Abstract
The aversive aspects of nicotine withdrawal are powerful motivational forces contributing to the tobacco smoking habit. We evaluated measures of affective and somatic aspects of nicotine withdrawal in C57BL/6J and BALB/cByJ mice. Nicotine withdrawal was induced by termination of chronic nicotine delivery through osmotic minipumps or precipitated with the nicotinic acetylcholine receptor (nAChR) antagonists mecamylamine or dihydro-β-erythroidine (DHβE). A rate-independent discrete-trial intracranial self-stimulation threshold procedure was used to assess brain reward function. Anxiety-like behavior and sensorimotor gating were assessed in the light-dark box and prepulse inhibition (PPI) tests, respectively. Acoustic startle response and somatic signs of withdrawal were also evaluated. Spontaneous nicotine withdrawal after 14-day exposure to 10–40 mg/kg/day nicotine induced no alterations in anxiety-like behavior, startle reactivity, PPI, or somatic signs in either strain, and no changes in thresholds in C57BL/6J mice. Extended 28-day exposure to 40 mg/kg/day nicotine induced threshold elevations, increased somatic signs, and anxiety-like behavior 24 h post-nicotine in C57BL/6J mice; thresholds returned to baseline levels by day 4 in nicotine-exposed mice. Mecamylamine or DHβE administration induced threshold elevations in nicotine-exposed C57BL/6J mice compared with saline-exposed mice. In conclusion, administration of relatively high nicotine doses over prolonged periods of time induces both the affective and somatic aspects of spontaneous nicotine withdrawal in the mouse, while exposure to nicotine for shorter periods of time is sufficient for nAChR antagonist-precipitated nicotine withdrawal. The current study is one of the first to demonstrate reward deficits associated with both spontaneous and nAChR antagonist-precipitated nicotine withdrawal in C57BL/6J mice.
Keywords: nicotine, withdrawal, brain stimulation reward, anxiety, light-dark box, startle, prepulse inhibition, somatic signs, mice, strains
Introduction
Tobacco smoking, which is attributed partially to the addictive properties of nicotine (Crooks and Dwoskin, 1997; Stolerman and Jarvis, 1995), is predicted to become the world’s largest health problem by the year 2020 (Murray and Lopez, 1997). The affective/emotional, as well as somatic, aspects of the nicotine withdrawal syndrome after smoking cessation contribute to the maintenance of the tobacco smoking habit (Hughes et al., 1994; Hughes et al., 1991b; Shiffman and Jarvik, 1976). Unfortunately, only 20–30% of smokers remain abstinent after 1 year (Alterman et al., 2001; Haas et al., 2004; Hughes et al., 1991a; Hunt and Bespalec, 1974). Animal models are important tools for promoting our understanding of the neurobiology of nicotine dependence as a first step toward identifying novel therapeutic targets for smoking cessation with better efficacy than current treatments. Mouse models of nicotine withdrawal are particularly useful for studying the genetic bases of nicotine dependence because of the opportunities that mice offer for genetic engineering approaches. However, nicotine withdrawal, and particularly the affective aspects of nicotine withdrawal, has not been extensively characterized in the mouse.
One of the affective symptoms of nicotine withdrawal in humans is anhedonia, defined as the inability to experience pleasure in rewarding stimuli (American Psychiatric Association, 2000; Hughes, 2007). In animals, the intracranial self-stimulation (ICSS) procedure provides a valid, reliable and quantitative measure of reward (i.e., brain reward thresholds) (Markou and Koob, 1993). Nicotine administration lowers brain reward thresholds, indicative of the reward-enhancing effects of nicotine (Bauco and Wise, 1994; Bespalov et al., 1999; Bozarth et al., 1998; Epping-Jordan et al., 1998; Harrison et al., 2001, 2002; Huston-Lyons et al., 1993; Ivanova and Greenshaw, 1997; Kenny and Markou, 2006; Semenova and Markou, 2003b; Skjei and Markou, 2003; Wise et al., 1998). By contrast, elevations in ICSS thresholds seen during nicotine withdrawal are interpreted as a decrease in the reward value of the stimulation and are an operational measure of the anhedonia characterizing nicotine withdrawal (Epping-Jordan et al., 1998; Hughes, 2007). Although the ICSS procedure has been established in mice (Cazala, 1980; Cazala and Guenet, 1980; Gill et al., 2004), the effects of chronic nicotine administration and nicotine withdrawal on brain reward function have not been reported in this species.
Another affective aspect of the nicotine withdrawal syndrome in humans is increased anxiety (American Psychiatric Association, 2000; Hughes, 2007). In mice, the light-dark box test is commonly used to assess anxiety-like behavior. This test is based on an approach-avoidance conflict situation involving the mouse’s conflicting motivations to explore a new environment and avoid intensely bright spaces (Crawley and Goodwin, 1980). In BKW mice, increased anxiety-like behavior in the light-dark box was seen during spontaneous nicotine withdrawal after 14 days of nicotine injections (twice daily, 0.1 mg/kg intraperitoneally, base; Costall et al. 1989). Another study reported increased anxiety-like behavior measured in the elevated plus maze 24 h after termination of administration of 24 and 48 mg/kg/day nicotine base for 14 days in C57BL/6, but not 129/SvEv, mice (Damaj et al. 2003). Finally, C57BL/6J mice showed a small increase in anxiety-like behavior in the light-dark box after termination of administration of 48 mg/kg/day nicotine base for 14 days (Jonkman et al., 2005).
When human subjects are in an anxious state, their acoustic startle response, reflecting reactivity to environmental stimuli, is increased (Bast and Feldon, 2003; Grillon, 2002). Therefore, increased startle reactivity may be considered an indirect measure of anxiety-like behavior associated with nicotine withdrawal. Humans undergoing nicotine withdrawal showed no changes in the acoustic startle response (Kumari and Gray, 1999; Mueller et al., 1998; Postma et al., 2001). Rodent startle studies indicated conflicting results in rats (Acri et al., 1991; Faraday et al., 1999; Faraday et al., 1998; Helton et al., 1993). Further, no effects in startle were seen after either acute nicotine administration in C57BL/6 mice (Gould et al., 2005) or during spontaneous nicotine withdrawal in C57BL/6J and DBA/2J mice (Jonkman et al., 2005; Semenova et al., 2003a). Thus, whether nicotine withdrawal is associated with increased startle reactivity in mice remains unclear.
Similarly, conflicting data exist about changes in prepulse inhibition (PPI) of the startle response during nicotine withdrawal. Prepulse inhibition of the acoustic startle response is a measure of sensorimotor gating and may reflect a pre-attentional process. Abnormalities in such a pre-attentional process may contribute to cognitive deficits seen in humans (Hatsukami et al., 1989; Jacobsen et al., 2005; Pomerleau, 1997) and mice (Davis and Gould, 2006; Davis et al., 2005) undergoing nicotine withdrawal. In humans, PPI is decreased during nicotine withdrawal (Kumari and Gray, 1999; Postma et al., 2001). In DBA/2J mice, only withdrawal from low doses of nicotine decreased PPI (Semenova et al., 2003a). This finding was not replicated in either DBA/2J or C57BL/6J mice in a later study (Jonkman et al., 2005) using higher nicotine doses than those in the original study (Semenova et al., 2003a).
Finally, in humans, the nicotine withdrawal syndrome is also characterized by ‘physical’ or somatic signs that include bradycardia, insomnia, gastrointestinal discomfort, and increased appetite (Hughes, 2007; Hughes et al., 1991b). In rats, nicotine withdrawal is associated with somatic signs, such as abdominal constrictions, facial fasciculations, increased eye blinks, and ptosis (Epping-Jordan et al., 1998; Harrison et al., 2002; Hildebrand et al., 1997; Malin et al., 1992; Semenova and Markou, 2003b; Watkins et al., 2000). In general, somatic signs of nicotine withdrawal in the mouse resemble well-described signs in rats. However, discrepancies exist in the types of somatic signs seen in different mouse strains, suggesting that the expression of nicotine withdrawal may not be uniform between different strains and even within a single mouse strain across laboratories (Balerio et al., 2004; Damaj et al., 2003; Isola et al., 1999; Jackson et al., 2008; Salas et al., 2004, 2007; Semenova et al., 2003a).
The current study systematically explored different aspects of nicotine withdrawal in two inbred strains of mice: C57BL/6J and BALB/cByJ. These two mouse strains exhibit different emotional reactivity in the light-dark box and elevated plus maze tests, with BALB/cByJ exhibiting higher emotional reactivity than C57BL/6J mice (Crawley et al., 1997; Griebel et al., 2000). As such, these two mouse strains may differ in the expression of affective aspects of nicotine withdrawal. In the present study, nicotine withdrawal was induced by termination of chronic nicotine delivery through subcutaneous osmotic minipumps (i.e., spontaneous nicotine withdrawal) or administration of the nicotinic acetylcholine receptor (nAChR) antagonists, mecamylamine or dihydro-β-erythroidine (DHβE; precipitated nicotine withdrawal). These nAChR antagonists induced the affective and/or somatic aspects of nicotine withdrawal in rats (Epping-Jordan et al., 1998; Hildebrand et al., 1997; Malin et al., 1998; Markou and Paterson, 2001; Skjei and Markou, 2003; Watkins et al., 2000) and mice (Damaj et al., 2003; Jackson et al., 2008; Salas et al., 2007; Salas et al., 2004).
Materials and Methods
Subjects
Adult experimentally naive C57BL/6J and BALB/cByJ mice (Jackson Laboratories, Bar Harbor, ME, USA) 8–10 weeks old (Experiment 1) and 12–16 weeks old (Experiment 2 and 3) were housed in a humidity- and temperature-controlled animal facility on a 12 h:12 h (lights off at 7 am) reverse light-dark cycle with ad libitum access to food and water except during testing. Behavioral testing was conducted during the dark phase of the light-dark cycle (unless otherwise required by the experimental design). All experiments were in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and the National Research Council’s Guide for Care and Use of Laboratory Animals, and were approved by the University of California, San Diego, Institutional Animal Care and Use Committee.
Drugs
Nicotine tartrate (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in sterile 0.9% saline solution and infused through subcutaneous osmotic minipumps for 14 or 28 days (model 2002 and 2004, respectively, Alzet, Palo Alto, CA, USA). Dihydro-β-erythroidine (DHβE; Sigma-Aldrich, St. Louis, MO, USA) and mecamylamine hydrochloride (Sigma-Aldrich, St. Louis, MO, USA) were dissolved in saline and injected subcutaneously in volumes of 0.1 ml/10 g.
Minipump implantation and removal
Mice were anesthetized with an isoflurane/oxygen vapor mixture (1–3%), and osmotic minipumps were inserted subcutaneously using aseptic surgery techniques. Minipumps were placed parallel to the spine at shoulder level with the flow moderator directed away from the wound. The wound was closed with 7 mm stainless steel wound clips (Reflex, Cellpoint Scientific, Gaithersburg, MD, USA). On either day 15 (Experiment 1 and 2) or day 29 (Experiment 3), minipumps were surgically removed under isoflurane anesthesia using aseptic surgery techniques.
Light-dark box: Apparatus and procedure
The light-dark box consisted of a dark compartment (27 × 15 cm) and a light compartment (27 × 29 cm) that were connected through an opening (10 × 10 cm). Testing was performed in a dark experimental room. A 40-watt light bulb was positioned above the light compartment, such that the light intensity in the middle of the light compartment was approximately 500 lux. At the beginning of the experiment, the mouse was placed in the dark compartment with its head facing away from the opening. A video camera directed at the dark compartment recorded all mouse behavior in the light-dark box for 5 min, during which time the experimenter left the room. The total time spent in the white compartment, the latency to the first transition to the light compartment, and the number of transitions were scored during observation of the videotapes at a later time. The mouse was considered to have entered the white compartment when all four paws were inside the white compartment. Similarly, the mouse was considered to have entered the dark compartment when all four paws were inside the dark compartment. After testing of each mouse, feces were removed, and the surface was wiped with a wet paper towel. This test provides the following measures: time spent in white compartment, latency to the first transition, and number of transitions.
Acoustic startle and prepulse inhibition: Apparatus and procedure
One acoustic startle apparatus was used that consisted of a 5.1 cm (outside diameter) Plexiglas cylinder mounted on a Plexiglas platform and enclosed in a ventilated sound-attenuated cubicle equipped with high-frequency loudspeakers (SR-LAB, San Diego Instruments, San Diego, CA, USA). Movements within the cylinder were detected and transduced by a piezoelectric accelerometer attached to the platform, digitized, and stored by the operating computer. The startle box was placed on a vibration isolation platform (Model BM-8, Minus K Technology, Inglewood, CA, USA) to minimize environmental vibration.
After the mice were placed in the illuminated startle chambers, a 70 dB background noise was presented for a 5 min acclimation period and continued throughout the test session. During the test session, all trial types were presented several times in a pseudorandom order for a total of 60 trials: 12 pulse-alone trials, 12 no-stimulus trials, twelve 74 dB prepulse+pulse trials, twelve 78 dB prepulse+pulse trials, and twelve 82 dB prepulse+pulse trials. In addition to the 60 trials, there were six pulse-alone trials, which were not included in the calculation of PPI values and which were presented at the beginning of the session, and six more pulse alone trials at the end of each test session to assess startle habituation during the session. The time between trials averaged 15 s (ranging from 12 to 30 s), and the total duration of the test session was approximately 25 min. The pulse-alone trial consisted of a 40 ms 120 dB pulse of broadband noise. The prepulse+pulse trials consisted of a 20 ms noise prepulse, a 100 ms delay, then a 40 ms 120 dB startle pulse. Prepulse intensities were 4, 8, and 12 dB above the 70 dB background level, corresponding to 74, 78, and 82 dB. The no-stimulus trial consisted of background noise only and allowed assessment by the piezoelectric accelerometer of general activity in the startle chamber when no acoustic stimuli were presented. Startle magnitude was calculated as the average response to all of the pulse-alone trials, excluding the first and last blocks of six pulse-alone trials. The amount of PPI at each prepulse intensity was calculated as a percentage score: % PPI = 100 — ([(startle response for prepulse+pulse) / (startle response for pulse-alone)] × 100).
Assessment of somatic signs of nicotine withdrawal
Mice were habituated to the plastic cylinders (diameter 22 cm, height 25 cm) prior to testing for two 20 min periods. Observation of somatic signs took place immediately after the light-dark box test and was 20 min in duration. Somatic signs scored included rearing, scratching, forelimb tremors, body shakes, headshakes, abdominal constrictions, jumps, genital licks, and grooming. Grooming was defined as face, head, or body stroking with the forelimbs or licking any part of the body for more than 3 s. Between observations, the cylinders were cleaned by changing the bedding. Somatic signs were analyzed as total number of somatic signs during each observation period, excluding rears that were analyzed separately. Rears were analyzed separately because decreases are typically seen in this measure, while increases are seen in all of the other assessed measures of somatic signs.
Intracranial self-stimulation: Surgery, apparatus, and procedure
For ICSS surgery, mice were anesthetized by inhalation of 1–3% isoflurane in oxygen and positioned in a stereotaxic frame (Kopf Instruments, Tujunga, CA, USA). Stainless steel bipolar electrodes (0.20 mm in diameter; 6 mm long; Plastics One, Roanoke, VA, USA) were implanted into the medial forebrain bundle at the level of the lateral hypothalamus (coordinates: 1.58 mm AP, 1.0 mm ML, 5.3 mm DV from flat skull; Paxinos and Franklin, 2001), as previously described (Gill et al., 2004). Four stainless steel screws (3.2 mm long, Plastics One, Roanoke, VA, USA) were fixed to the skull to keep the electrode in place together with the application of a resin ionomer (Den-Mat, Santa Maria, CA, USA) and dental acrylic (Ortho-Jet, Lang Dental, Wheeling, IL, USA). Mice were allowed seven post-surgery recovery days.
ICSS training and testing were conducted in four Plexiglas operant chambers (30.5 × 24 × 27 cm; Med Associates, St. Albans, VT, USA). Each operant chamber was enclosed within a light- and sound-attenuated chamber (40 × 60 × 63.5 cm). Intracranial stimulation was delivered by constant current stimulators (Stimtech model 1200c, San Diego Instruments, San Diego, CA, USA). Subjects were connected to the stimulation circuit through flexible bipolar leads (Plastics One, Roanoke, VA, USA) attached to gold-contact swivel commutators (model SL2C, Plastics One, Roanoke, VA, USA) mounted above the operant chamber. The operant response required by the subjects was a simple one-quarter turn of a wheel manipulandum (5.5 cm in diameter, 4 cm in width) that extended 1.5 cm out of one wall of the operant chamber. The stimulation parameters, data collection, and all test session functions were controlled by a microcomputer.
The ICSS procedure used here was described previously for mice (Gill et al., 2004) and is adapted from the discrete-trial current threshold procedure in rats (Kornetsky and Esposito, 1979; Markou and Koob, 1992). Initially, mice were trained to turn the wheel manipulandum on a fixed-ratio 1 schedule of reinforcement. After successful acquisition of this schedule (two sessions where the mouse received 200 reinforcers in less than 10 min), mice were tested in the discrete-trial current-threshold procedure. Each trial began with the delivery of a noncontingent electrical stimulus followed by a 7.5 s response window within which the subject could make a response to receive a second contingent stimulus identical in all parameters to the initial noncontingent stimulus. A response during this time window was labeled a positive response, while the lack of a response was labeled a negative response. During a 2 s period immediately after a positive response, additional responses had no consequences. The intertrial interval that followed either a positive response or the end of the response window (in the case of a negative response) had an average duration of 10 s (ranging from 7.5 s to 12.5 s). Responses that occurred during the intertrial interval were labeled time-out responses and resulted in a further 12.5 s delay of the onset of the next trial. During training on the discrete-trial procedure, the duration of the intertrial interval and delay periods induced by time-out responses were gradually increased until both reached a duration of 10 s (ranging from 1–10 s during training). The animals were subsequently tested on the current-threshold procedure in which stimulation current intensities were varied according to the classical psychophysical method of limits. A test session consisted of four alternating series of descending and ascending current intensities starting with a descending series. Blocks of three trials were presented to the subject at a given stimulation intensity, and the intensity changed by 5 µA steps between blocks of trials. The initial stimulus intensity was set at approximately 30–40 µA above the baseline current-threshold for each animal. Each test session typically lasted 30–40 min and provided two dependent variables for behavioral assessment: threshold and response latency. The threshold value of each series was defined as the midpoint in microamperes between the current intensity level at which the animal made two or more positive responses out of the three stimulus presentations, and the level where the animal made less than two positive responses. The animal's estimated current threshold for each test session was the mean of the four series’ thresholds. The response latency was defined as the average time in seconds that elapsed between the delivery of the electrical stimulus and the turning of the wheel manipulandum for all of the trials that led to a positive response.
Experimental design
Experiment 1: Effects of withdrawal from chronic nicotine/saline exposure (0, 10, 20, 30, 40 mg/kg/day base, 14 days) on light-dark box performance, somatic signs, acoustic startle response, and prepulse inhibition of the acoustic startle response in C57BL/6J and BALB/cByJ mice
C57BL/6J (n = 9/group) and BALB/cByJ (n = 8–9/group) mice were prepared with 14-day minipumps delivering 10, 20, 30, or 40 mg/kg/day nicotine base or saline. Mice were habituated to the somatic-signs behavioral observational area on days 9 and 11 of minipump exposure. On days 1, 3, and 5 after minipump removal, mice were subjected to a battery of tests. First, the 5 min light-dark box test was conducted, followed by a 20 min observation of somatic signs. Then the 25 min acoustic startle/PPI test was performed.
Experiment 2: Effects of DHβE-precipitated and spontaneous nicotine withdrawal on ICSS performance in C57BL/6J mice exposed to 0 and 40 mg/kg/day nicotine base for 14 days
Naive mice (n = 11) were prepared with ICSS electrodes in the lateral hypothalamus and trained in the ICSS procedure. After establishment of stable ICSS thresholds (less than 10% variation over 5 days), mice were prepared with 14-day minipumps delivering saline (n = 5) or 40 mg/kg/day nicotine base (n = 6) and tested in the ICSS procedure twice daily throughout chronic nicotine/saline exposure. Although 14-day exposure to 40 mg/kg/day nicotine did not result in robust withdrawal signs in the measures assessed in Experiment 1, this relatively high nicotine dose was selected because it was well tolerated by the mice, and to address the primary question of this experiment whether DHβE would precipitate nicotine withdrawal. DHβE was administered subcutaneously using a within-subjects Latin square design at doses of 0, 0.75, 1.5, and 3 mg/kg salt on days 7, 9, 11, and 13 of nicotine/saline exposure before the first of the two daily ICSS sessions. Mice were placed into the operant chambers 20 min after DHβE/vehicle injection. DHβE induced transient decreases in locomotor activity immediately after injection that appeared to dissipate by 20 min post-injection. Baseline for evaluating the effects of each DHβE/vehicle administration was defined as the threshold (or latency) of the first session of the day preceding the DHβE/vehicle administration; this was done so that both baseline thresholds and drug-influenced thresholds were derived from the first daily ICSS session. The second daily ICSS session served to maintain stable ICSS performance. Inspection of the data indicated that DHβE injections had no effect on ICSS performance during the second daily session. After 14 days of nicotine/saline exposure, pumps were removed, and mice were tested in the ICSS procedure at 6, 8, 12, 16, 24, 32, 48, 52, 72, 76, 96, 100, and 120 h after pump removal.
Experiment 3: Effects of mecamylamine-precipitated and spontaneous nicotine withdrawal on ICSS performance, and the effects of spontaneous nicotine withdrawal on light-dark box performance and somatic signs in C57BL/6J mice after 28 days of exposure to 0 and 40 mg/kg/day nicotine base
The purpose of this experiment was to assess the effects of longer exposure to nicotine than that used in the previous two experiments on the various aspects of nicotine withdrawal. Naive mice (n = 23) were prepared with electrodes and trained in the ICSS procedure. After establishment of stable ICSS performance, mice were prepared with 28-day minipumps delivering saline (n = 12) or 40 mg/kg/day nicotine base (n = 11) and tested once daily. Mecamylamine was administered on days 9, 11, 13, and 15 of exposure to nicotine/saline because 28-day pump priming required additional 32 h to start full drug delivery compared with that of the 14-day minipumps (Experiment 2). Thus, the days selected to administer DHβE and mecamylamine were approximately equivalent between the two experiments in terms of days of full exposure to nicotine/saline. Mecamylamine was administered subcutaneously using a within-subjects Latin square design at doses of 0, 1.5, 3, and 6 mg/kg salt. Mice were tested immediately after injection with mecamylamine. No adverse effects were seen immediately after mecamylamine administration in pilot work. ICSS thresholds and response latencies after mecamylamine/vehicle administration were expressed as the percentage of the previous day’s baseline values. Mice were habituated to the cylinders for somatic signs observation on days 25 and 27 of nicotine/saline exposure. On day 29, pumps were removed, and mice were tested in the ICSS procedure at 3, 6, 8, 12, 16, 24, 32, 48, 52, 72, 76, 96, 100, and 120 h after pump removal. After completion of the ICSS test at the 24 h time-point, the mice were also tested in the light-dark box for 5 min followed immediately by a 20 min observation of somatic signs.
Statistical analyses
All analyses were performed using the Biomedical Computer Programs for Personal Computers Statistical Package (BMDP, Los Angeles, CA, USA). Data were analyzed using appropriate one-, two-, or three-way analysis of variance (ANOVA) with Strain and Nicotine Dose or Nicotine Exposure as between-subject factors and Withdrawal Day or Nicotine/Saline Exposure Day as within-subjects factors. Newman-Keuls post hoc analyses followed statistically significant interaction effects in the ANOVAs. The level of significance was set at 0.05.
In terms of ICSS data analyses, for evaluation of the effects of chronic nicotine/saline exposure, thresholds and response latencies were expressed as percentage of baseline values obtained during the last three daily sessions before pump implantation. To evaluate the effects of DHβE and mecamylamine on ICSS performance, thresholds and response latencies were expressed as percentage of baseline values (see experimental design above for definition of baseline thresholds and response latencies for each experiment). To evaluate ICSS performance during spontaneous nicotine/saline withdrawal, thresholds and response latencies were expressed as percentage of baseline values obtained during the last three ICSS daily sessions before pump removal. For experiment 3, time-points of withdrawal data were combined for analysis (6 + 8 h, 12 + 16 h, 24 + 32 h, 48 + 52 h, 72 + 76 h, 96 + 100 h) to provide a more robust and reliable estimate of the effects of nicotine/saline withdrawal. ICSS thresholds of mice tend to vary more across measurements than thresholds of rats (Semenova and Markou, unpublished observations). For the testing of a priori hypotheses that nicotine withdrawal induced threshold elevations and increased the number of somatic signs, group comparisons were made using t-tests. Additionally, the non-parametric χ2 test was used to compare the percentage of mice that showed threshold elevations more than 10% from baseline levels.
Results
Experiment 1: Effects of withdrawal from chronic nicotine exposure (0, 10, 20, 30, 40 mg/kg/day base, 14 days) on light-dark box performance, somatic signs, acoustic startle response, and prepulse inhibition of the acoustic startle response in C57BL/6J and BALB/cByJ mice
No statistically reliable overall increase in anxiety-like behavior was observed during days 1, 3, and 5 of nicotine withdrawal in C57BL/6J mice in the light-dark box test (Table 1). Two-way ANOVAs revealed no significant interaction between Nicotine Dose and Withdrawal Day for any of the measures. There were only significant main effects of Nicotine Dose on latency to enter the light compartment (F(4,40) = 2.79, p < 0.05) and significant main effects of Withdrawal Day on time spent in the light compartment (F(2,80) = 36.66, p < 0.001), latency to enter the light compartment (F(2,80) = 5.75, p < 0.01), and number of transitions (F(2,80) = 41.08, p < 0.001). Due to the high anxiety-like levels exhibited by the BALB/cByJ mice under the light-dark box conditions used in the present study (i.e., many mice of this strain, including saline-treated controls, never entered the light compartment), no meaningful results were derived from this strain in this test.
Table 1.
Effects of withdrawal from 14-day chronic nicotine/saline exposure on light-dark box measures in C57BL/6J mice
| Strain | Nicotine Dose | Day 1 | Day 3 | Day 5 |
|---|---|---|---|---|
| Time spent in light compartment (s) | ||||
| C57BL/6Ja | 0 | 116.22 ± 12.83 | 162.89 ± 3.97 | 116.78 ± 17.62 |
| 10 | 125.89 ± 11.98 | 113.56 ± 3.75 | 145.11 ± 19.68 | |
| 20 | 145.00 ± 11.19 | 129.56 ± 8.02 | 120.67 ± 6.93 | |
| 30 | 120.11 ± 9.54 | 137.33 ± 10.33 | 138.67 ± 9.95 | |
| 40 | 143.00 ± 9.18 | 89.67 ± 14.05 | 169.11 ± 8.28 | |
| Latency to enter light compartment (s) | ||||
| C57BL/6Ja, b | 0 | 39.11 ± 17.86 | 15.56 ± 3.58 | 20.33 ± 9.79 |
| 10 | 18.89 ± 2.43 | 10.67 ± 2.35 | 6.67 ± 1.21 | |
| 20 | 18.44 ± 2.49 | 15.00 ± 2.03 | 9.22 ± 2.59 | |
| 30 | 51.33 ± 14.13 | 57.00 ± 31.68 | 17.78 ± 5.8 | |
| 40 | 26.22 ± 5.55 | 14.56 ± 3.14 | 8.22 ± 1.76 | |
| Transitions between compartment | ||||
| C57BL/6Ja | 0 | 17.78 ± 2.58 | 30.33 ± 2.02 | 19.11 ± 2.99 |
| 10 | 20.89 ± 2.65 | 17.33 ± 1.39 | 22.44 ± 3.96 | |
| 20 | 23.67 ± 3.41 | 27.00 ± 3.21 | 18.67 ± 1.61 | |
| 30 | 17.00 ± 1.31 | 27.67 ± 1.63 | 23.89 ± 3.72 | |
| 40 | 25.56 ± 2.62 | 15.44 ± 2.82 | 29.44 ± 4.10 | |
Nicotine doses are expressed as mg/kg/day base. Mice were tested on 1, 3, and 5 days of nicotine/saline withdrawal. Data are presented as mean values ± S.E.M.
indicates a significant main effect of Withdrawal Day (p < 0.01)
indicates a significant effect of Nicotine Dose in two-way ANOVA (p < 0.05).
No statistically significant interactions were observed. The unit of measurement for time spent in the light compartment and latency to enter the light compartment is seconds (s).
Nicotine withdrawal did not lead to an overall significant change in somatic signs or rears in either C57BL/6J or BALB/cByJ mice (Table 2). Specifically, a three-way ANOVA on somatic signs with the factors Strain, Nicotine Dose and Withdrawal Day revealed a significant effect of Strain (F(79,1) = 26.81, p < 0.001) but no three-way interaction (F(8,158) = 0.82, n.s.). Follow-up two-way ANOVAs on each mouse strain separately revealed a significant main effect of Nicotine Dose in C57BL/6J mice on somatic signs (F(4,40) = 2.7, p < 0.05) but not rears. However, Newman-Keuls post hoc tests did not show differences in somatic signs between groups. In BALB/cByJ mice, follow-up two-way ANOVAs revealed no significant effect of Nicotine Dose on the number of somatic signs or rears.
Table 2.
Effects of withdrawal from 14-day chronic nicotine/saline exposure on somatic signs and rears in C57BL/6J and BALB/cByJ mice
| Strain | Nicotine Dose | Day 1 | Day 3 | Day 5 |
|---|---|---|---|---|
| Somatic signs | ||||
| C57BL/6Ja | 0 | 17.56 ± 4.31 | 16.11 ± 2.12 | 15.89 ± 3.16 |
| 10 | 15.44 ± 2.26 | 14.89 ± 2.12 | 17.00 ± 3.60 | |
| 20 | 17.78 ± 2.45 | 19.56 ± 1.99 | 14.00 ± 2.07 | |
| 30 | 25.22 ± 4.37 | 21.22 ± 3.38 | 20.78 ± 15.22 | |
| 40 | 18.44 ± 3.27 | 14.00 ± 2.07 | 15.22 ± 1.85 | |
| BALB/cByJ | 0 | 18.89 ± 2.91 | 20.78 ± 3.14 | 18.67 ± 2.81 |
| 10 | 29.00 ± 6.86 | 42.44 ± 10.54 | 29.67 ± 4.07 | |
| 20 | 27.78 ± 3.62 | 28.78 ± 5.71 | 29.89 ± 7.60 | |
| 30 | 30.75 ± 8.74 | 29.50 ± 8.72 | 31.50 ± 5.83 | |
| 40 | 33.44 ± 8.71 | 38.11 ± 6.30 | 40.56 ± 7.00 | |
| Rears | ||||
| C57BL/6J | 0 | 136.33 ± 8.19 | 141.44 ± 9.02 | 139.67 ± 11.87 |
| 10 | 110.11 ± 8.07 | 123.00 ± 5.22 | 122.56 ± 5.65 | |
| 20 | 143.33 ± 10.47 | 130.56 ± 7.29 | 139.56 ± 6.16 | |
| 30 | 122.44 ± 17.17 | 124.78 ± 13.19 | 115.89 ± 14.81 | |
| 40 | 156.78 ± 9.88 | 139.00 ± 9.86 | 135.44 ± 13.29 | |
| BALB/cByJ | 0 | 121.78 ± 16.31 | 111.00 ± 19.44 | 113.89 ± 10.47 |
| 10 | 134.22 ± 8.59 | 149.00 ± 9.61 | 129.78 ± 12.50 | |
| 20 | 137.67 ± 8.59 | 144.89 ± 11.71 | 139.22 ± 7.85 | |
| 30 | 153.25 ± 15.11 | 129.25 ± 11.79 | 140.38 ± 16.00 | |
| 40 | 145.89 ± 18.00 | 157.22 ± 15.79 | 163.22 ± 15.56 | |
Nicotine doses are expressed as mg/kg/day base. Mice were tested on 1, 3, and 5 days of nicotine/saline withdrawal. Data are presented as mean values ± S.E.M.
indicates a significant effect of Nicotine Dose in a follow-up two-way ANOVA (p < 0.05) on data from the C57BL/6J mice, after a significant main effect of Strain in the overall three-way ANOVA on somatic signs (p < 0.001). No statistically significant effects on rears in either strain were observed.
Spontaneous nicotine withdrawal did not induce statistically reliable increases in the acoustic startle response in either C57BL/6J or BALB/cByJ mice (Table 3). Specifically, a three-way ANOVA revealed a significant Strain × Withdrawal Day interaction (F(2,158) = 6.93, p < 0.01) and main effects of Strain (F(1,79) = 49.09, p < 0.001) and Withdrawal Day (F(2,158) = 4.12, p < 0.001) but no main effect of Nicotine Dose and no Strain × Nicotine Dose × Withdrawal Day interaction on startle reactivity. Further, follow-up two-way ANOVAs on the two strains separately with Nicotine Dose and Withdrawal Day as within- and between-subjects factors, respectively, showed an effect of Withdrawal Day on startle response in BALB/cByJ (F(2,78) = 6.46, p < 0.01) but not in C57BL/6J mice. A three-way ANOVA performed on all PPI data revealed that there was a significant effect of Prepulse Intensity (F(2,158) = 21.96, p < 0.0001) but no other significant or interaction effects between the factors Strain, Nicotine Dose, Withdrawal Day, and Prepulse Intensity.
Table 3.
Effects of withdrawal from 14-day chronic nicotine/saline exposure on the acoustic startle response in C57BL/6J and BALB/cByJ mice
| Strain | Nicotine Dose | Day 1 | Day 3 | Day 5 |
|---|---|---|---|---|
| C57BL/6J | 0 | 184.80 ± 28.66 | 128.78 ± 19.29 | 155.96 ± 17.60 |
| 10 | 191.21 ± 18.70 | 194.28 ± 15.97 | 181.48 ± 18.58 | |
| 20 | 173.73 ± 15.72 | 190.37 ± 23.24 | 171.49 ± 20.13 | |
| 30 | 168.70 ± 18.75 | 203.06 ± 25.44 | 136.94 ± 23.89 | |
| 40 | 211.03 ± 29.93 | 213.96 ± 27.03 | 179.52 ± 18.44 | |
| BALB/cByJ | 0 | 272.69 ± 15.59 | 302.69 ± 25.11 | 284.99 ± 21.40 |
| 10 | 263.63 ± 46.19 | 325.54 ± 43.50 | 319.77 ± 43.12 | |
| 20 | 297.53 ± 36.46 | 319.38 ± 31.27 | 310.32 ± 26.11 | |
| 30 | 245.71 ± 24.05 | 274.88 ± 36.08 | 250.70 ± 22.40 | |
| 40 | 248.02 ± 34.01 | 294.13 ± 53.17 | 306.72 ± 41.32 | |
Nicotine doses are expressed as mg/kg/day base. Mice were tested on 1, 3, and 5 days of nicotine/saline withdrawal. Data are presented as mean values ± S.E.M. There was a significant Strain × Nicotine Dose × Withdrawal Day interaction (p < 0.05), a significant main effect of Strain (p < 0.05), and a significant main effect of Withdrawal Day (p < 0.05) in a three-way ANOVA.
Experiment 2: Effects of DHβE-precipitated and spontaneous nicotine withdrawal on ICSS performance in C57BL/6J mice exposed to 0 and 40 mg/kg/day nicotine base for 14 days
ANOVAs performed on ICSS threshold and response latency data collected during days 1–6 of chronic nicotine administration did not reveal any significant main or interaction effects (data not shown). DHβE administration during chronic nicotine exposure induced significant elevations in ICSS thresholds in nicotine-treated mice compared with saline-treated mice (F(1,18) = 5.85, p < 0.05; Figure 1A). Although Newman-Keuls post hoc tests did not reveal to which factors this significant main effect may be attributable, pre-planned t-test comparisons indicated significant elevations in ICSS thresholds in nicotine-treated rats compared with saline-treated rats after administration of 3 mg/kg DHβE (p < 0.05). Spontaneous nicotine withdrawal after 14 days of nicotine exposure did not alter brain reward thresholds (Table 5) or response latencies (data not shown), as indicated by the absence of any statistically significant effects in the ANOVA.
Figure 1.
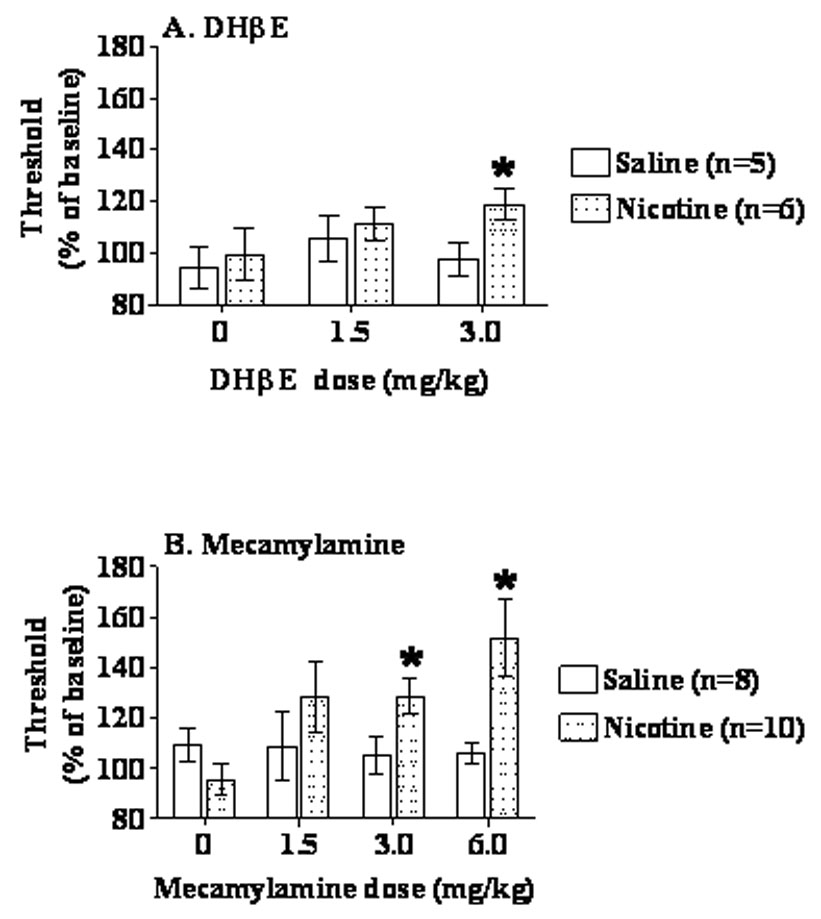
Effects of DHβE- (A) and mecamylamine- (B) precipitated nicotine/saline withdrawal (exposure 40 mg/kg/day nicotine base or saline) on ICSS thresholds in C57BL/6J mice. Data are presented as percentage of baseline thresholds (mean ± S.E.M.). Asterisk indicates a significant difference between nicotine- and saline-treated mice (*p < 0.05).
Table 5.
Effects of withdrawal from 14-day chronic nicotine/saline exposure (40 mg/kg/day base) on brain reward thresholds in C57BL/6J mice
| Hours of nicotine/saline withdrawal | Nicotine (n = 6) | Saline (n = 4) |
|---|---|---|
| 6–8 | 102.03 ± 4.36 | 106.81 ± 11.32 |
| 12–16 | 96.03 ± 6.65 | 103.63 ± 9.99 |
| 24–32 | 104.25 ± 4.10 | 109.46 ± 10.26 |
| 48 | 104.08 ± 4.80 | 115.34 ± 13.18 |
| 52 | 94.49 ± 6.52 | 105.61 ± 15.95 |
| 72–76 | 94.53 ± 7.97 | 114.19 ± 13.93 |
| 96–100 | 92.51 ± 7.23 | 104.91 ± 6.76 |
| 120 | 84.95 ± 7.00 | 91.72 ± 3.46 |
Data are presented as percentage of baseline thresholds (mean ± S.E.M.). There were no significant changes in brain reward thresholds during spontaneous nicotine withdrawal.
Experiment 3: Effects of mecamylamine-precipitated and spontaneous nicotine withdrawal on ICSS performance, and the effects of spontaneous nicotine withdrawal on light-dark box performance and somatic signs in C57BL/6J mice after 28 days of exposure to 0 and 40 mg/kg/day nicotine base
One saline-treated mouse was excluded from the spontaneous withdrawal data analyses because it became sick after pump removal, but the mouse was included in the mecamylamine-precipitated withdrawal analyses. Three saline-treated mice and one nicotine-treated mouse were excluded from the ICSS analyses due to unstable baseline thresholds, but these mice were included in the analyses of light-dark box and somatic signs data. One nicotine-treated mouse was excluded from the light-dark box analyses as an outlier because it exhibited behavior more than two standard deviations from the mean of all mice tested.
Chronic nicotine exposure lowered ICSS thresholds (Figure 2A) and had no effect on response latencies (data not shown). Data from days 1–8 and days 16–27 were analyzed separately. Mecamylamine injections were given on days 9–15 of nicotine/saline exposure with interspersed mecamylamine-free days (see Methods). An ANOVA performed on ICSS threshold data from days 1–8 of chronic nicotine/saline administration showed no effect of Nicotine/Saline Exposure or Nicotine/Saline Exposure Day and no interaction effects. An ANOVA performed on days 16–27 of chronic nicotine/saline administration revealed significant main effects of Nicotine/Saline Exposure (F(1,16) = 6.11, p < 0.05), no effect of Nicotine/Saline Exposure Day, and no interaction effect. Post hoc analyses revealed that nicotine-treated mice had significantly lowered thresholds compared with saline-treated mice only on days 23 and 24 (p < 0.05), although that trend continued through nicotine exposure (Figure 2). No effect of chronic nicotine/saline administration on response latencies was observed (data not shown).
Figure 2.
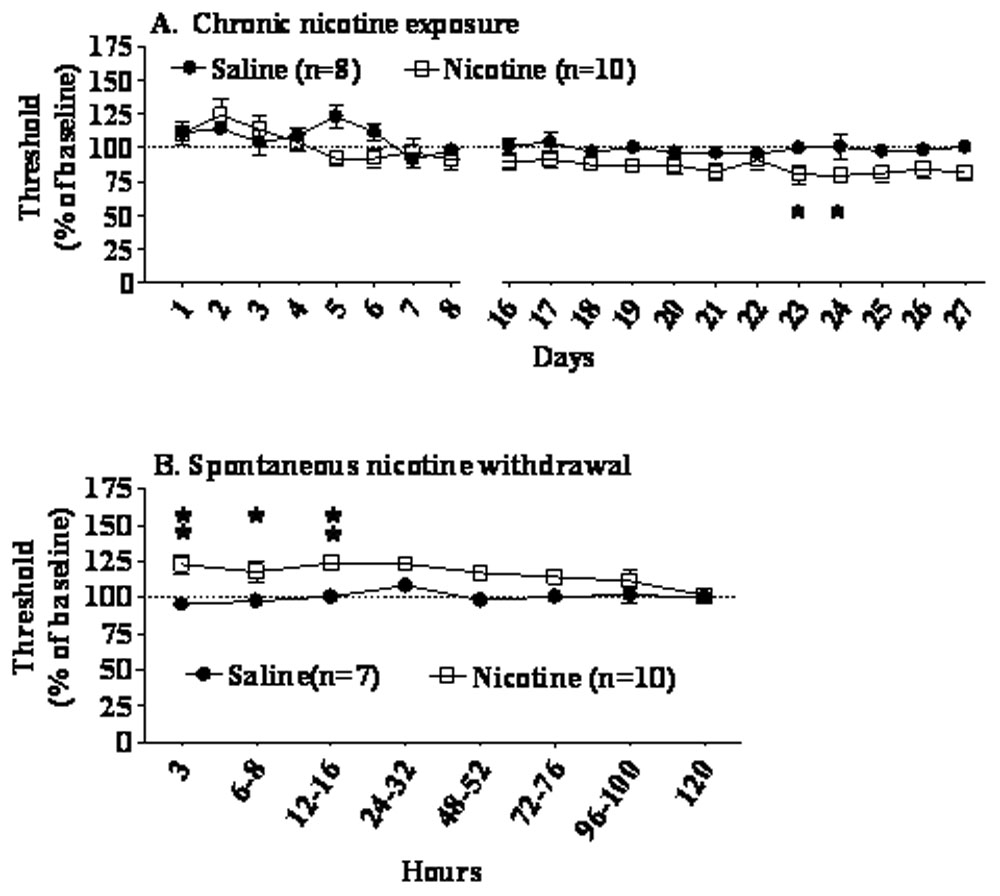
Effects of chronic administration of nicotine (40 mg/kg/day base, 28 days) or saline (A), and effects of nicotine/saline withdrawal (B) on ICSS thresholds in C57BL/6J mice. Data are presented as percentage of baseline thresholds (mean ± S.E.M.). Asterisks indicate significant differences between nicotine- and saline-treated mice (*p < 0.05, **p < 0.01).
Mecamylamine administration during chronic nicotine/saline administration elevated ICSS thresholds in nicotine-treated rats compared with saline-treated control subjects (Nicotine/Saline Exposure × Mecamylamine Dose interaction: F(3,48) = 3.29, p < 0.05; main effect of Nicotine/Saline Exposure: F(1,16) = 5.89, p < 0.05; trend toward main effect of Mecamylamine Dose: F(3,48) = 2.55, p < 0.07) (Figure 1B). Post hoc tests indicated significant elevations in ICSS thresholds after administration of 3 mg/kg (t-test, p < 0.05) and 6 mg/kg (Newman-Keuls test, p < 0.05) of mecamylamine in nicotine-treated compared with saline-treated mice.
Spontaneous withdrawal from 28 days of nicotine exposure induced a significant elevation in brain reward thresholds in C57BL/6J mice (Nicotine/Saline Exposure × Withdrawal Time interaction: F(7,105) = 2.41, p < 0.05; main effect of Nicotine/Saline Exposure: F(1,15) = 9.10, p < 0.01) (Figure 2B). The main effect of Nicotine/Saline Exposure is also graphically demonstrated by an area-under-the-curve analysis where the threshold values exhibited during the 3–120 h assessment were added for each group (Figure 3). Post hoc tests showed significant elevations in ICSS thresholds in nicotine-withdrawing mice compared with saline-withdrawing mice at 16 h post-pump removal (p < 0.05). Further, analyses of individual mice performance showed that during 3–76 h post-pump removal, 80–70% of nicotine-withdrawing mice, but only 0–28% of saline-withdrawing mice, exhibited more than 10% threshold elevations (χ2 test, p < 0.05, Figure 3). No changes in response latency during nicotine/saline withdrawal were observed (data not shown).
Figure 3.
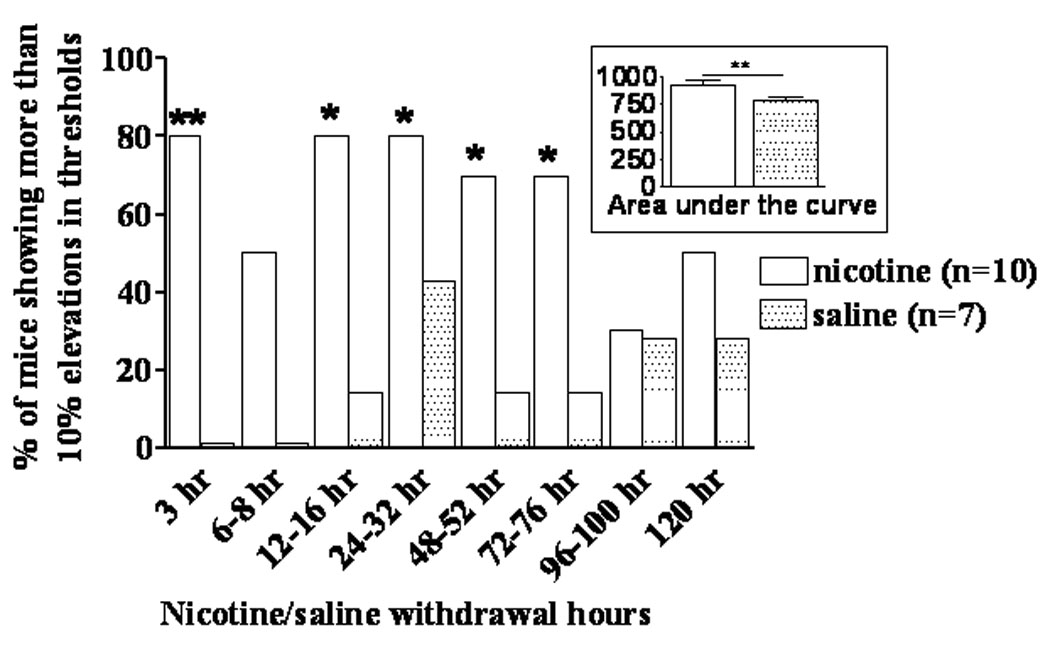
Percentage of mice that showed more than 10% elevations in ICSS thresholds in both the nicotine- and saline-treated groups (χ2 test, *p < 0.05, **p < 0.01). Inset: area-under-the-curve calculated as a sum of threshold values exhibited during the 3–120 h threshold assessment time-points in nicotine- and saline-exposed mice (main effect of Nicotine/Saline in the ANOVA, **p < 0.01).
Anxiety-like behavior was significantly increased in nicotine-withdrawing mice compared with saline-withdrawing mice 24 h post-pump removal as reflected in all measures of the light-dark box (Figure 4). One-way ANOVAs and post hoc comparisons revealed decreases in time spent in the light compartment (F(1,19) = 8.05, p < 0.01, Figure 4A) and the number of transitions between the light and dark compartments (F(1,19) = 8.79, p < 0.01, Figure 4C) and an increase in latency to enter the light compartment (F(1, 19) = 6.47, p < 0.05, Figure 4B) in nicotine-withdrawing mice compared with saline-withdrawing mice.
Figure 4.
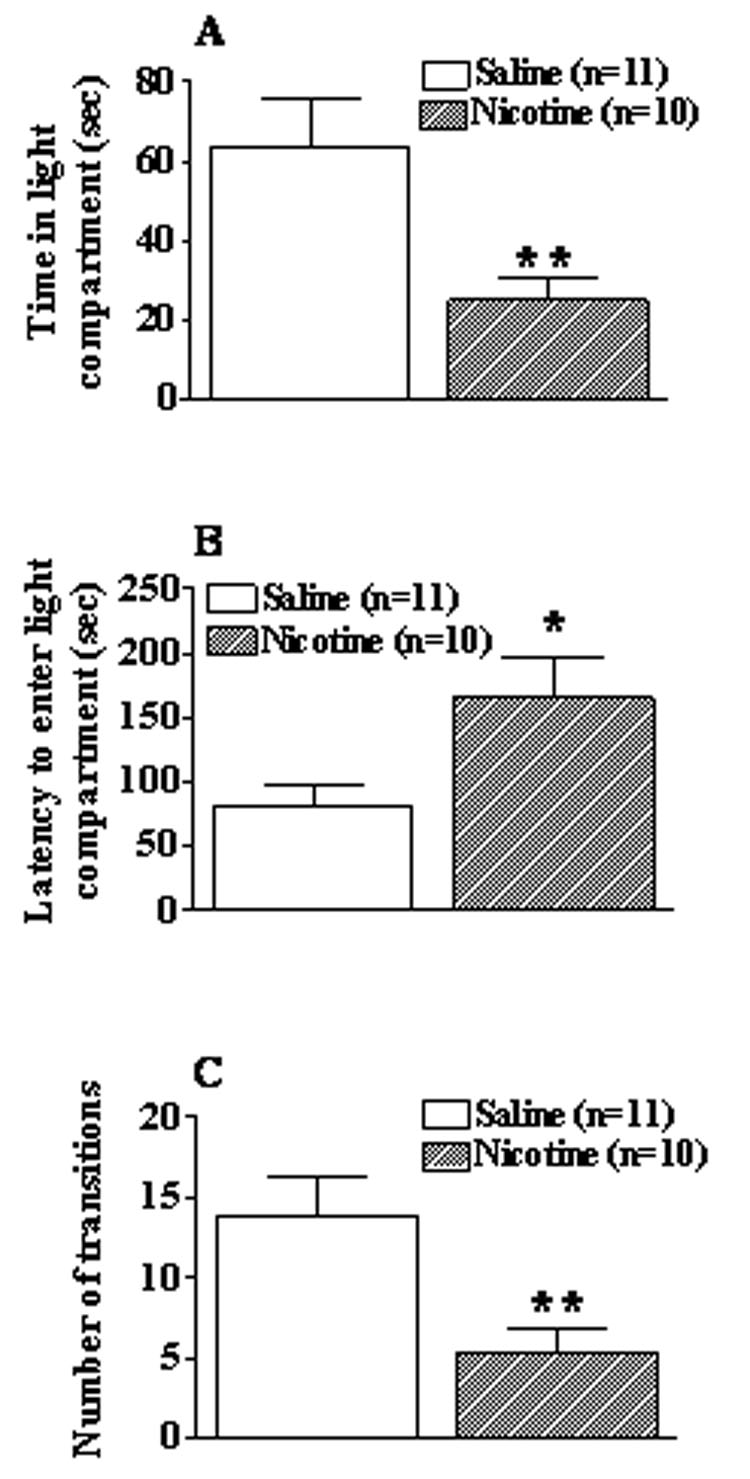
Effects of 24 h withdrawal from 28-day exposure to 40 mg/kg/day of nicotine (28 days) or saline on anxiety-like behavior in C57BL/6J mice. Light-dark box measures: (A) time spent in light compartment, (B) latency to enter light compartment, and (C) number of transitions between compartments. Data are presented as mean ± S.E.M. Asterisk indicates a significant difference between nicotine- and saline-withdrawing mice (*p < 0.05).
Behavioral observations at 24 h post-pump removal showed that nicotine withdrawal induced “physical” dependence in C57BL/6J mice. ANOVA and post hoc analyses confirmed a statistically significant increase in somatic signs (F(1, 20) = 16.44, p < 0.001) and a significant decrease in rears (F(1,20) = 9.14, p < 0.01) in nicotine-withdrawing compared with saline-withdrawing mice (Figure 5).
Figure 5.
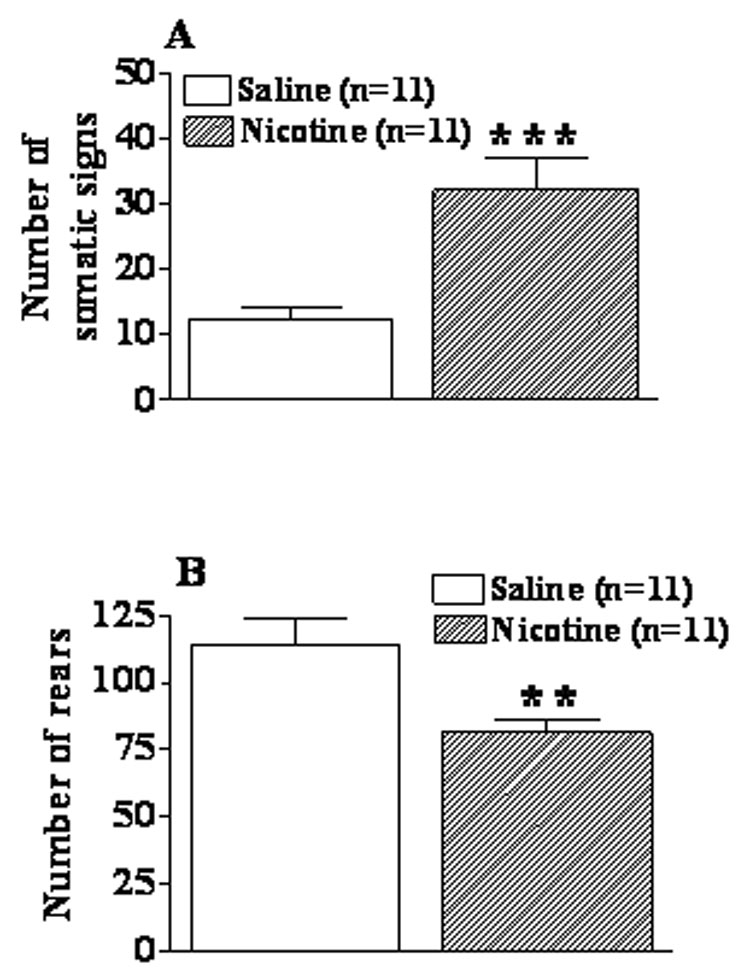
Effect of 24 h withdrawal from nicotine (40 mg/kg/day, 28 days) or saline on somatic signs (A) and rears (B) in C57BL/6J mice. Data are presented as mean ± S.E.M. Asterisks indicate a significant difference between nicotine- and saline-withdrawing mice (*p < 0.05, **p < 0.01).
Discussion
The results of the present study indicated that cessation of 14-day exposure to 10–40 mg/kg/day nicotine base did not produce clear and reliable nicotine withdrawal in either C57BL/6J or BALB/cByJ mice. Specifically, no alterations were observed in anxiety-like behavior measured in the light-dark box in C57BL/6J mice during nicotine withdrawal, while this test was not suitable for BALB/cByJ mice. Startle reactivity, PPI, or somatic signs were not altered in either mouse strain during spontaneous nicotine withdrawal. Finally, no changes were observed in brain reward function, measured only in C57BL/6J mice, during spontaneous nicotine withdrawal with 40 mg/kg/day nicotine dose and 14-day exposure time. When exposure to the highest nicotine dose (40 mg/kg/day base) was extended to 28 days, both affective and somatic aspects of nicotine withdrawal were observed in C57BL/6J mice. Specifically, ICSS thresholds were elevated, indicating deficits in brain reward function. Further, 24 h post-nicotine, increased anxiety-like behavior was clearly observed in C57BL/6J mice as reflected in decreased time spent in the light compartment, decreased number of transitions between compartments, and increased latency to enter the light compartment of the light-dark box. Somatic signs of withdrawal were significantly increased, indicating the development of “physical” dependence. Finally, administration of mecamylamine, and to a lesser extent DHβE, induced elevations in ICSS threshold in C57BL/6J mice after exposure for 7 days to 40 mg/kg/day nicotine, but not saline, indicating deficits in brain reward function.
Effects of nicotine withdrawal after 14-day exposure to nicotine
Light-dark box
Termination of 14-day exposure to 10, 20, 30 or 40 mg/kg/day nicotine base did not induce anxiety-like behavior in the light-dark box test during 1, 3, and 5 days post-pump removal in C57BL/6J mice. These results extend previous findings from our laboratory (Jonkman et al., 2005) that showed no changes in light-dark box measures at 24 h after termination of exposure to 24 mg/kg/day nicotine base for 14 days, but a significant decrease in time spent in the light compartment 24 h after termination of exposure to 48 mg/kg/day nicotine base for 14 days. BALB/cByJ mice, even saline-exposed control mice, did not generally enter the light compartment, thus preventing the evaluation of the effects of termination of chronic nicotine administration on performance in this test. In the present studies, this high level of anxiety-like behavior of BALB/cByJ mice may be explained by the relatively high level of illumination (i.e., light intensity of 500 lux). Nevertheless, previous studies have also reported poor performance and high anxiety-like behavior in the light-dark box in BALB/c mice under baseline conditions in anxiety tests (Crawley and Davis, 1982; Crawley et al., 1997; Guillot and Chapouthier, 1996; Lepicard et al., 2000).
Somatic signs
Exposure to all nicotine doses (10, 20, 30, or 40 mg/kg/day) for 14 days did not induce significant and reliable increases in somatic signs during spontaneous withdrawal in either BALB/cByJ or C57BL/6J mice. A previous study by Damaj and colleagues (2003) reported that exposure to 24 mg/kg/day nicotine base for 14 days induced significant increases in somatic signs during spontaneous nicotine withdrawal in ICR mice and during mecamylamine-precipitated nicotine withdrawal in C57BL/6J mice. However, despite the use of similar and higher nicotine doses and 14-day exposure time as well as the same C57BL/6J mouse strain, results from the present studies did not replicate some of the findings by Damaj and colleagues (2003). Nevertheless, we observed significant increases in somatic signs during spontaneous nicotine withdrawal after 28 days of exposure to nicotine (see below). These differences in the results between the Damaj and colleagues study (2003) and our study may be attributed to the fact that in the present study we investigated spontaneous withdrawal while Damaj and colleagues (2003) explored mecamylamine-precipitated withdrawal in C57BL/6J mice. Therefore, immediate nAChR blockade by nAChR antagonist administration may have a different effect on somatic signs than gradual clearance of nicotine from the system that occurs during spontaneous withdrawal. Further, important differences have been reported in the types of somatic signs seen in different studies, suggesting that the expression of nicotine withdrawal may not be uniform between and even within strains. Specifically, Damaj and colleagues (2003) observed paw tremor, head shakes, backing, and writhing in ICR and C57BL/6J mice, but not in 129/SvEv mice, during mecamylamine-precipitated nicotine withdrawal (24 mg/kg/day nicotine base for 14 days), indicating strain differences in the expression of somatic component of precipitated nicotine withdrawal. Isola and colleagues (1999) identified two groups of Swiss-Webster mice that exhibited different spontaneous and mecamylamine-precipitated somatic signs of nicotine withdrawal. In this study, Swiss-Webster mice were administered 2 mg/kg nicotine base subcutaneously four times daily for 14 days. One group of mice exhibited primarily escape-like activity (rearing and jumping), while another group demonstrated stereotypic-like activity characterized by chewing, scratching, head shakes, body shakes, and facial tremor. Semenova and colleagues (2003a) observed increased jumping activity only in DBA/2J mice after withdrawal from 14-day exposure to 6 mg/kg/day of nicotine base. Balerio and colleagues (2004) observed paw tremor, wet-dog shakes, genital licks, and scratches in the first study of precipitated withdrawal, and only paw tremor and teeth chattering in the second study during mecamylamine-precipitated withdrawal in CD1 mice treated chronically with 8.77 mg/kg/day nicotine base for 6 days.
Taken together, these literature findings indicate that genetic factors may be powerful determinants of the expression of the somatic aspects of nicotine withdrawal. Further, this literature review indicates that mice in the present study received a high enough nicotine dose to induce the somatic component of nicotine withdrawal comparable or larger than that reported in the literature. The duration of nicotine exposure may be an important factor for the induction of the somatic aspects of nicotine dependence/withdrawal, and this factor was explored in subsequent studies reported here.
Acoustic startle response
C57BL/6J and BALB/cByJ mice did not differ in their startle response under baseline conditions in the present study, consistent with previous reports (Bullock et al., 1997; Logue et al., 1997; Paylor and Crawley, 1997; Willott et al., 2003). For all nicotine doses tested, neither the BALB/cByJ nor the C57BL/6J strain showed significant and reliable increases in startle after termination of 14-day exposure to nicotine (10–40 mg/kg/day). However, it is possible that there may be increases in startle reactivity during nicotine withdrawal after longer exposure to nicotine or in other mouse strains. For example, there was decreased startle in Sprague-Dawley rats (Acri et al., 1991), increased startle in Long–Evans rats (Helton et al., 1993), and no changes in Wistar rats (Jonkman et al., 2007). Importantly, recent a personal communication with Dr. Kurt Rasmussen (Jonkman et al., 2007) indicated that the startle boxes used in the Helton studies were transparent, and thus startle responses were assessed under bright light conditions. Such conditions may have led to a potentiated startle response in control rats, presumably due to the stressful conditions of exposure to bright light in rats (Walker and Davis, 1997). Most relevant to the present discussion are recent findings from our laboratory showing that only light-potentiated startle is increased in nicotine-withdrawing rats, while startle in dark conditions remains unchanged (Jonkman et al., 2007). Thus, in addition to strain differences (Bullock et al., 1997; Logue et al., 1997; Paylor and Crawley, 1997; Willott et al., 2003), the degree of stress induced by the particular testing conditions in the various laboratories may affect the observed startle response. That is, nicotine withdrawal appears to potentiate responses to stressors rather than induce a reliable anxiety-like state under low stress conditions (Jonkman et al., 2007).
Prepulse inhibition of the acoustic startle response
C57BL/6J and BALB/cByJ mice did not differ in PPI under baseline conditions in the present study, consistent with previous reports (Bullock et al., 1997; Logue et al., 1997; Paylor and Crawley, 1997; Willott et al., 2003). Further, neither strain of mice showed deficits in PPI on days 1, 3, and 5 after termination of 14-day nicotine exposure. These results extend previous results from our laboratory showing a similar lack of changes in PPI after exposure to nicotine (24 or 48 mg/kg/day) for 14 days in C57BL/6J mice (Jonkman et al., 2005; Spielewoy and Markou, 2004). By contrast, deficits in PPI have been seen in DBA/2J mice exposed to 6 mg/kg/day nicotine base for 14 days (Semenova et al., 2003a). Similar strain differences in changes in PPI during nicotine withdrawal have been reported in rats. Specifically, PPI was decreased in Sprague-Dawley rats (Acri et al., 1991), whereas increased PPI was seen in Long-Evans rats during nicotine withdrawal (Helton et al., 1993; however, see Discussion above).
Taken together, the results indicate that 14-day exposure to nicotine at the dose range of 10–40 mg/kg/day did not produced reliable changes in anxiety-like behavior measured in the light-dark box and acoustic startle tests. Further, at these doses, no deficits in PPI or increases in somatic signs during spontaneous nicotine withdrawal in either C57BL/6J or BALB/cByJ mice were seen. Therefore, subsequent studies determined whether longer exposure to higher nicotine doses may produce more robust and more easily observed nicotine withdrawal signs than those seen with the doses and exposure times reported above.
Effects of nicotine withdrawal after 28-day exposure to nicotine
Intracranial self-stimulation
Chronic 28-day, but not 14-day, exposure to 40 mg/kg/day of nicotine lowered ICSS thresholds in C57BL/6J mice, demonstrating the reward-enhancing effects of nicotine. This finding is consistent with previous observations in rats showing threshold lowering during the first 1–2 days of exposure to nicotine via subcutaneous osmotic minipumps (Cryan et al., 2003; Harrison et al., 2001; Semenova and Markou, 2003b), an effect also seen after bolus administration of acute nicotine in rats (Cryan et al., 2003; Harrison et al., 2002; Semenova and Markou, 2003b). However, in the present study in mice, nicotine-induced threshold lowering was not observed until days 23–27 of nicotine exposure, probably due to the mildly aversive effects of the relatively high nicotine dose used that may require longer exposure for tolerance to develop to such potentially aversive effects of nicotine before the reward-enhancing effects of nicotine were revealed. A similar reward-enhancing effect (e.g., lowering of brain reward thresholds) was observed after acute administration of cocaine or SKF-82958, a dopamine D1 receptor agonist, in Swiss-Webster mice (Gilliss et al., 2002).
To our knowledge, this is one of the first demonstrations that termination of 28-day, but not 14-day, nicotine exposure (40 mg/kg/day) resulted in elevations in ICSS thresholds for 4 days, indicating a decrease in brain reward function during spontaneous nicotine withdrawal in C57BL/6J mice. Further, the present study demonstrated that administration of either of two nAChR antagonists, DHβE or mecamylamine, precipitated nicotine withdrawal as reflected in threshold elevations in nicotine-treated mice at doses that have no effect in saline-treated control mice. Interestingly, the shorter 7-day exposure to nicotine was sufficient to induce threshold elevations during nAChR antagonist-precipitated withdrawal compared with spontaneous withdrawal that required longer nicotine exposure before it emerged (i.e., 28 days). Repeated exposure to either DHβE or mecamylamine-precipitated withdrawal may influence the effects of spontaneous nicotine withdrawal on brain reward function. However, nAChR antagonist precipitated withdrawal is unlikely to explain the differential results between the 14- and 28-day exposure groups because both groups had similar nAChR antagonist precipitated nicotine withdrawal experiences (DHβE in Experiment 2 and mecamylamine in Experiment 3). Thus, the results suggest that the length of exposure to nicotine is an important factor for the induction of ICSS threshold elevations upon cessation of nicotine administration. These results are entirely consistent with the results of studies of spontaneous and nAChR antagonist-precipitated nicotine withdrawal in rats (Epping-Jordan et al., 1998; Harrison et al., 2002; Kenny and Markou, 2006; Semenova and Markou, 2003b; Watkins et al., 2000). Nevertheless, it should be noted that larger nicotine doses (i.e., 40 mg/kg/day) and longer exposure times (i.e., 28 days) were required in mice for these effects to emerge during spontaneous nicotine withdrawal than those required in rats (i.e., 3.16 mg/kg/day nicotine base for 7–14 days). Nicotine metabolism rates are approximately 10 times faster in mice than in rats or humans (Petersen et al., 1984). Thus, taken into consideration nicotine metabolism in rats and mice, the nicotine doses that induced withdrawal in the two species appear roughly equipotent.
In C57BL/6J mice, spontaneous nicotine withdrawal after a 28-day exposure to 40 mg/kg/day nicotine led to significant increases in anxiety-like behavior measured in the light-dark box test. Specifically, time spent in the light compartment and the number of transitions between compartments was decreased, whereas the latency to enter the light compartment was increased. Finally, after prolonged nicotine exposure (28 days; 40 mg/kg/day), the number of somatic signs was increased, whereas the number of rears was decreased in C57BL/6J mice. Taken together, these results demonstrate that anxiety-like behavior and somatic withdrawal signs are observed during spontaneous nicotine withdrawal in mice when mice are exposed to a high nicotine dose for a prolonged period of time.
Because of the invasive nature of the acoustic startle test, we did not assess startle response and PPI after 28 days of exposure to 40 mg/kg/day. We did not wish for the measurement of startle to interfere with our assessment of ICSS thresholds. Thus, it remains a possibility that prolonged nicotine exposure to high nicotine doses may induce PPI deficits and increases in the acoustic startle response in C57BL/6J or BALB/cByJ mice.
Conclusions
In summary, the main finding of this study was that prolonged administration of a relatively high nicotine dose (40 mg/kg/day base for 28 days) was necessary to induce a spontaneous nicotine withdrawal syndrome in C57BL/6J mice. The current study is one of the first studies to demonstrate elevations in ICSS thresholds associated with both spontaneous and nAChR antagonist-precipitated nicotine withdrawal in C57BL/6J mice. Additionally, spontaneous nicotine withdrawal after prolonged nicotine administration (40 mg/kg/day for 28 days) was associated with increased anxiety-like behavior assessed in the light-dark box and increased number of somatic signs. In contrast, C57BL/6J and BALB/cByJ mice withdrawn from chronic nicotine administration at the same or lower nicotine doses, but after shorter (14 days) nicotine exposure, did not exhibit reliable increases in somatic signs of nicotine withdrawal [in contrast to earlier reported findings in the same and other mouse strains; (Acri et al., 1991; Balerio et al., 2004; Berrendero et al., 2002; Castañé et al., 2002; Damaj et al., 2003)] and did not exhibit increases in anxiety-like behavior or alterations in PPI or startle reactivity. In conclusion, the results demonstrate that administration of relatively high nicotine doses over prolonged periods of time induces both the affective and somatic aspects of spontaneous nicotine withdrawal in the mouse, while exposure to nicotine for shorter periods of time is sufficient for nAChR antagonist-precipitated nicotine withdrawal.
Table 4.
Effect of withdrawal from 14-day chronic nicotine exposure on prepulse inhibition in C57BL/6J and BALB/cByJ mice
| Strain | Nicotine Day 1Dose | Day 1 | Day 3 | Day 5 | ||||
|---|---|---|---|---|---|---|---|---|
| Prepulse Intensity 74 dBd | ||||||||
| C57BL/6J | 0 | 24.59 ± 7.15 | 17.33 ± 16.81 | 36.89 ± 7.14 | ||||
| 10 | 37.52 ± 6.20 | 43.04 ± 3.87 | 39.71 ± 6.27 | |||||
| 20 | 32.86 ± 4.52 | 37.02 ± 4.93 | 34.00 ± 5.04 | |||||
| 30 | 34.00 ± 6.65 | 36.85 ± 5.47 | 26.63 ± 9.07 | |||||
| 40 | 15.13 ± 6.54 | 34.82 ± 6.51 | 37.42 ± 4.44 | |||||
| BALB/cByJ | 0 | 43.10 ± 3.36 | 43.73 ± 3.29 | 40.67 ± 7.14 | ||||
| 10 | 37.33 ± 5.85 | 47.51 ± 2.91 | 36.38 ± 4.29 | |||||
| 20 | 42.13 ± 4.67 | 41.96 ± 3.78 | 43.42 ± 4.27 | |||||
| 30 | 43.37 ± 4.13 | 45.17 ± 4.35 | 36.77 ± 5.99 | |||||
| 40 | 39.56 ± 4.71 | 41.65 ± 4.99 | 41.89 ± 6.08 | |||||
| Prepulse Intensity 78 dB | ||||||||
| C57BL/6J | 0 | 45.79 ± 5.46 | 43.69 ± 11.01 | 57.39 ± 4.92 | ||||
| 10 | 50.87 ± 5.94 | 49.23 ± 5.58 | 51.44 ± 6.69 | |||||
| 20 | 40.71 ± 4.99 | 56.16 ± 5.24 | 52.23 ± 4.26 | |||||
| 30 | 55.77 ± 3.73 | 51.73 ± 6.70 | 47.84 ± 8.15 | |||||
| 40 | 36.42 ± 5.01 | 45.93 ± 6.48 | 47.59 ± 4.47 | |||||
| BALB/cByJ | 0 | 49.99 ± 2.91 | 48.94 ± 3.69 | 49.82 ± 3.96 | ||||
| 10 | 46.08 ± 4.74 | 51.94 ± 4.49 | 49.41 ± 4.25 | |||||
| 20 | 49.14 ± 5.76 | 48.32 ± 3.71 | 45.16 ± 3.87 | |||||
| 30 | 51.71 ± 4.30 | 48.88 ± 4.58 | 43.42 ± 7.30 | |||||
| 40 | 50.65 ± 4.60 | 43.41 ± 6.03 | 43.45 ± 5.74 | |||||
| Prepulse Intensity 82 dBa, b, c | ||||||||
| C57BL/6J | 0 | 52.92 ± 4.98 | 47.05 ± 10.29 | 65.60 ± 4.82 | ||||
| 10 | 59.19 ± 5.10 | 57.65 ± 4.94 | 55.24 ± 4.72 | |||||
| 20 | 53.48 ± 5.17 | 62.57 ± 4.64 | 62.00 ± 4.48 | |||||
| 30 | 57.51 ± 3.43 | 58.38 ± 5.67 | 53.24 ± 7.53 | |||||
| 40 | 39.485.28 ± | 49.74 ± 6.79 | 57.01 ± 4.38 | |||||
| BALB/cByJ | 0 | 57.45 ± 4.22 | 58.70 ± 3.83 | 56.98 ± 3.92 | ||||
| 10 | 52.38 ± 4.26 | 59.41 ± 2.42 | 52.93 ± 3.67 | |||||
| 20 | 55.89 ± 5.02 | 53.63 ± 3.74 | 51.01 ± 3.16 | |||||
| 30 | 64.68 ± 4.08 | 54.13 ± 3.98 | 55.34 ± 3.96 | |||||
| 40 | 58.70 ± 4.41 | 51.39 ± 2.18 | 55.38 ± 4.68 | |||||
Nicotine doses are expressed as mg/kg/day base. Mice were tested on 1, 3, and 5 days of nicotine/saline withdrawal. Data are presented as mean values ± S.E.M.
indicates a significant Strain × Nicotine Dose × Withdrawal Day interaction.
indicates a significant Nicotine Dose × Withdrawal Day interaction.
indicates a significant Strain × Withdrawal Day interaction.
indicates a significant main effect of Strain in a three-way ANOVA (all p < 0.05).
Acknowledgements
This work was supported by National Institute on Drug Abuse (NIDA) grant R01 DA023209 to AM and the Tobacco-Related Disease Research Program (TRDRP) from the State of California grant 14IT-0053 to SS. The authors are grateful to Mr. Sietse Jonkman and Dr. Brook Henry for their previous work on nicotine withdrawal in mice in our laboratory. Finally, the authors wish to thank Mrs. Jessica Benedict for technical assistance and Mr. Michael Arends for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acri JB, Grunberg NE, Morse DE. Effects of nicotine on the acoustic startle reflex amplitude in rats. Psychopharmacology (Berl) 1991;104:244–248. doi: 10.1007/BF02244186. [DOI] [PubMed] [Google Scholar]
- Alterman AI, Gariti P, Mulvaney F. Short- and long-term smoking cessation for three levels of intensity of behavioral treatment. Psychol Addict Behav. 2001;15:261–264. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th edn, text rev. Washington, DC: American Psychiatric Press; 2000. [Google Scholar]
- Balerio G, Aso E, Berrendero F, Murtra P, Maldonado R. Δ9-tetrahydrocannabinol decreases somatic and motivational manifestations of nicotine withdrawal in mice. Eur J Neurosci. 2004;20:2737–2748. doi: 10.1111/j.1460-9568.2004.03714.x. [DOI] [PubMed] [Google Scholar]
- Bast T, Feldon J. Hippocampal modulation of sensorimotor processes. Prog Neurobiol. 2003;70:319–345. doi: 10.1016/s0301-0082(03)00112-6. [DOI] [PubMed] [Google Scholar]
- Bauco P, Wise RA. Potentiation of lateral hypothalamic and midline mesencephalic brain stimulation reinforcement by nicotine: examination of repeated treatment. J Pharmacol Exp Ther. 1994;271:294–301. [PubMed] [Google Scholar]
- Berrendero F, Kieffer BL, Maldonado R. Attenuation of nicotine-induced antinociception, rewarding effects and dependence in µ-opioid receptor knock-out mice. J Neurosci. 2002;22:10935–10940. doi: 10.1523/JNEUROSCI.22-24-10935.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bespalov A, Lebedev A, Panchenko G, Zvartau E. Effects of abused drugs on thresholds and breaking points of intracranial self-stimulation in rats. Eur Neuropsychopharmacol. 1999;9:377–383. doi: 10.1016/s0924-977x(99)00008-5. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Pudiak CM, KuoLee R. Effect of chronic nicotine on brain stimulation reward. I. Effect of daily injections. Behav Brain Res. 1998;96:185–188. doi: 10.1016/s0166-4328(98)00050-3. [DOI] [PubMed] [Google Scholar]
- Bullock AE, Slobe BS, Vazquez V, Collins AC. Inbred mouse strains differ in the regulation of startle and prepulse inhibition of the startle response. Behav Neurosci. 1997;111:1353–1360. doi: 10.1037//0735-7044.111.6.1353. [DOI] [PubMed] [Google Scholar]
- Castañé A, Valjent E, Ledent C, Parmentier M, Maldonado R, Valverde O. Lack of CB1 cannabinoid receptors modifies nicotine behavioural responses, but not nicotine abstinence. Neuropharmacology. 2002;43:857–867. doi: 10.1016/s0028-3908(02)00118-1. [DOI] [PubMed] [Google Scholar]
- Cazala P. Effect of clonidine and phentolamine on self-stimulation behavior in the dorsal and ventral regions of the lateral hypothalamus in mice. Psychopharmacology (Berl) 1980;68:173–177. doi: 10.1007/BF00432137. [DOI] [PubMed] [Google Scholar]
- Cazala P, Guenet JL. The recombinant inbred strains: a tool for the genetic analysis of differences observed in the self-stimulation behaviour of the mouse. Physiol Behav. 1980;24:1057–1060. doi: 10.1016/0031-9384(80)90047-5. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Davis LG. Baseline exploratory activity predicts anxiolytic responsiveness to diazepam in five mouse strains. Brain Res Bull. 1982;8:609–612. doi: 10.1016/0361-9230(82)90087-9. [DOI] [PubMed] [Google Scholar]
- Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Crawley J, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Crooks PA, Dwoskin LP. Contribution of CNS nicotine metabolites to the neuropharmacological effects of nicotine and tobacco smoking. Biochem Pharmacol. 1997;54:743–753. doi: 10.1016/s0006-2952(97)00117-2. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Bruijnzeel AW, Skjei KL, Markou A. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology (Berl) 2003;168:347–358. doi: 10.1007/s00213-003-1445-7. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther. 2003;307:526–534. doi: 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. The effects of DHβE and MLA on nicotine-induced enhancement of contextual fear conditioning in C57BL/6 mice. Psychopharmacology (Berl) 2006;184:345–352. doi: 10.1007/s00213-005-0047-y. [DOI] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci. 2005;25:8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Faraday MM, O'Donoghue VA, Grunberg NE. Effects of nicotine and stress on startle amplitude and sensory gating depend on rat strain and sex. Pharmacol Biochem Behav. 1999;62:273–284. doi: 10.1016/s0091-3057(98)00159-2. [DOI] [PubMed] [Google Scholar]
- Faraday MM, Rahman MA, Scheufele PM, Grunberg NE. Nicotine administration impairs sensory gating in Long-Evans rats. Pharmacol Biochem Behav. 1998;61:281–289. doi: 10.1016/s0091-3057(98)00094-x. [DOI] [PubMed] [Google Scholar]
- Gill BM, Knapp CM, Kornetsky C. The effects of cocaine on the rate independent brain stimulation reward threshold in the mouse. Pharmacol Biochem Behav. 2004;79:165–170. doi: 10.1016/j.pbb.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Gilliss B, Malanga CJ, Pieper JO, Carlezon WA., Jr Cocaine and SKF-82958 potentiate brain stimulation reward in Swiss-Webster mice. Psychopharmacology (Berl) 2002;163:238–248. doi: 10.1007/s00213-002-1153-8. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Rukstalis M, Lewis MC. Atomoxetine and nicotine enhance prepulse inhibition of acoustic startle in C57BL/6 mice. Neurosci Lett. 2005;377:85–90. doi: 10.1016/j.neulet.2004.11.073. [DOI] [PubMed] [Google Scholar]
- Griebel G, Belzung C, Perrault G, Sanger DJ. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl) 2000;148:164–170. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology. Biol Psychiatry. 2002;52:958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- Guillot PV, Chapouthier G. Intermale aggression and dark/light preference in ten inbred mouse strains. Behav Brain Res. 1996;77:211–213. doi: 10.1016/0166-4328(95)00163-8. [DOI] [PubMed] [Google Scholar]
- Haas AL, Munoz RF, Humfleet GL, Reus VI, Hall SM. Influences of mood, depression history, and treatment modality on outcomes in smoking cessation. J Consult Clin Psychol. 2004;72:563–570. doi: 10.1037/0022-006X.72.4.563. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Gasparini F, Markou A. Nicotine potentiation of brain stimulation reward reversed by DHβE and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology (Berl) 2002;160:56–66. doi: 10.1007/s00213-001-0953-6. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Liem YT, Markou A. Fluoxetine combined with a serotonin-1A receptor antagonist reversed reward deficits observed during nicotine and amphetamine withdrawal in rats. Neuropsychopharmacology. 2001;25:55–71. doi: 10.1016/S0893-133X(00)00237-2. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Fletcher L, Morgan S, Keenan R, Amble P. The effects of varying cigarette deprivation duration on cognitive and performance tasks. J Subst Abuse. 1989;1:88–93. [PubMed] [Google Scholar]
- Helton DR, Modlin DL, Tizzano JP, Rasmussen K. Nicotine withdrawal: a behavioral assessment using schedule controlled responding, locomotor activity, and sensorimotor reactivity. Psychopharmacology (Berl) 1993;113:205–210. doi: 10.1007/BF02245698. [DOI] [PubMed] [Google Scholar]
- Hildebrand BE, Nomikos GG, Bondjers C, Nisell M, Svensson TH. Behavioral manifestations of the nicotine abstinence syndrome in the rat: peripheral versus central mechanisms. Psychopharmacology (Berl) 1997;129:348–356. doi: 10.1007/s002130050200. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9:315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Gust SW, Keenan R, Fenwick JW, Skoog K, Higgins ST. Long-term use of nicotine vs placebo gum. Arch Internal Med. 1991a;151:1993–1998. [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Bickel WK. Nicotine withdrawal versus other drug withdrawal syndromes: similarities and dissimilarities. Addiction. 1994;89:1461–1470. doi: 10.1111/j.1360-0443.1994.tb03744.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal: a replication and extension. Arch Gen Psychiatry. 1991b;48:52–59. doi: 10.1001/archpsyc.1991.01810250054007. [DOI] [PubMed] [Google Scholar]
- Hunt WA, Bespalec DA. An evaluation of current methods of modifying smoke behaviour. J Clin Psychol. 1974;30:431–438. doi: 10.1002/1097-4679(197410)30:4<431::aid-jclp2270300402>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Huston-Lyons D, Sarkar M, Kornetsky C. Nicotine and brain-stimulation reward: interactions with morphine, amphetamine and pimozide. Pharmacol Biochem Behav. 1993;46:453–457. doi: 10.1016/0091-3057(93)90378-7. [DOI] [PubMed] [Google Scholar]
- Isola R, Vogelsberg V, Wemlinger TA, Neff NH, Hadjiconstantinou M. Nicotine abstinence in the mouse. Brain Res. 1999;850:189–196. doi: 10.1016/s0006-8993(99)02131-9. [DOI] [PubMed] [Google Scholar]
- Ivanova S, Greenshaw AJ. Nicotine-induced decreases in VTA electrical self-stimulation thresholds: blockade by haloperidol and mecamylamine but not scopolamine or ondansetron. Psychopharmacology (Berl) 1997;134:187–192. doi: 10.1007/s002130050441. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther. 2008 doi: 10.1124/jpet.107.132977. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westervel M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Jonkman S, Henry B, Semenova S, Markou A. Mild anxiogenic effects of nicotine withdrawal in mice. Eur J Pharmacol. 2005;516:40–45. doi: 10.1016/j.ejphar.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Jonkman S, Risbrough VB, Geyer MA, Markou A. Spontaneous nicotine withdrawal potentiates the effects of stress in rats. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301607. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31:1203–1211. doi: 10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU. Euphorigenic drugs: Effects on the reward pathways of the brain. Fed Proc. 1979;38:2473–2476. [PubMed] [Google Scholar]
- Kumari V, Gray JA. Smoking withdrawal, nicotine dependence and prepulse inhibition of the acoustix startle reflex. Psychopharmacology (Berl) 1999;141:11–15. doi: 10.1007/s002130050800. [DOI] [PubMed] [Google Scholar]
- Lepicard EM, Joubert C, Hagneau I, Perez-Diaz F, Chapouthier G. Differences in anxiety-related behavior and response to diazepam in BALB/cByJ and C57BL/6J strains of mice. Pharmacol Biochem Behav. 2000;67:739–748. doi: 10.1016/s0091-3057(00)00419-6. [DOI] [PubMed] [Google Scholar]
- Logue SF, Owen EH, Rasmussen DL, Wehner JM. Assessment of locomotor activity, acoustic and tactile startle, and prepulse inhibition of startle in inbred mouse strains and F1 hybrids: implications of genetic background for single gene and quantitative trait loci analyses. Neuroscience. 1997;80:1075–1086. doi: 10.1016/s0306-4522(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Newlin-Maultsby P, Roberts LK, Lanier JG, Carter VA, Cunningham JS, Wilson OB. Rodent model of nicotine abstinence syndrome. Pharmacol Biochem Behav. 1992;43:779–784. doi: 10.1016/0091-3057(92)90408-8. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Upchurch TP, Shenoi M, Rajan N, Schweinle WE. Nicotine abstinence syndrome precipitated by the competitive nicotinic antagonist dihydro-β-erythroidine. Pharmacol Biochem Behav. 1998;60:609–613. doi: 10.1016/s0091-3057(98)00028-8. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav. 1992;51:111–119. doi: 10.1016/0031-9384(92)90211-j. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob G. Intracranial self-stimulation thresholds as a measure of reward. In: Sahgal A, editor. Behavioural Neuroscience: A Practical Approach. vol. 2. Oxford: IRL Press; 1993. pp. 93–115. [Google Scholar]
- Markou A, Paterson NE. The nicotinic antagonist methyllycaconitine has differential effects on nicotine self-administration and nicotine withdrawal in the rat. Nicotine Tob Res. 2001;3:361–373. doi: 10.1080/14622200110073380. [DOI] [PubMed] [Google Scholar]
- Mueller V, Mucha RF, Pauli P. Dependence on smoking and the acoustic startle response in healthy smokers. Pharmacol Biochem Behav. 1998;59:1031–1038. doi: 10.1016/s0091-3057(97)00508-x. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2nd edn. London: Academic Press; 2001. [Google Scholar]
- Paylor R, Crawley JN. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology (Berl) 1997;132:169–180. doi: 10.1007/s002130050333. [DOI] [PubMed] [Google Scholar]
- Petersen DR, Norris KJ, Thompson JA. A comparative study of the disposition of nicotine and its metabolites in three inbred strains of mice. Drug Metab Dispos. 1984;12:725–731. [PubMed] [Google Scholar]
- Pomerleau CS. Co-factors for smoking and evolutionary psychobiology. Addiction. 1997;92:397–408. [PubMed] [Google Scholar]
- Postma P, Kumari V, Sharma T, Hines M, Gray JA. Startle response during smoking and 24 h after withdrawal predicts successful smoking cessation. Psychopharmacology (Berl) 2001;156:360–367. doi: 10.1007/s002130100829. [DOI] [PubMed] [Google Scholar]
- Salas R, Main A, Gangitano D, De Biasi M. Decreased withdrawal symptoms but normal tolerance to nicotine in mice null for the α7 nicotinic acetylcholine receptor subunit. Neuropharmacology. 2007;53:863–869. doi: 10.1016/j.neuropharm.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the β4 nicotinic acetylcholine receptor subunit. J Neurosci. 2004;24:10035–10039. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova S, Bespalov A, Markou A. Decreased prepulse inhibition during nicotine withdrawal in DBA/2J is reversed by nicotine self-administration. Eur J Pharmacol. 2003a;472:99–110. doi: 10.1016/s0014-2999(03)01904-6. [DOI] [PubMed] [Google Scholar]
- Semenova S, Markou A. Clozapine treatment attenuated somatic and affective signs of nicotine and amphetamine withdrawal in subsets of rats exhibiting hyposensitivity to the initial effects of clozapine. Biol Psychiatry. 2003b;54:1249–1264. doi: 10.1016/s0006-3223(03)00240-3. [DOI] [PubMed] [Google Scholar]
- Shiffman SM, Jarvik ME. Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology (Berl) 1976;50:35–39. doi: 10.1007/BF00634151. [DOI] [PubMed] [Google Scholar]
- Skjei KL, Markou A. Effects of repeated withdrawal episodes, nicotine dose, and duration of nicotine exposure on the severity and duration of nicotine withdrawal in rats. Psychopharmacology (Berl) 2003;168:280–292. doi: 10.1007/s00213-003-1414-1. [DOI] [PubMed] [Google Scholar]
- Spielewoy C, Markou A. Strain-specificity in nicotine attenuation of phencyclidine-induced disruption of prepulse inhibition in mice: relevance to smoking in schizophrenia patients. Behav Genet. 2004;34:343–354. doi: 10.1023/B:BEGE.0000017878.75206.fd. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berl) 1995;117:2–10. doi: 10.1007/BF02245088. discussion 14–20. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Anxiogenic effects of high illumination levels assessed with the acoustic startle response in rats. Biol Psychiatry. 1997;42:461–471. doi: 10.1016/S0006-3223(96)00441-6. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Stinus L, Koob GF, Markou A. Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. J Pharmacol Exp Ther. 2000;292:1053–1064. [PubMed] [Google Scholar]
- Willott JF, Tanner L, O'Steen J, Johnson KR, Bogue MA, Gagnon L. Acoustic startle and prepulse inhibition in 40 inbred strains of mice. Behav Neurosci. 2003;117:716–727. doi: 10.1037/0735-7044.117.4.716. [DOI] [PubMed] [Google Scholar]
- Wise R, Marcangione C, Bauco P. Blockade of the reward-potentiating effects of nicotine on lateral hypothalamic brain stimulation by chlorisondamine. Synapse. 1998;29:72–79. doi: 10.1002/(SICI)1098-2396(199805)29:1<72::AID-SYN6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]


