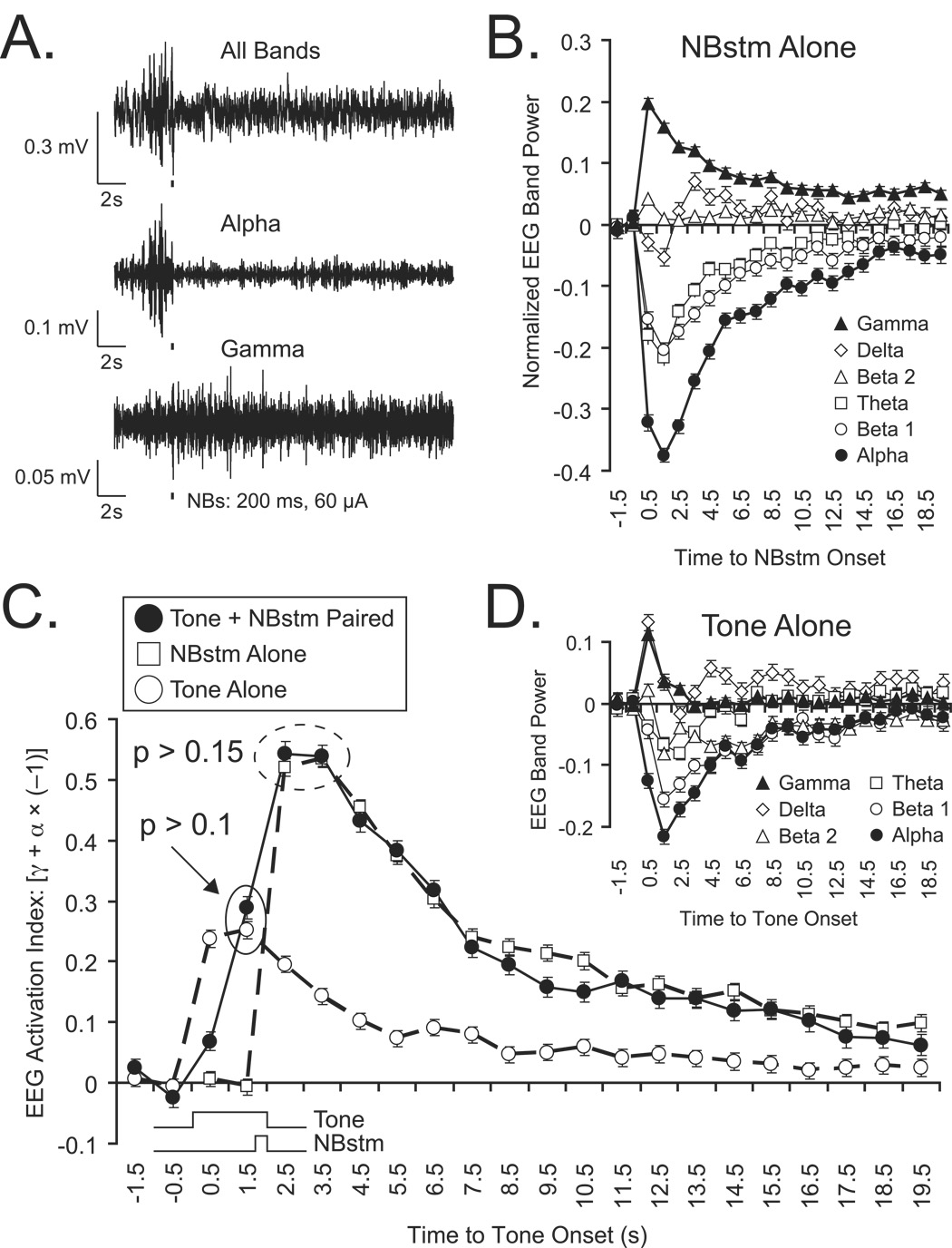
As NBstm was next presented during the place-preference test (below), it was important to determine its effectiveness after this phase of the experiment, because a negative finding (i.e., no effect of NBstm on place-preference) might be attributable to a loss of its potency rather than to its lack of motivational effect. As noted in Methods, the subjects also received 200 randomly-presented CS tones and NBstm following place-preference testing. Examples of the EEG changes elicited by NBstm alone (“All Bands” and Alpha and Gamma activity) are presented in Figure 3A and group data are presented in Figure 3B. The same pattern of EEG activation was found as that observed during tone-NBstm pairing, i.e, increased gamma and decreased alpha activity with other frequency bands exhibiting lesser changes (compare with Figures 2A,B). To determine the relative effectiveness of NBstm during pairing and after place-preference testing, we compared their respective “EEG Activation Indices” (Methods). Figure 3C presents the mean EAI values. Note their similarity, differing only in the earlier increase elicited by tone during pairing trials. The peak values for both the training period and the assessment after the place-preference test are similar. A statistical comparison of their EAIs revealed no significant differences between the ability of NBstm to activate the auditory cortex during training and after the place-preference test (t(2) = 2.92, p > 0.15, two-tailed). Therefore, the effectiveness of NBstm was not diminished after tone-NBstm pairing.
Figure 3.
Effectiveness of NBstm and tone alone on the auditory cortex after completion of the place-preference test, and comparison with EEG activation during tone-NBstm pairing. Subjects received 200 presentations each of tone and NBstm randomly. (A) Example of EEG activation by NBstm (200 ms, 100 Hz, 60 µA bipolar stimulation) observed for “All Bands”, “Alpha” and “Gamma” bands, as described in Figure 2. As for EEG activation during tone-NBstm pairing, there is a shift from higher voltage, slower waves to lower voltage, faster waves (“All Bands”) with pronounced decrease in alpha activity and increase in gamma activity. Thick narrow bar indicates 200 ms period of NBstm. (B) Group mean spectral changes in EEG induced by NBstm alone. NB stimulation-induced spectral changes in the EEG relative to pre-stimulation period (first 2 s out of 4 s immediately preceding NBstm, computed as in Figure 2. (C) Comparison of EEG activation during training and after place-preference test. The graph shows the mean “EEG Activation Index” (EAI) for tone + NBstim during pairing, NBstm alone and also tone alone after place-preference test. Note that there was no significant difference between the peak magnitudes of the EAI values (p > 0.15 for designated data points), indicating that NBstm potency had not diminished after induction of specific memory during training. During tone-NBstm pairing, both stimuli contribute to the magnitude of EEG activation. However, the contributions of the tone preceding presentation of the NBstm can be observed in the first two data points following tone onset. This probably reflects conditioned EEG activation (McLin, Miasnikov and Weinberger, 2003). Tone alone after the place-preference test still elicits considerable EEG activation although obtained in the absence of pairing (i.e, potential extinction period). Its peak EAI is not significantly different from activation to tone during pairing with NBstm (p > 0.10), suggesting conditioned EEG activation is robust. (D) EEG spectral changes to tone alone after place-preference test. Although smaller in magnitude, note the similarity of the EEG effects of tone alone with the tone-NBstm pairing (Figure 2B) and NBstm alone (Figure 3B).
Tone also produced EEG activation. The rise-time of the EAI for tone-elicited activation after the place-preference test was faster than during training and their peak magnitudes were not significantly different (t(12) = 1.62, p > 0.10) (Figure 3C). This might be surprising because random tone and NBstm constitute a potential extinction session. Together, these findings suggest that conditioned tone activation was robust.
NB Induction of Specific Behavioral Memory
Conditioning produced CS-specific changes in respiration. Figure 4A shows an example of respiratory waveforms before and after conditioning at the CS frequency and at a lower and higher frequency. Following pairing, there was a strong response (interruption of respiration) at the CS frequency while there was little change at lower (2.75 kHz) and higher (15.00 kHz) frequencies.
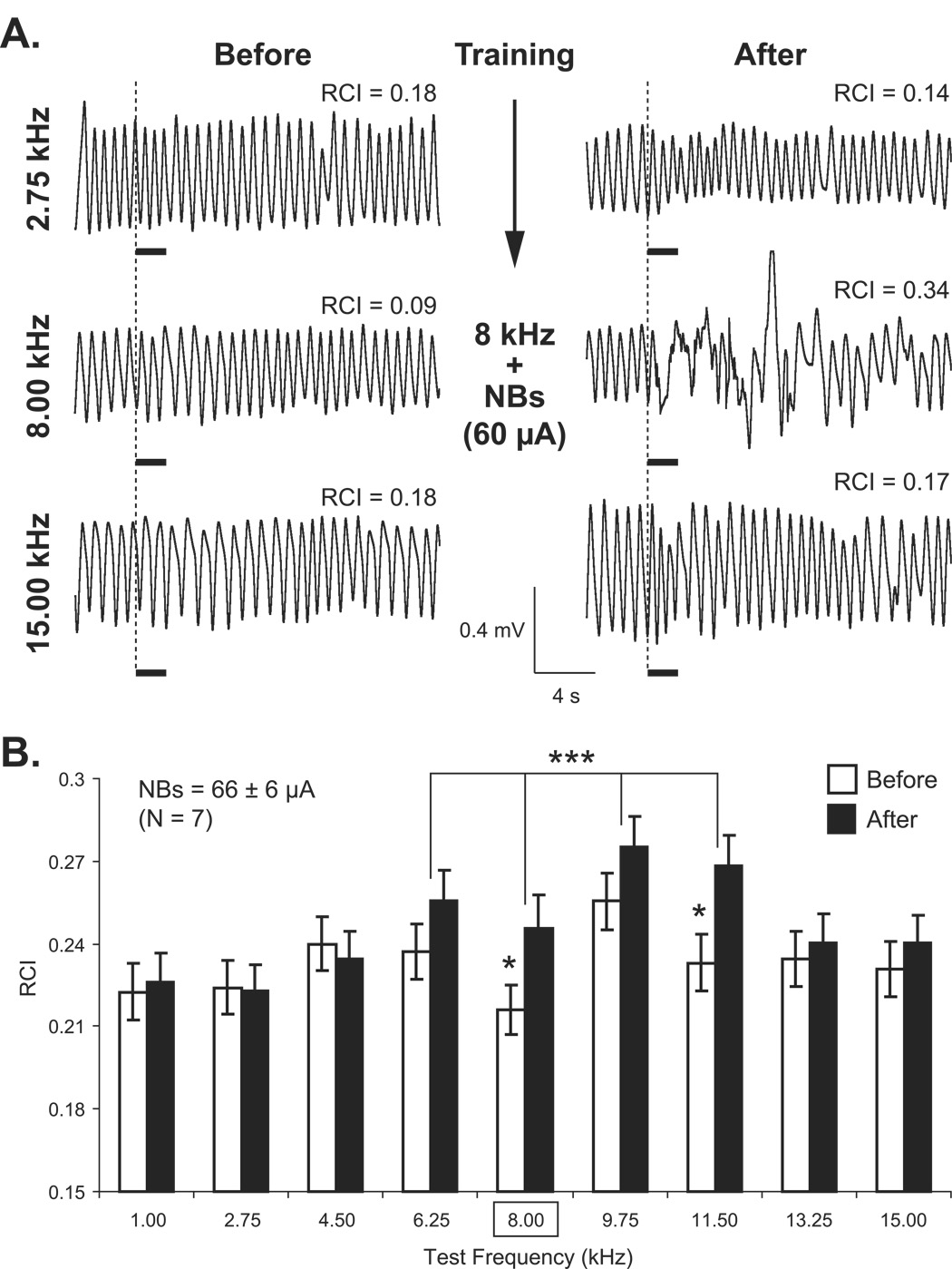
Figure 4.
Respiration frequency generalization gradients. (A) Sample respiratory waveforms obtained from an NB-Mem subject. Shown are responses to three frequencies, the CS (8.00 kHz) and a lower (2.75 kHz) and higher (15.00 kHz) frequencies on the pre-training day (Before) and 24 h post-training (After). RCI values are the quantified values for each record. Note the large disruption of respiration only at the CS frequency after training (RCI = 0.34). The thick horizontal bars indicate tone presentation. (B) CS-specific induced memory as indexed by differential responses to tone after pairing with NBstm. Group mean respiration responses (RCI) (mean ± SE) for all test tone frequencies (X-axis, square frame for 8 kHz denotes CS frequency during training) for the NB-Mem group. Post-training generalization gradients (“After”, black bars) exhibited CS-specific changes for the group (bracket combining responses at 6.25–11.50 kHz; p < 0.001). The individual frequencies, the CS (8.0 kHz, p < 0.05) and the nearby frequency of 11.5 kHz (p < 0.02) each elicited significantly larger responses following pairing. In the figure, statistically significant paired comparisons (post hoc Tukey’s tests) are indicated with asterisks: *p < 0.05 and ***p < 0.005.
Group data are presented in Figure 4B. Baseline behavioral responses to various frequencies (i.e., the magnitude of disruption of respiration) were not significantly different from each other (F(8, 1391) = 1.37, p > 0.05); the smallest response was at 8.0 kHz, the tone later selected to be the CS. Pairing this frequency with NBstm produced a significant change in Post-training responses (F(8, 1391) = 2.85, p < 0.004). Post-hoc Tukey’s tests revealed the presence of significant increases in response at the CS frequency of 8.00 kHz compared to baseline (t(300) = 1.99, p < 0.05, 2-tailed) and 11.50 kHz (t(313) = 2.36, p < 0.02, 2-tailed). Response to the frequency band closest to one octave that includes the CS frequency (6.25–11.50, 0.88 octave) was also significantly increased relative to baseline (Figure 4B bracket: t(1236) = 3.42, p < 0.001). Thus, we conclude that pairing a tone with NBstm induced specific associative memory.
Place-Preference Test
Figure 5 illustrates the arena apparatus and summarizes the place-preference findings. The initial question was whether the arena was biased in some way. An ANOVA for the combined NB-Mem and Cont groups revealed no difference either in the time spent in different quadrants (F(3, 52) = 0.44, p > 0.05) or the number of visits to various quadrants (F(3, 52) = 0.185, p > 0.05) (Figure 5B). Given an unbiased arena, we next asked whether the groups behaved differently during baseline. There were no significant differences between the NB-Mem and the Cont groups for the total time spent within all quadrants (557 ± 53 s and 588 ± 28 s, respectively; t(12) = 1.35, p > 0.05) or the number of quadrants entered (56.0 ± 12.8 and 50.6 ± 12.1, respectively; t(12) = 0.82, p > 0.05).
Figure 5.
Place Preference data obtained from animals in NB-Mem and Cont groups. (A) Schematic representation of the arena (Methods). (B) Relative baseline time spent and number of visits for each quadrant (percent of time spent and number of visits for each quadrant). There was no quadrant bias (see Results). (C) The quadrants were ranked based both on the relative time each particular subject spent in them and the relative number of visits during the baseline (Day 1) expressed as percent of total time and visits to all quadrants. The ranking were the same for time spent and number of visits. Preferences were idiosyncratic to each rat, but the NB-Mem and Cont groups did not differ in the relative amount of time spent in or number of visits to the most to least attended quadrant (see Results). The quadrant where an animal spent the second least relative (in percent) time was selected (arrow) for confinement + NBstm (NB-Mem group) or only confinement (Cont group). (D) Group behavior for the confinement quadrant. Shown are the percent differences in time spent and number of visits between the groups (Cont minus NB-Mem) before (Day 1) and following (Day 3) treatment. The denominators for each measure were the total time spent in and the total number of visits to all four quadrants (same as shown on Y-axes in Fig. 5B and C). See also Table 1. Analysis of treatment effects was accomplished by 2 × 2 repeated measures ANOVA on absolute values (see text). There were no significant effects.
The next question was how to determine the quadrant for confinement (and NBstm in the NB-Mem group) during Day 2. An analysis of time spent and number of visits to each quadrant showed that rats had individual preferences. We therefore ranked the quadrant selection of each subject and found that the time spent in any quadrant was closely related to the number of visits to that quadrant, regardless of the degree of preference (Figure 5C). There was no significant difference between groups either in the percent of time that they allocated to the respective rank-ordered quadrants (i.e., most favored, second most-favored, second least-favored, least favored) (F(1, 48) = 0.26, p > 0.05) or the percentage of visits to the most-favored to least-favored quadrants (F(1, 48) = 1.93, p > 0.05). We selected the second least preferred quadrant for confinement on Day 2. An analysis of baseline behavior for this quadrant revealed no difference between groups, either for time spent (NB-Mem = 101 ± 36 s; Cont = 95 ± 32 s; t(12) = 0.35, p > 0.05) or number of entries (NB-Mem = 12.3 ± 5.3; Cont = 11.9 ± 3.5; t(12) = 1.77, p > 0.05). Thus, the arena quadrants were not differentially biased, and while animals had idiosyncratic preferences, the groups exhibited neither differences in relative allocation of time from most to least-favored nor in their behavior with respect to the second-least favored quadrant in which they were to be confined on Day 2.
The behavior on Day 3 was used to seek differential place-preference between the groups, i.e., to determine whether NBstm in the NB-Mem group was either rewarding or aversive, based on time spent and entries into the NBstm quadrant, compared to the Cont group. The effects of confinement for the NB-Mem and Cont groups are summarized in Table 1, which provides both absolute and percent values for both time spent and number of visits. To evaluate the results of the place-preference test, we performed a 2 × 2 ANOVA (doubly multivariate General Linear Model Repeated Measures design, SPSS v.15) on the absolute values, where the dependent variables represented measurements of more than one variable for the different levels of the within-subjects factors (i.e. “Time spent in selected quadrant” and “Number of visits to selected quadrants” as measured “Before” and “After” confinement with or without NBstm). The analyses showed that the “Group” factor (NBstm vs. Cont) was not significant (F(2,11 = 1.093, p = 0.369, the “Time” factor (Day 1 vs. Day 3) was not significant (F(2,11) = 2.852, p = 0.101), and the interaction (“Time-Group”) was not significant (F(2,11) = 1.702, p = 0.227).
Table 1.
Time spent in and the number of visits to the confinement quadrant with and without NB stimulation.
| Groups | Time Spent in Quadrant | Visits to Quadrants | ||
|---|---|---|---|---|
| Before (Day 1) | After (Day 3) | Before (Day 1) | After (Day 3) | |
| NBstm | 101 ± 36 s 18.7 ± 7.0 % |
108 ± 46 s 19.6 ± 8.8 % |
12.3 ± 5.3 # 21.2 ± 6.1 % |
11.9 ± 4.3 # 22.8 ± 4.1 % |
| Control | 95 ± 32 s 16.1 ± 5.6 % |
161 ± 125 s 27.6 ± 21.2 % |
11.9 ± 3.5 # 23.7 ± 5.0 % |
8.9 ± 3.9 # 25.4 ± 6.6 % |
Values (mean ± s.d.) are presented both in absolute units (seconds (s) for time spent and number (#) for visits) and relative units (percent (%) of time spent and of number of visits. All percent values were calculated relative to the total amount of time spent in the four quadrants of the arena and the total number of entries into the four quadrants, respectively; they were calculated for each subject and then combined to obtain group data. Percent values complement data in Figure 5D which illustrates percent differences between groups in time spent and visits, in concordance with Figures 5B and 5C that summarize relative behavior in all four maze quadrants. The effect of treatment (confinement with or without NB stimulation) for time spent and number of visits was determined by 2 × 2 repeated measures ANOVA on absolute values. There was no significant treatment effect (see text).
DISCUSSION
Validity of the Findings
The findings may be summarized as follows. First, pairing a tone with stimulation of the NB induces specific associative behavioral memory. Second, the same NBstm that induces memory within animals does not bias these subjects either for or against a place in an arena where they received such stimulation after training. One interpretation of these findings is that NBstm that is sufficient to induce specific memory is motivationally neutral, i.e., neither rewarding nor punishing. This interpretation supports the view that within the neural mechanics of natural memory formation, the NB is “downstream” of motivational systems. In this model (Weinberger, Ashe, Metherate, McKenna, Diamond, & Bakin, 1990; Weinberger & Bakin, 1998), motivational systems would activate the nucleus basalis to release acetylcholine in the cortex and elsewhere, which in turn would promote the storage of experiences that are currently represented as a distributed network throughout the brain.
The NB is not part of any known motivational system (Pennartz, 1995). Nevertheless, the present interpretation of motivational neutrality rests on the validity of the current findings. The first issue is whether or not the specific changes in behavior consequent to tone–NB pairing are truly associative, as a non-associative control group was not included. We did not include such a control group because prior studies using paired NBstm have been shown to produce associative memory. That is, non-associative control groups failed to develop specific behavioral memory, whether assessed by changes in respiration or heart rate, or whether employing 2,000 trials over days or 200 trials in a single day (McLin et al., 2002a; Miasnikov et al., 2006; Weinberger et al., 2006). In fact, subjects receiving random tone and NBstm develop decreased responses to the CS frequency (Weinberger et al., 2006), which is characteristic of habituation (Condon & Weinberger, 1991; Thompson & Spencer, 1966). In summary, as the use of NBstm is known to require pairing to induce associative specific memory, and as the present findings show NB induction of specific memory, it is reasonable to conclude that memory induced in this study is associative.
A second issue concerns the Cont group. Perhaps they might have been a better control had they received tone and NBstm randomly, before being tested for place-preference. However, we specifically chose not to do so because animals given unpaired stimulation are likely to learn that the tone is a signal that NBstm is not immediately forthcoming (Rescorla, 1967). Indeed, even for random tone and NBstm, animals have the opportunity to learn that presentation of the tone is a poor predictor of NBstm. Such “negative” learning is illustrated by decreased responses found in such groups, as noted above. Thus, while “negative” learning may be of interest, it would interfere with the interpretation of present findings. For example, had an unpaired group later spent more time in the NBstm quadrant than the NB-Mem group, then the latter group would have spent less time in that place and, by comparison, could be thought to have exhibited avoidance of their stimulation quadrant, indicating that NBstm was aversive. Thus, it was important to use a control group that itself had no opportunity to learn about NBstm. Rather, the Cont group served as a control for handling, surgery, the presence of a head pedestal and any restrictions of movement due to the connection to an overhead cable. Without these controls, the arena behavior of the NB-Mem group might have been attributable to one or more of these factors.
A third issue concerns the sensitivity of the place-preference test. Pilot data indicated that the arena was not biased and the lack of pre-confinement differences between the NB-Mem and Cont groups indicated that they were properly matched. Although place-preference tests have been successfully used widely (Bardo et al., 1995), the current study found no preference for the place of NBstm. This is a negative finding and, of course, it is impossible to “prove” a negative. Therefore, a caveat is that NBstm that is sufficient to induce memory appears to be neither rewarding nor punishing within the domain of the current assessment test. It is, of course, possible that some other behavioral test might reveal a positive or negative valence.
Finally, the lack of effect might reflect “saturation” of NBstm. That is, its use during tone-NBstm pairing might have rendered it ineffectual during the subsequent place-preference stimulation period. According to this account, although the NB was stimulated during the place-preference test, it could not manifest the motivational properties that it actually had possessed during previous tone-NBstm pairing. However, the physiological effectiveness of NBstm was not diminished after the place-preference test as the same NBstm produced the same amount of EEG activation (Figure 3C). Therefore, the failure to obtain evidence of motivational significance actually indicates that the NBstm which induced behavioral memory is motivationally neutral.
Relation to Previous Findings
Although the induction of associative behavioral memory by properly-timed stimulation of the NB apparently has not yet been studied in other laboratories, the present failure to find a direct positive or negative motivational role for the NB is consistent with the results of several other lines of inquiry. For example, the NB exhibits tuning to pure tone frequencies, indicating that it minimally receives non-motivational signals (Chernyshev & Weinberger, 1998). Additionally, studies of learning and memory have reported that the NB rapidly develops associatively-induced discharges to auditory stimuli that serve as conditioned stimuli in fear conditioning, showing that it exhibits plasticity to stimuli that signal primary reinforcers (Maho, Hars, Edeline, & Hennevin, 1995). Also, direct tests of primary reinforcers fail to reveal responses in the NB. For example, neither positive (juice) nor negative (salt water) reinforcers elicit any responses within the NB of monkeys (Wilson & Rolls, 1990). Furthermore, while the NB does respond to visual or acoustic stimuli that signal reinforcers, it does not respond to the reinforcers themselves (Wilson & Ma, 2004). Overall, the findings support a signal function, rather than a motivational function, for the nucleus basalis.
Future Directions
Although not a focus of this study, the EEG activation index of tone-alone (at the end of the experiment) was as large, and it rise-time was faster, than those of tone-elicited activation during tone-NBstm pairing (Figure 3C). As tone-alone data were obtained during random presentation of NBstim, this constituted a potential extinction session, which would be expected to diminish tone conditioned activation. Thus, these incidental observations suggest that conditioned EEG activation induced by NBstm is very robust. Future studies should better characterize such conditioned effects, including the dynamics of their development during pairing, their relationship to the specificity of behavioral memory and their strength relative to EEG conditioning with natural unconditioned stimuli.
Properly-timed activation of the nucleus basalis appears to be sufficient to promote the storage of memory. The current findings are agnostic with respect to the roles of other neuromodulators or brain systems in the storage of memory. We assume that most of memory storage is distributed and that various regions and systems of the brain have particular, but still largely unidentified, functions. For example, they could be substrates for the various components of an experience, may it be the sensory, emotional or reflect other aspects of memory.
It would now be appropriate to investigate the involvement of other key neuromodulatory regions in the “direct” induction of behavioral memory. Thus, pairing a tone with stimulation of the locus coeruleus or the raphe would shed light on the extent to which norepinephrine and serotonin, respectively, can support the induction of specific associative behavioral memory, and whether such support includes control of the degree of encoded detail (Weinberger et al., 2006). Such experiments should greatly expand the understanding of how these major neuromodulators are involved in learning and memory.
On the other hand, stimulation of the VTA to release dopamine would be expected to induce memory because it taps into a motivational system (Schultz, 2001). Indeed, tone paired with VTA stimulation does induce specific plasticity in the auditory cortex (Bao, Chan, & Merzenich, 2001). There are no reports concerning whether tone paired with VTA stimulation can induce specific associative auditory memory, but this would also be expected if such stimulation is simply another way to pair a tone with a motivationally significant reinforcer. Thus, the fact of the induction of specific cortical plasticity, or perhaps more interestingly, the induction of specific memory, cannot itself reveal whether the role of such direct intervention into the brain is motivational support for mnemonic processes, or is itself likely to be downstream of reward/punishment systems and probably more directly linked to memory formation.
This brain stimulation approach provides an avenue of inquiry complementary to pharmacological studies while providing for mimicry of the natural action of each neuromodulatory system, by engagement of their critical cell bodies and release of their transmitters at normally-distributed synaptic targets (Rasmusson et al., 1994). Such studies will also need to be supplemented by pharmacological interventions to determine the extent to which memory induction by electrical stimulation requires the engagement of particular receptors or receptor sub-types in various regions. With regard to the current findings, it remains to be determined if the induction of specific associative behavioral memory by stimulation of the nucleus basalis depends upon cholinergic mechanisms. This is an essential step because activation of the NB, with presumptive release of ACh in the cerebral cortex and other regions, might induce memory through non-cholinergic mechanisms yet to be discovered.
ACKNOWLEDGEMENTS
This research was funded by a research grant from the National Institute of Deafness and Other Communication Disorders, DC-02938. We wish to thank Jacquie Weinberger, Julia Martinson and Gabriel K. Hui for their assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: This PDF receipt will only be used as the basis for generating PubMed Central (PMC) documents. PMC documents will be made available for review after conversion (approx. 2–3 weeks time). Any corrections that need to be made will be done at that time. No materials will be released to PMC without the approval of an author. Only the PMC documents will appear on PubMed Central -- this PDF Receipt will not appear on PubMed Central.
REFERENCES
- Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(20):11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412(6842):79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Rowlett JK, Harris MJ. Conditioned place preference using opiate and stimulant drugs: A meta-analysis. Neuroscience and Biobehavioral Reviews. 1995;19(1):39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Bigl V, Woolf NJ, Butcher LL. Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: A combined fluorescent tracer and acetylcholinesterase analysis. Brain Research Bulletin. 1982;8(6):727–749. doi: 10.1016/0361-9230(82)90101-0. [DOI] [PubMed] [Google Scholar]
- Bjordahl TS, Dimyan MA, Weinberger NM. Induction of long-term receptive field plasticity in the auditory cortex of the waking guinea pig by stimulation of the nucleus basalis. Behavioral Neuroscience. 1998;112(3):467–479. doi: 10.1037//0735-7044.112.3.467. [DOI] [PubMed] [Google Scholar]
- Casamenti F, Deffenu G, Abbamondi AL, Pepeu G. Changes in cortical acetylcholine output induced by modulation of the nucleus basalis. Brain Research Bulletin. 1986;16(5):689–695. doi: 10.1016/0361-9230(86)90140-1. [DOI] [PubMed] [Google Scholar]
- Celesia GG, Jasper HH. Acetylcholine released from cerebral cortex in relation to state of activation. Neurology. 1966;16(11):1053–1063. doi: 10.1212/wnl.16.11.1053. [DOI] [PubMed] [Google Scholar]
- Chernyshev BV, Weinberger NM. Acoustic frequency tuning of neurons in the basal forebrain of the waking guinea pig. Brain Research. 1998;793(1–2):79–94. doi: 10.1016/s0006-8993(98)00163-2. [DOI] [PubMed] [Google Scholar]
- Condon CD, Weinberger NM. Habituation produces frequency-specific plasticity of receptive fields in the auditory cortex. Behavioral Neuroscience. 1991;105(3):416–430. doi: 10.1037//0735-7044.105.3.416. [DOI] [PubMed] [Google Scholar]
- Detari L, Juhasz G, Kukorelli T. Effect of stimulation of vagal and radial nerves on neuronal activity in the basal forebrain area of anaesthetized cats. Acta Physiologica Hungarica. 1983;61(3):147–154. [PubMed] [Google Scholar]
- Detari L, Juhasz G, Kukorelli T. Firing properties of cat basal forebrain neurones during sleep–wakefulness cycle. Electroencephalography and Clinical Neurophysiology. 1984;58(4):362–368. doi: 10.1016/0013-4694(84)90062-2. [DOI] [PubMed] [Google Scholar]
- Detari L, Juhasz G, Kukorelli T. Neuronal firing in the pallidal region: Firing patterns during sleep–wakefulness cycle in cats. Electroencephalography and Clinical Neurophysiology. 1987;67(2):159–166. doi: 10.1016/0013-4694(87)90039-3. [DOI] [PubMed] [Google Scholar]
- Deutsch JA. The cholinergic synapse and the site of memory. Science. 1971;174(11):788–794. doi: 10.1126/science.174.4011.788. [DOI] [PubMed] [Google Scholar]
- Dimyan MA, Weinberger NM. Basal forebrain stimulation induces discriminative receptive field plasticity in the auditory cortex. Behavioral Neuroscience. 1999;113(4):691–702. doi: 10.1037//0735-7044.113.4.691. [DOI] [PubMed] [Google Scholar]
- Edeline J-M, Hars B, Maho C, Hennevin E. Transient and prolonged facilitation of tone-evoked responses induced by basal forebrain stimulations in the rat auditory cortex. Experimental Brain Research. 1994;97(3):373–386. doi: 10.1007/BF00241531. [DOI] [PubMed] [Google Scholar]
- Edeline J-M, Maho C, Hars B, Hennevin E. Non-awaking basal forebrain stimulation enhances auditory cortex responsiveness during slow-wave sleep. Brain Research. 1994;636(2):333–337. doi: 10.1016/0006-8993(94)91033-2. [DOI] [PubMed] [Google Scholar]
- Fisher A, Brandeis R, Chapman S, Pittel Z, Michaelson DM. M1 muscarinic agonist treatment reverses cognitive and cholinergic impairments of apolipoprotein E-deficient mice. Journal of Neurochemistry. 1998;70(5):1991–1997. doi: 10.1046/j.1471-4159.1998.70051991.x. [DOI] [PubMed] [Google Scholar]
- Flood JF, Landry DW, Jarvik ME. Cholinergic receptor interactions and their effects on long-term memory processing. Brain Research. 1981;215(1–2):177–185. doi: 10.1016/0006-8993(81)90500-x. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Rakovska A, Benton RS, Pazzagli M, Bianchi L, Pepeu G. Effects of novelty and habituation on acetylcholine, GABA, and glutamate release from the frontal cortex and hippocampus of freely moving rats. Neuroscience. 2001;106(1):43–53. doi: 10.1016/s0306-4522(01)00266-4. [DOI] [PubMed] [Google Scholar]
- Hasenohrl RU, Oitzl MS, Huston JP. Conditioned place preference in the corral: A procedure for measuring reinforcing properties of drugs. Journal of Neuroscience Methods. 1989;30(2):141–146. doi: 10.1016/0165-0270(89)90060-5. [DOI] [PubMed] [Google Scholar]
- Introini-Collison IB, McGaugh JL. Modulation of memory by post-training epinephrine: Involvement of cholinergic mechanisms. Psychopharmacology. 1988;94(3):379–385. doi: 10.1007/BF00174693. [DOI] [PubMed] [Google Scholar]
- Jasper HH, Tessier J. Acetylcholine liberation from cerebral cortex during paradoxical (REM) sleep. Science. 1971;172(983):601–602. doi: 10.1126/science.172.3983.601. [DOI] [PubMed] [Google Scholar]
- Jimenez-Capdeville ME, Dykes RW, Myasnikov AA. Differential control of cortical activity by the basal forebrain in rats: A role for both cholinergic and inhibitory influences. Journal of Comparative Neurology. 1997;381(1):53–67. [PubMed] [Google Scholar]
- Johnston MV, McKinney M, Coyle JT. Evidence for a cholinergic projection to neocortex from neurons in basal forebrain. Proceedings of the National Academy of Sciences of the United States of America. 1979;76(10):5392–5396. doi: 10.1073/pnas.76.10.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhasz G, Detari L, Kukorelli T. Effects of hypnogenic vagal stimulation on thalamic neuronal activity in cats. Brain Research Bulletin. 1985;15(5):437–441. doi: 10.1016/0361-9230(85)90032-2. [DOI] [PubMed] [Google Scholar]
- Kametani H, Kawamura H. Alterations in acetylcholine release in the rat hippocampus during sleep–wakefulness detected by intracerebral dialysis. Life Sciences. 1990;47(5):421–426. doi: 10.1016/0024-3205(90)90300-g. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998a;279(5357):1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Plasticity of temporal information processing in the primary auditory cortex. Nature Neuroscience. 1998b;1(8):727–731. doi: 10.1038/3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Pandya PK, Engineer ND, Moucha R. Cortical network reorganization guided by sensory input features. Biological Cybernetics. 2002;87(5–6):333–343. doi: 10.1007/s00422-002-0352-z. [DOI] [PubMed] [Google Scholar]
- Kukorelli T, Feuer L, Juhasz G, Detari L. Effect of glutaurine on sleep–wakefulness cycle and aggressive behaviour in the cat. Acta Physiologica Hungarica. 1986;67(1):31–35. [PubMed] [Google Scholar]
- Luiten PG, Gaykema RP, Traber J, Spencer DG., Jr Cortical projection patterns of magnocellular basal nucleus subdivisions as revealed by anterogradely transported Phaseolus vulgaris leucoagglutinin. Brain Research. 1987;413(2):229–250. doi: 10.1016/0006-8993(87)91014-6. [DOI] [PubMed] [Google Scholar]
- Ma X, Suga N. Augmentation of plasticity of the central auditory system by the basal forebrain and/or somatosensory cortex. Journal of Neurophysiology. 2003;89(1):90–103. doi: 10.1152/jn.00968.2001. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. The psychology of animal learning. New York: Academic Press; 1974. [Google Scholar]
- Maho C, Hars B, Edeline J-M, Hennevin E. Conditioned changes in the basal forebrain: Relations with learning-induced cortical plasticity. Psychobiology. 1995;23(1):10–25. [Google Scholar]
- Marrosu F, Portas C, Mascia MS, Casu MA, Fa M, Giagheddu M, Imperato A, Gessa GL. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep–wake cycle in freely moving cats. Brain Research. 1995;671(2):329–332. doi: 10.1016/0006-8993(94)01399-3. [DOI] [PubMed] [Google Scholar]
- McLin DE, 3rd, Miasnikov AA, Weinberger NM. Induction of behavioral associative memory by stimulation of the nucleus basalis. Proceedings of the National Academy of Sciences of the United States of America. 2002a;99(6):4002–4007. doi: 10.1073/pnas.062057099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLin DE, 3rd, Miasnikov AA, Weinberger NM. The effects of electrical stimulation of the nucleus basalis on the electroencephalogram, heart rate, and respiration. Behavioral Neuroscience. 2002b;116(5):795–806. [PubMed] [Google Scholar]
- McLin DE, Miasnikov AA, Weinberger NM. CS-specific gamma, theta, and alpha EEG activity detected in stimulus generalization following induction of behavioral memory by stimulation of the nucleus basalis. Neurobiology of Learning and Memory. 2003;79(2):152–176. doi: 10.1016/s1074-7427(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: An overview based on an alternative nomenclature (Ch1–Ch6) Neuroscience. 1983;10(4):1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Nucleus basalis stimulation elicits neocortical activation and facilitates thalamocortical synaptic transmission: Intracellular and extracellular recordings in rat auditory cortex. Abs. No. 411.8. Society for Neuroscience Abstracts. 1992;18(2):975. [Google Scholar]
- Metherate R, Ashe JH. Nucleus basalis stimulation facilitates thalamocortical synaptic transmission in the rat auditory cortex. Synapse. 1993;14(2):132–143. doi: 10.1002/syn.890140206. [DOI] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Weinberger NM. Rapid induction of specific associative behavioral memory by stimulation of the nucleus basalis in the rat. Neurobiology of Learning and Memory. 2006;86(1):47–65. doi: 10.1016/j.nlm.2005.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, McLin D, 3rd, Weinberger NM. Muscarinic dependence of nucleus basalis induced conditioned receptive field plasticity. NeuroReport. 2001;12(7):1537–1542. doi: 10.1097/00001756-200105250-00047. [DOI] [PubMed] [Google Scholar]
- Miasnikov AA, Weinberger NM. 2003 Abstract Viewer. Washington, DC: Society for Neuroscience; 2003. Rapid formation of behavioral memory to tone paired with nucleus basalis stimulation in rat. Prog. No. 621.13. [Google Scholar]
- Mostofsky DI, editor. Stimulus generalization. Palo Alto, CA: Stanford University Press; 1965. [Google Scholar]
- Panos JJ, Rademacher DJ, Renner SL, Steinpreis RE. The rewarding properties of NMDA and MK-801 (dizocilpine) as indexed by the conditioned place preference paradigm. Pharmacology, Biochemistry, and Behavior. 1999;64(3):591–595. doi: 10.1016/s0091-3057(99)00155-0. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. In: Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. Anrep GV, translator. New York: Dover Publications; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos GT, Watson C, editors. The rat brain in stereotaxic coordinates. 3rd ed. San Diego, CA: Academic Press; 1997. [Google Scholar]
- Pennartz CM. The ascending neuromodulatory systems in learning by reinforcement: Comparing computational conjectures with experimental findings. Brain Research Reviews. 1995;21(3):219–245. doi: 10.1016/0165-0173(95)00014-3. [DOI] [PubMed] [Google Scholar]
- Phillis JW, York DH. Pharmacological studies on a cholinergic inhibition in the cerebral cortex. Brain Research. 1968;10(3):297–306. doi: 10.1016/0006-8993(68)90201-1. [DOI] [PubMed] [Google Scholar]
- Power AE, Vazdarjanova A, McGaugh JL. Muscarinic cholinergic influences in memory consolidation. Neurobiology of Learning and Memory. 2003;80(3):178–193. doi: 10.1016/s1074-7427(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD, Clow K, Szerb JC. Frequency-dependent increase in cortical acetylcholine release evoked by stimulation of the nucleus basalis magnocellularis in the rat. Brain Research. 1992;594(1):150–154. doi: 10.1016/0006-8993(92)91041-c. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD, Clow K, Szerb JC. Modification of neocortical acetylcholine release and electroencephalogram desynchronization due to brainstem stimulation by drugs applied to the basal forebrain. Neuroscience. 1994;60(3):665–677. doi: 10.1016/0306-4522(94)90495-2. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD, Szerb IC, Jordan JL. Differential effects of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid and N-methyl-D-aspartate receptor antagonists applied to the basal forebrain on cortical acetylcholine release and electroencephalogram desynchronization. Neuroscience. 1996;72(2):419–427. doi: 10.1016/0306-4522(95)00523-4. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Pavlovian conditioning and its proper control procedures. Psychological Review. 1967;74(1):71–80. doi: 10.1037/h0024109. [DOI] [PubMed] [Google Scholar]
- Rudy JW. Scopolamine administered before and after training impairs both contextual and auditory-cue fear conditioning. Neurobiology of Learning and Memory. 1996;65(1):73–81. doi: 10.1006/nlme.1996.0008. [DOI] [PubMed] [Google Scholar]
- Russell RW, Escobar ML, Booth RA, Bermudez-Rattoni F. Accelerating behavioral recovery after cortical lesions. II. In vivo evidence for cholinergic involvement. Behavioral and Neural Biology. 1994;61(1):81–92. doi: 10.1016/s0163-1047(05)80047-0. [DOI] [PubMed] [Google Scholar]
- Rye DB, Wainer BH, Mesulam MM, Mufson EJ, Saper CB. Cortical projections arising from the basal forebrain: a study of cholinergic and noncholinergic components employing combined retrograde tracing and immunohistochemical localization of choline acetyltransferase. Neuroscience. 1984;13(3):627–643. doi: 10.1016/0306-4522(84)90083-6. [DOI] [PubMed] [Google Scholar]
- Sahraei H, Pirzadeh-Jahromi G, Noorbakhshnia M, Asgari A, Haeri-Rohani A, Khoshbaten A, Poorheidari GR, Sepehri H, Ghoshooni H, Zarrindast MR. Involvement of nucleus accumbens in L-arginine-induced conditioned place preference in rats. Behavioural Pharmacology. 2004;15(7):473–480. doi: 10.1097/00008877-200411000-00003. [DOI] [PubMed] [Google Scholar]
- Salinas JA, Introini-Collison IB, Dalmaz C, McGaugh JL. Posttraining intraamygdala infusions of oxotremorine and propranolol modulate storage of memory for reductions in reward magnitude. Neurobiology of Learning and Memory. 1997;68(1):51–59. doi: 10.1006/nlme.1997.3776. [DOI] [PubMed] [Google Scholar]
- Schultz W. Reward signaling by dopamine neurons. Neuroscientist. 2001;7(4):293–302. doi: 10.1177/107385840100700406. [DOI] [PubMed] [Google Scholar]
- Stratton L, Petrinovich L. Post-trial injections of an anti-cholinesterase drug and maze learning in two strains of rats. Psychopharmacoligia. 1963;5:47–54. doi: 10.1007/BF00405574. [DOI] [PubMed] [Google Scholar]
- Szerb J. The effect of tertiary and quaternary atropine on cortical acetylcholine output and on the electroencephalogram in cats. Canadian Journal of Physiology and Pharmacology. 1964;42:303–314. doi: 10.1139/y64-036. [DOI] [PubMed] [Google Scholar]
- Szymusiak R, McGinty D. Sleep-related neuronal discharge in the basal forebrain of cats. Brain Research. 1986;370(1):82–92. doi: 10.1016/0006-8993(86)91107-8. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Spencer WA. Habituation: A model phenomenon for the study of neuronal substrates of behavior. Psychological Review. 1966;73(1):16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Tremblay N, Warren RA, Dykes RW. Electrophysiological studies of acetylcholine and the role of the basal forebrain in the somatosensory cortex of the cat. II. Cortical neurons excited by somatic stimuli. Journal of Neurophysiology. 1990;64(4):1212–1222. doi: 10.1152/jn.1990.64.4.1212. [DOI] [PubMed] [Google Scholar]
- Weinberger NM, Ashe JH, Metherate R, McKenna TM, Diamond DM, Bakin J. Retuning auditory cortex by learning: A preliminary model of receptive field plasticity. Concepts in Neuroscience. 1990;1(1):91–132. [Google Scholar]
- Weinberger NM, Bakin JS. Learning-induced physiological memory in adult primary auditory cortex: Receptive fields plasticity, model, and mechanisms. Audiology and Neuro-otology. 1998;3(2–3):145–167. doi: 10.1159/000013787. [DOI] [PubMed] [Google Scholar]
- Weinberger NM, Miasnikov AA, Chen JC. The level of cholinergic nucleus basalis activation controls the specificity of auditory associative memory. Neurobiology of Learning and Memory. 2006;86(3):270–285. doi: 10.1016/j.nlm.2006.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson FA, Ma YY. Reinforcement-related neurons in the primate basal forebrain respond to the learned significance of task events rather than to the hedonic attributes of reward. Cognitive Brain Research. 2004;19(1):74–81. doi: 10.1016/j.cogbrainres.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Wilson FA, Rolls ET. Neuronal responses related to reinforcement in the primate basal forebrain. Brain Research. 1990;509(2):213–231. doi: 10.1016/0006-8993(90)90546-n. [DOI] [PubMed] [Google Scholar]