Abstract
Trafficking of AMPA subtype glutamate receptors (AMPARs) from intracellular compartments to synapses is thought to be a major mechanism underlying the expression of long-term potentiation (LTP), a cellular substrate for learning and memory. However, it remains unclear whether the AMPAR trafficking that takes place during LTP is due to a targeted insertion of AMPARs directly into the synapse or delivery to extrasynaptic sites followed by translocation into the synapse. Here, we provide direct physiological evidence that LTP induced by a theta-burst pairing and tetanic stimulation protocols causes the rapid delivery of AMPARs to a perisynaptic site. Perisynaptic AMPARs do not normally detect synaptically released glutamate but can do so when the glial glutamate transporter EAAT1 is inhibited. AMPARs can be detected at this perisynaptic site before, but not after, the full expression of LTP. The appearance of perisynaptic AMPARs requires postsynaptic exocytosis, PKA signaling, and the C-terminal region of GluR1 subunit of AMPARs but not actin polymerization. Actin polymerization after LTP induction is required to retain AMPARs at the perisynaptic site after their appearance. Low-frequency stimulation given shortly after LTP induction leads to activity-dependent removal of perisynaptic AMPARs and suppresses the subsequent expression of LTP. These results demonstrate that AMPARs are rapidly trafficked to perisynaptic sites shortly after LTP induction and suggest that the delivery and maintenance of perisynaptic AMPARs may serve as a checkpoint in the expression of LTP.
Keywords: actin, dendritic spine, trafficking, TBOA, two-photon imaging
Activity-induced modification of neuronal connections is essential for the development of the nervous system and may underlie some forms of learning and memory. One widely examined form of synaptic plasticity is long-term potentiation (LTP). LTP leads to the synaptic insertion of glutamate receptors of the AMPA subtype (AMPARs) (1–4). Recent studies also suggest that this synaptic incorporation of AMPARs may stabilize spine modifications associated with LTP (5, 6). Postsynaptic exocytosis (7, 8), PKA activity (9), and AMPARs containing the GluR1 subunit (10) are implicated in the synaptic incorporation of AMPARs during LTP, but it remains unclear whether LTP leads to the direct insertion of AMPARs at the postsynaptic density (PSD) or to insertion at extrasynaptic regions followed by translocation into the PSD.
Consistent with the latter idea, AMPAR targeting from intracellular compartments to the cell surface can occur via mechanisms that are distinct from AMPAR targeting from the cell surface to the synapse (11). By visualizing the movement of transfected AMPAR subunits or specific domains of AMPAR subunits in culture, trafficking of AMPARs can be observed in response to LTP induced by synaptic activity or chemical induction protocols. Using this approach, studies have found that AMPARs containing GluR1 can be inserted into the plasma membrane from intracellular stores (2). Lateral mobility between extrasynaptic and synaptic regions on the cell surface has also been observed by using tagged AMPARs (12). These studies support a model in which AMPARs mobilized during LTP are first delivered to the extrasynaptic region and then translocate into the synapse.
However, it remains unclear whether similar mechanisms are involved in trafficking of native AMPARs in situ, in response to LTP induced by Hebbian induction protocols. Under these conditions, physiological approaches are a straightforward method to assess the function of synaptic AMPARs. However, extrasynaptic AMPARs are difficult to assess physiologically, because the methods of applying exogenous glutamate, such as iontophoresis or photolytic uncaging, lack the spatial resolution required to selectively probe AMPAR delivery to the extrasynaptic sites that are of the greatest interest, the perisynaptic regions immediately adjacent to the PSD.
An alternative approach to observe perisynaptic AMPARs would be to alter the spread of synaptically released glutamate so that it spills out of the synaptic cleft to engage these AMPARs. The spread of glutamate after exocytosis is normally limited by a family of excitatory amino acid transporters (EAAT1-5), so it might be possible to recruit perisynaptic AMPARs by blocking glutamate uptake. Using this approach, we provide physiological evidence that native AMPARs are delivered to perisynaptic sites in response to a Hebbian LTP induction protocol. The delivery of perisynaptic AMPARs precedes the full expression of LTP, and these AMPARs leave the perisynaptic site coincident with the development of synaptic potentiation. Manipulations that inhibit the delivery or maintenance of AMPARs at the perisynaptic site prevent the subsequent expression of LTP. Thus, our study identifies a previously uncharacterized perisynaptic site that is not sensitive to synaptically released glutamate under normal conditions, where AMPARs accumulate after LTP induction.
Results
AMPARs Are Inserted to Perisynaptic Sites in a NMDA Receptor (NMDAR)-Dependent Manner.
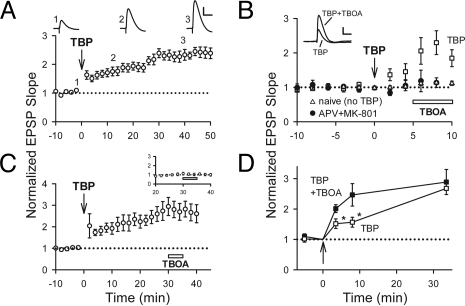
Pyramidal neurons in the CA1 region of acute hippocampal slices from young rats were recorded by using whole-cell patch–clamp, and synaptic responses were evoked by electrical stimulation of presynaptic inputs delivered through a glass microelectrode. We have shown previously that NMDAR-dependent LTP can be induced by pairing theta-burst stimulation (TBS) of presynaptic inputs with a modest postsynaptic depolarization, so that the excitatory postsynaptic potentials (EPSPs) during the burst are suprathreshold (5). This theta-burst pairing (TBP) is Hebbian in the sense that presynaptic bursts are temporally correlated with postsynaptic spikes, and this activity pattern is thought to mimic the firing patterns seen in hippocampus during behavioral learning in vivo (13). After TBP, there was an immediate increase in EPSPs followed by a gradual increase that developed over 15–30 min (Fig. 1A; EPSP slopes were 243 ± 17% of baseline at 45–50 min after TBP, n = 16). This slow development of LTP is frequently reported in studies that use TBP-based induction protocols (5, 14–16). The reason TBP-LTP is so slow to develop remains obscure but does not depend on activity after the induction protocol (5). This makes it unlikely that it is due to NMDAR or AMPAR activity after LTP induction, consistent with prior reports (17, 18).
Fig. 1.
Activity-dependent accumulation of AMPARs at a perisynaptic site. (A) LTP induced by TBP develops gradually. Sample traces (average of five EPSPs) were acquired at the times indicated. (B) TBOA enhanced EPSPs when applied shortly after TBP (squares) but not at naïve synapses (triangles). The enhancement by TBOA was blocked by APV+MK-801 (circles). Sample EPSPs are shown (Inset). (C) TBOA did not affect EPSPs 30 min after TBP. Inset shows the portion of responses around TBOA application (open bar), normalized to the average EPSP size for 5 min preceding TBOA application. (D) EPSPs in the presence of TBOA potentiated rapidly to a stable level after TBP (filled), whereas EPSPs in control conditions (TBP only) gradually reached the same level (open). *, P < 0.05. These data were from B and C. [Scale bars: 50 ms and 5 mV (A), 50 ms and 3 mV (B).]
We have shown that LTP induced by TBP is reduced by inhibiting postsynaptic exocytosis (5), consistent with the involvement of AMPAR insertion. The delivery of receptors to the spine surface is distinct from their synaptic incorporation (11, 19, 20), so we reasoned that the slow development of TBP-LTP might be due to rapid delivery of AMPARs to the spine surface but slow synaptic incorporation of AMPARs. If this were the case, a population of perisynaptic AMPARs should be present for several minutes after LTP induction but preceding the full expression of LTP. These perisynaptic AMPARs would be silent under normal conditions but might be recruited if synaptic glutamate were allowed to spill out of the synaptic cleft by blocking the glutamate uptake (21).
To test this idea, we examined whether the EPSP could be facilitated after TBP when glutamate uptake was blocked by DL-threo-β-benzyloxyaspartic acid (TBOA), a broad spectrum inhibitor of glutamate transporters (21). TBOA (100 μM) increased the size of EPSPs shortly after TBP (Fig. 1B; 230 ± 35%; n = 6; P < 0.05), but it had no effect on EPSPs from naive synapses (Fig. 1B; 111 ± 6%, n = 7). TBOA also had no effect, at least during short applications, on spine size or neuronal activity [supporting information (SI) Fig. S1]. When NMDARs were blocked by DL-2-amino-5-phosphonopentanoic acid (APV) plus (+)-MK-801 hydrogen maleate (MK-801) to inhibit LTP induction, TBOA had no effect on the EPSP shortly after TBP (Fig. 1B; 113 ± 14%, n = 6). Thus, TBP induces a NMDAR-dependent enhancement in EPSP that is observed only when glutamate uptake is impaired.
If TBOA allows us to monitor perisynaptic AMPARs that are coupled to the expression of LTP, we would expect that these AMPARs should be present in the perisynaptic region transiently. We would also expect that the EPSP slope in the presence of TBOA should remain constant as LTP develops. Consistent with these predictions, TBOA had no significant effect on the EPSP 30 min after TBP (Fig. 1C Inset; 114 ± 16%, n = 6), when LTP is fully developed. Moreover, in the presence of TBOA the slopes of EPSPs were similar at 30 sec, 5 min, or 30 min after TBP (Fig. 1D). Thus, our data are compatible with the notion that perisynaptic AMPARs translocate into the synapse over time; however, we cannot exclude the possibility that perisynaptic AMPARs are removed from the perisynaptic region to a nonsynaptic location over a time course that is similar to that underlying the synaptic delivery of a distinct set of AMPARs.
These findings are generally consistent with the idea that TBP induces the insertion of perisynaptic AMPARs that do not detect synaptically released glutamate unless glutamate uptake is impaired. However, an alternative is that TBP induces the insertion of AMPARs directly at the synapse but also up-regulates glutamate transporters. In this scenario, LTP would be expressed rapidly but suppressed shortly after TBP by enhanced uptake; the gradual “development” of LTP would be a slow loss of this suppression. LTP induction is not thought to alter glutamate uptake near the synaptic cleft, because synaptic transporter currents in astrocytes are unchanged after LTP (22, 23). However, it has been reported that bulk uptake of radiolabeled glutamate in CA1 is enhanced after LTP induction because of the increased function of EAAT3 shortly after induction and increased expression of EAAT2 at later periods (24, 25). Thus, if TBOA facilitates the EPSP via blockade of EAAT2 or EAAT3, this might be explained by increased function of EAATs rather than newly inserted AMPARs.
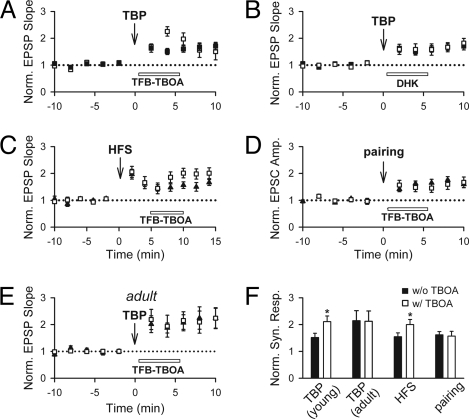
To test this idea, we defined the transporters that underlie the effect of TBOA with subtype-selective EAAT inhibitors. EAAT1-3 are expressed in the hippocampus, with EAAT1 and EAAT2 expressed primarily in astrocytes and EAAT3 in neurons (26). Thus, we can identify the EAAT(s) involved in the effect of TBOA by the use of TFB-TBOA, an analogue of TBOA that is selective for EAAT1/2, and dihydrokainic acid (DHK), a specific inhibitor of EAAT2. As was seen with TBOA, TFB-TBOA enhanced the EPSPs after TBP (Fig. 2A; 225 ± 18%, n = 8; P < 0.01 compared with control LTP) but had no effect on naïve synapses (SI Text and Fig. 2A); thus, the effect of TBOA can be effectively mimicked by inhibiting only EAAT1/2. In contrast, the specific EAAT2 inhibitor DHK did not cause a resolvable enhancement of the EPSP after TBP (Fig. 2B; 158 ± 21%, n = 7; P = 0.97). Thus, the effect of TBOA after TBP is mediated primarily, if not entirely, by EAAT1 and is unrelated to the previously reported increase in EAAT2/3 activity after LTP (SI Text, Note 1).
Fig. 2.
The enhancement of the EPSP is mediated by inhibition of EAAT1. (A) Inhibition of EAAT1/2 with TFB-TBOA yielded an enhancement in synaptic responses when added 30 seconds after TBP when compared with responses in the absence of TBOA (open symbols denote experiments with TBOA, filled symbols denote control experiments without TBOA). (B) Selective inhibition of EAAT2 with DHK had little effect on the EPSP. (C) TFB-TBOA enhanced EPSPs when added 5 min after HFS. (D) TFB-TBOA had little effect on EPSCs when added 30 sec after voltage pairing. (E) TFB-TBOA had little effect on EPSPs when added 30 sec after TBP in adult hippocampal neurons. (F) Summary of the effects of TFB-TBOA on synaptic responses under different LTP induction protocols. *, P < 0.05.
The properties of LTP may vary depending on the induction protocol used to induce LTP. To address whether the above findings on the perisynaptic AMPARs can be generalized to other LTP induction protocols, we induced LTP with high-frequency tetanic stimulation (HFS). One train of HFS (100 Hz, 300 ms) induced a posttetanic potentiation (PTP) followed by LTP (SI Text, Fig. 2B). TFB-TBOA enhanced EPSPs when added 30 sec after HFS (data not shown). However, it was difficult to be certain whether this was associated with LTP or with the robust PTP that was also induced by HFS. We therefore repeated this experiment with TFB-TBOA applied 5 min after HFS, when PTP has largely decayed. Under these circumstances, TFB-TBOA still significantly enhanced the EPSP (Fig. 2C; 201 ± 18%, n = 7; P < 0.05 compared with that without TFB-TBOA). Furthermore, TFB-TBOA had little effect on EPSPs when added 30 min after HFS (188 ± 18%, n = 7; P = 0.77 compared with that without TFB-TBOA).
We next examined whether the effect of TFB-TBOA is observed in response to LTP induced by pairing presynaptic stimulation with sustained postsynaptic depolarization in cells held in voltage clamp (27). LTP under these circumstances was rapidly expressed and reached a plateau almost immediately after pairing (Fig. S2C; ref. 28). TFB-TBOA had no effect on EPSCs when applied 30 sec (Fig. 2D) or 5 min (data not shown) after pairing.
We also examined whether the appearance of perisynaptic AMPARs after LTP is developmentally regulated, by repeating TBP induction in hippocampal slices taken from adult (3- to 4-month-old) rats. TFB-TBOA had no effect on EPSPs when added 30 sec after TBP (Fig. 2E; 206 ± 38%, n = 6; P = 0.87 compared with that without TFB-TBOA). The gradual enhancement in EPSPs after TBP was also absent under these conditions (Fig. S2D). A summary of the above results is shown in Fig. 2F.
Thus, the ability of TBOA to recruit AMPARs after LTP induction depends on the induction protocol and the maturational state of the synapses. We note with interest that the effect of TBOA is absent after induction protocols that unambiguously lead to a rapid development of LTP (TBP in slices from adult animals and pairing in slices from young animals) and present after induction protocols in which the time course over which LTP develops is gradual (TBP in slices from young animals).
Delivery of Perisynaptic AMPARs.
If AMPAR trafficking to the perisynaptic region is due to mobilization from intracellular compartments, then we would expect that the TBOA-induced facilitation of the EPSP after TBP should occur via postsynaptic exocytosis. To test this, we loaded postsynaptic neurons with the light chain of botulinum toxin (BoTox; 0.5 μM), a neurotoxin that prevents vesicle fusion. This toxin prevents the full expression of LTP (Fig. S3; ref. 5), and application of TBOA 30 sec after TBP had no effect in neurons loaded with BoTox [Fig. 3A; 103 ± 15%, n = 8 (TBOA); 104 ± 7% (no TBOA; Fig. S3)]. Heat-inactivated BoTox did not prevent LTP (Fig. S3) or the effect of TBOA after TBP (Fig. 3A).
Fig. 3.
Accumulation of perisynaptic AMPARs requires postsynaptic exocytosis, PKA signaling, and the C-terminal of GluR1 subunit. (A) TBOA did not enhance EPSPs after TBP in BoTox-loaded neurons (filled) but did in neurons loaded with heat-inactivated BoTox (open). (B) TBOA also had no effect on the EPSP after TBP in neurons loaded with PKI (open), compared with control experiments in the absence of TBOA (filled). (C) Postsynaptic loading of the pep-1 peptide abolished the effect of TBOA (open), compared with control experiments in the absence of TBOA (filled).
Various protein kinases have been shown to be involved in the synaptic incorporation of AMPARs (29–32). However, PKA is of particular interest in the delivery of AMPARs to extrasynaptic regions, because phosphorylation of the PKA site of GluR1 has been suggested to drive the delivery of AMPARs to extrasynaptic sites (31, 32). To test whether PKA is required for the delivery of perisynaptic AMPARs, we loaded the postsynaptic neurons with the peptide inhibitor PKI (20 μM) (33). PKI also led to a significant reduction in LTP (Fig. S4; ref. 5) and blocked the effect of TBOA [Fig. 3B; 139 ± 19%, n = 6 (TBOA); 138 ± 25% (control)].
The above results are consistent with the notion that delivery of AMPARs to the perisynaptic site underlies the expression of LTP. However, because BoTox and PKI are likely to affect many targets other than AMPARs, we next used a more selective manipulation. Several studies have implicated the GluR1 subunit in delivery or retention of AMPARs at the extrasynaptic surface (31, 34), and it has been shown that delivery of GluR1-containing AMPARs to synapses during LTP requires protein–protein interactions with the C-terminal region of the GluR1 subunit (35, 36). To see whether the delivery of AMPARs to the perisynaptic region would occur if this interaction was prevented, we loaded pyramidal neurons with a synthetic peptide (pep-1) corresponding to the C-terminal region of the GluR1 subunit. Pep-1 has been shown to block LTP when loaded into postsynaptic neurons (36), presumably by acting as a pseudosubstrate that competes for the binding targets of the GluR1 C terminus. Consistent with previous reports, we found that postsynaptic loading of pep-1 reduced the magnitude of LTP (Fig. S5A). Importantly, it also rendered TBOA ineffective [Fig. 3C; 113 ± 5%, n = 10 (TBOA); 114 ± 11% (control)], indicating that pep-1 prevented the appearance of AMPARs at the perisynaptic site. Pep-1 did not affect either basal synaptic transmission or NMDAR-mediated responses (Fig. S5 B and C).
The impairments in LTP in the BoTox, PKI, or pep-1 experiments were not due to the longer baseline recording (15 min) used to load a sufficient amount of toxin or peptide, because LTP was not affected in control neurons with the same long baseline recording (5).
Actin Polymerization Is Required to Maintain Perisynaptic AMPARs.
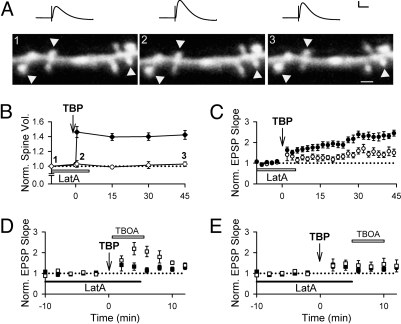
Synaptic plasticity during TBP is associated with a rapid expansion of the spine head that persists for >30 min (5). Several studies implicate actin in spine dynamics (37), and changes in the actin cytoskeleton take place with the induction of LTP (38–41). Because actin is also thought to act as a tether for AMPARs via interactions with cytoskeletal binding proteins (42, 43), changes in the cytoskeleton might also be important for trafficking of perisynaptic AMPARs. We therefore prevented the changes in the actin cytoskeleton that occur during TBP by using latrunculin A (latA, 0.1 μM) at a concentration that is sufficient to prevent actin polymerization without leading to substantial depolymerization (39). As expected, spine expansion was abolished when latA was bath-applied before and during TBP (Fig. 4 A and B; 102 ± 2%, n = 77 spines/8 cells; ref. 41), compared with a robust expansion of the spines in control neurons (Fig. 4B; 142 ± 6% at 45 min after TBP; n = 97 spines/10 cells; ref. 5). The presence of latA also significantly impaired the expression of LTP (Fig. 4 A and C; 153 ± 14%, n = 6). LatA had no effect on AMPAR EPSPs, NMDAR EPSPs, or spine volume in naïve synapses (Fig. S6). Thus, latA selectively prevents the cytoskeletal reorganization that takes place during LTP.
Fig. 4.
Actin polymerization is not required for the delivery of perisynaptic AMPARs. (A) A sample experiment in which both EPSPs and spine morphology were monitored simultaneously. Perfusion of latA before and during TBP abolished expansion of the spines (arrowheads). Sample EPSPs are shown on top of the images. (B) Population data showed that latA abolishes spine expansion (open), in contrast to the robust spine expansion observed in control neurons (filled). (C) LTP was impaired when TBP was delivered in the presence of latA (open), compared with the LTP in control neurons (filled). (D) TBOA enhanced synaptic responses when added 30 sec after TBP in the latA-treated neurons (open). Responses from control neurons without TBOA are shown in filled symbols. This enhancement was absent when TBOA was added 5 min after TBP (E; open), when compared with responses from control neurons without TBOA (E; filled). [Scale bars: 1 μm, 50 ms and 5 mV (A).]
Are AMPARs still delivered to the perisynaptic space in the presence of latA? Incubation of slices with latA before LTP induction did not affect the TBOA-induced enhancement when TBOA was applied 30 sec after TBP (Fig. 4D; 204 ± 38%, n = 6; P = 0.59 compared with control TBOA experiments). However, when TBOA was added 5 min after TBP, it no longer had any effect on the EPSP (Fig. 4E; 137 ± 25%, n = 8; P < 0.05 compared with control TBOA experiments), in marked contrast to our findings with untreated slices. Thus, AMPARs are delivered to the perisynaptic region even though latA is present; however, in the presence of latA, these AMPARs are not retained in this region.
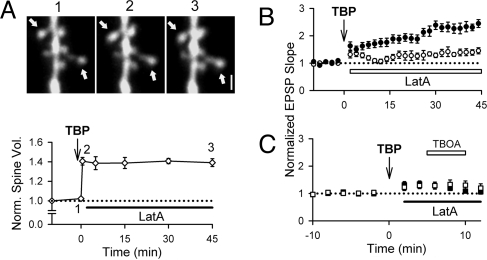
The experiments with latA demonstrate that AMPAR trafficking after TBP is dissociable from spine expansion (6, 44), because TBOA enhances the EPSP even in the absence of spine expansion. This has two implications. First, it means that the effect of TBOA cannot be due to changes in the extracellular space caused by spine expansion. Second, it suggests that LTP might be blocked even in the presence of persistent structural plasticity, if latA was applied after the spine expansion but before the full expression of LTP. To test this, we added latA 2 min after TBP. Spine expansion was persistent even in the continued presence of latA (Fig. 5A; 139 ± 4%; n = 67 spines/7 cells), but LTP was significantly reduced (Fig. 5B; 142 ± 12%, n = 9). The TBOA-induced synaptic enhancement was absent when TBOA was added 5 min after TBP [Fig. 5C; 120 ± 7%, n = 13 (TBOA); 128 ± 9% (no TBOA)]. Because perisynaptic AMPARs are present 30 sec after TBP (Fig. 1D), this confirms that latA prevented the retention of AMPARs in the perisynaptic region even when applied after TBP. These results indicate that spine expansion is not necessary to deliver AMPARs to the perisynaptic region, nor is it sufficient to retain AMPARs in the perisynaptic region or allow LTP expression.
Fig. 5.
Persistent actin polymerization is required for the maintenance of perisynaptic AMPARs. (A) latA applied 2 min after TBP did not disrupt the spine expansion that took place immediately after TBP, as shown in sample images (Upper; expanded spines shown with arrows) and population data (Lower). (B) LatA applied 2 min after TBP greatly reduced LTP, even though the spine expansion persisted. (C) TBOA did not enhance synaptic transmission when added at 5 min after TBP (open), compared with control neurons in the absence of TBOA (filled). [Scale bar: 1 μm (A).]
Activity-Dependent Removal of Perisynaptic AMPARs.
Our results with latA suggest that the retention of AMPARs in the perisynaptic region can be disrupted pharmacologically, but we wondered whether this process could be engaged physiologically. Moderate synaptic activity can reverse LTP when given shortly after LTP induction (45, 46), and low-frequency stimulation (LFS; 1 Hz, 5 min) suppresses the gradual expression of LTP if applied shortly (≤15 min) after TBP (5). This suppression requires activation of protein phosphatase-1/2A (PP-1/2A) and does not occur at naïve synapses (5). If LTP involves the translocation of perisynaptic AMPARs into the synapse, then LFS might suppress LTP by causing the loss of perisynaptic AMPARs before their synaptic incorporation.
To test whether LFS removes perisynaptic AMPARs, LFS was delivered at 30 sec after TBP. LFS prevented the expression of LTP (Fig. 6A; 123 ± 9%, n = 7), as expected. It also abolished the TBOA-induced synaptic enhancement [Fig. 6B; 105 ± 14%, n = 7 (TBOA); 99 ± 9% (no TBOA)], suggesting that perisynaptic AMPARs are subject to activity-dependent removal. To address whether PP-1/2A mediates this removal, we loaded pyramidal neurons with okadaic acid (OA, 1 μM), a PP-1/2A inhibitor. Both LTP (Fig. 6C; 206 ± 17%, n = 6) and the enhancement of the EPSP by TBOA [Fig. 6D; 207 ± 38%, n = 4 (TBOA); 128 ± 26% (no TBOA)] were unaffected by LFS in these neurons, confirming that both processes require PP-1/2A.
Fig. 6.
LFS removes perisynaptic AMPARs and suppresses the expression of LTP. (A) LFS suppressed the expression of LTP when delivered to synaptic inputs 30 sec after TBP (open), compared with LTP in control neurons (filled). (B) LFS also abolished the TBOA-induced enhancement of EPSPs (open), compared with control neurons in the absence of TBOA (filled). (C) In neurons loaded with okadaic acid (OA), LTP was no longer suppressed by LFS after TBP. (D) TBOA enhanced EPSPs after LFS in neurons loaded with OA (open), compared with control neurons loaded with OA but without the addition of TBOA (filled). (E) Summary of synaptic responses in the presence of TBOA under various conditions. Comparisons were made to the responses after TBP in control neurons (second open/filled bar from left, “TBP”). The results were separated into two groups: TBOA added at 30 sec (open bars), or 5 min (filled bars) after TBP. *, <0.05; **, <0.01.
Discussion
Our study suggests that TBP and HFS cause the transient appearance of AMPARs in a perisynaptic region via a mechanism that requires postsynaptic exocytosis and PKA but not actin polymerization or spine expansion. It is likely that these perisynaptic AMPARs contain GluR1 subunits, because the delivery of AMPARs to the perisynaptic region is also blocked by pseudosubstrate peptides mimicking the GluR1 C-terminal; however, pep-1 could interfere with trafficking of other proteins that use the same binding motif. AMPARs are maintained at the perisynaptic sites for 10–15 min and disappear from these sites with a time course that matches the gradual development of LTP. The retention of AMPARs at the perisynaptic site can be disrupted by preventing actin polymerization and by moderate synaptic activity. A summary of these results is shown in Fig. 6E.
Previous studies that assess receptor movement biochemically or using epitope-tagged receptors have indicated that GluR1-containing AMPARs are delivered to extrasynaptic sites (31, 32, 34), and extrasynaptic AMPARs can be activated using exogenous agonists (47, 48). However, our study defines the trafficking of native AMPARs around the perisynaptic region in response to activity-dependent LTP. The term “perisynaptic” here is defined functionally, because the postsynaptic area in which AMPARs would detect synaptically released glutamate if not for uptake mechanisms. The physical scale of this region is difficult to define precisely but is likely to be in the submicron range based on estimates of extracellular tortuosity and diffusion (49, 50).
Our use of uptake blockers to monitor perisynaptic AMPARs implicitly assumes that the spread and uptake of synaptically released glutamate are both constant. Synaptic uptake currents on astrocytes are not altered with LTP (22, 23), but it has been proposed that LTP can induce increased uptake of bulk glutamate via EAAT2 and EAAT3 (24, 25). The effect of TBOA that we have observed does not require either of these transporters, ruling out the findings of refs. 24 and 25 as an explanation for our results. The effect of TBOA also does not appear to be due to changes in the extracellular geometry associated with LTP, because it persists even when the spine expansion that normally occurs with LTP is blocked.
Our results are compatible with recent models in which AMPAR trafficking during LTP occurs in two steps: delivery to the surface membrane outside of the synapse, followed by translocation into the PSD (Fig. S7; refs. 3–5 in SI Text). The properties of perisynaptic AMPARs as assessed with TBOA match those expected if these same receptors translocate into the synapse: the perisynaptic AMPARs are present transiently, and disappear during the same time window in which LTP develops after TBP. Moreover, every manipulation that we have examined that disrupts delivery or retention of perisynaptic AMPARs also prevents the eventual expression of LTP. However, it remains to be seen whether the same AMPARs that are inserted perisynaptically also translocate into the synapse.
We have also found that the maintenance of AMPARs in the perisynaptic region can be disrupted. Extrasynaptic AMPARs are thought to be highly mobile in the plasma membrane (12), suggesting that AMPARs must be restricted to the perisynaptic region if they are to persist there for several minutes. Actin polymerization is critical for this maintenance of perisynaptic AMPARs, and LFS can drive their removal from the perisynaptic region. The LFS used here to drive the removal of perisynaptic AMPARs is less intense than that required to induce the removal of synaptic AMPARs during LTD (ref. 6 in SI Text), suggesting that perisynaptic AMPARs are more vulnerable to mobilization than those anchored in the PSD.
The ability of TBOA to recruit perisynaptic AMPARs after LTP is sensitive to both the induction protocol used and the developmental stage of the animal. In slices from young animals, recruitment of perisynaptic AMPARs occurs after TBP and HFS but not after a pairing protocol; in slices from adult animals, this recruitment could not be elicited even after TBP. We note with interest that when LTP is expressed gradually, perisynaptic AMPARs are present for >5 min after LTP induction. In contrast, the perisynaptic AMPARs are not present during this time window when LTP develops rapidly after induction. It is difficult to assess whether a similar correlation is observed during LTP induced by HFS because of the concomitant induction of PTP.
For those conditions in which LTP induction is rapid and not associated with an effect of TBOA, two alternative situations are compatible with our data. It might be that AMPARs are delivered to the perisynaptic region but incorporated into synapses too quickly for us to detect using TBOA. Alternatively, AMPARs might be inserted directly into the postsynaptic density without trafficking through the perisynaptic region via a distinct trafficking mechanism. It is difficult to distinguish between these alternatives at present, because little is known about the process of AMPAR translocation to synapses from perisynaptic sites.
In summary, we have provided direct physiological evidence that the induction of LTP is associated with the delivery of AMPARs to a perisynaptic region, and we have defined mechanisms that regulate the delivery and maintenance of AMPARs at this perisynaptic site. Our results suggest that the trafficking of AMPARs through the perisynaptic region may be an important intermediate step during the development of LTP.
Materials and Methods
Slice Preparation and Recordings.
Hippocampal slices were obtained from 13- to-18-day-old rat pups (Sprague–Dawley). Preparation and recordings were done after standard procedures described in SI Text, Note 2.
Image Acquisition and Analysis.
Imaging was performed on a two-photon laser scanning system modified from an Olympus Fluoview FV 300, driven by a Chameleon two-photon laser (Coherent) tuned to 810 nm. Emitted fluorescence was collected by two photomultiplier tubes (PMTs) and summed electronically. Laser power at the entry of the microscope was 30–40 MW and monitored continuously. Imaging and analysis procedures were based on our previous works (ref. 6 in SI Text, Note 3; ref. 5).
Statistical Analysis.
All data were expressed as mean ± SEM. Paired Student's t tests, Wilcoxon's signed ranks tests, and Wilcoxon's rank sum tests were used as appropriate. P < 0.05 was used to determined significance.
Supplementary Material
Acknowledgments.
We thank Drs. P. Adams, R. Blitzer, G. Huntley, and M.-m. Poo for comments on an earlier version of the manuscript. Q.Z. is supported by grants from the Whitehall Foundation and Ellison Medical Foundation, and M.F. is supported by the National Institutes of Health (Grants NS045101, MH077120, and AG026051).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802978105/DCSupplemental.
References
- 1.Bliss TV, Collingride GL. A synaptic model of memory: Long- term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 2.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 3.Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 4.Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y, Wang XB, Frerking M, Zhou Q. Spine expression and stabilization associated with long-term potentiation. J Neurosci. 2008;28:5740–5751. doi: 10.1523/JNEUROSCI.3998-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopec CD, Real E, Kessels HW, Malinow R. GliuR1 links structural and functional plasticity at excitatory synapses. J Neurosci. 2007;27:13706–13718. doi: 10.1523/JNEUROSCI.3503-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lledo PM, Zhang X, Sudhof TC, Malenka RC, Nicoll RA. Postsynaptic membrane fusion and long-term potentiation. Science. 1998;279:399–403. doi: 10.1126/science.279.5349.399. [DOI] [PubMed] [Google Scholar]
- 8.Lu W, et al. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 9.Esteban JA, et al. PKA phosphorylation of AMPA receptors subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- 10.Shi SH, et al. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, et al. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- 12.Triller A, Choquet D. Surface trafficking of receptors between synaptic and extrasynaptic membranes: And yet they do move! Trends Neurosci. 2005;28:133–139. doi: 10.1016/j.tins.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Otto T, Eichenbaum H, Wiener SI, Wible CG. Learning-related patterns of CA1 spike trains parallel stimulation parameters optimal for inducing hippocampal long-term potentiation. Hippocampus. 1991;1:181–192. doi: 10.1002/hipo.450010206. [DOI] [PubMed] [Google Scholar]
- 14.Magee JC, Johnston D. A synaptically controlled, associated signal for Hebbian plasticity in hippocampal neurons. Science. 1997;275:209–213. doi: 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- 15.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation. Proc Natl Acad Sci USA. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pike FG, Meredith RM, Olding AW, Paulsen O. Rapid report: Postsynaptic bursting is essential for “Hebbian” induction of associative long- term potentiation at excitatory synapses in rat hippocampus. J Physiol. 1999;518:571–576. doi: 10.1111/j.1469-7793.1999.0571p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adesnik H, Nicoll RA. Conservation of glutamate receptor 2-containing AMPA receptors during long-term potentiation. J Neurosci. 2007;27:4598–4602. doi: 10.1523/JNEUROSCI.0325-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gray EE, Fink AE, Sariñana J, Vissel B, O'Dell TJ. Long-term potentiation in the hippocampal CA1 region does not require insertion and activation of GluR2-lacking AMPA receptors. J Neurophysiol. 2007;98:2488–2492. doi: 10.1152/jn.00473.2007. [DOI] [PubMed] [Google Scholar]
- 19.Zamanillo D, et al. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284:1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]
- 20.Adesnik H, Nicoll RA, England PM. Photoinactivation of native AMPA receptor reveals their real-time trafficking. Neuron. 2005;48:977–985. doi: 10.1016/j.neuron.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 21.Diamond JS. Neuronal glutamate transporters limit activation of NMDA receptors by neurotransmitter spillover on CA1 pyramidal cells. J Neurosci. 2001;21:8328–8338. doi: 10.1523/JNEUROSCI.21-21-08328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lüscher C, Malenka RC, Nicoll RA. Monitoring glutamate release during LTP with glial transporter currents. Neuron. 1998;21:435–441. doi: 10.1016/s0896-6273(00)80552-8. [DOI] [PubMed] [Google Scholar]
- 23.Diamond JS, Bergles DE, Jahr CE. Glutamate release monitored with astrocyte transporter currents during LTP. Neuron. 1998;21:425–433. doi: 10.1016/s0896-6273(00)80551-6. [DOI] [PubMed] [Google Scholar]
- 24.Levenson J, et al. Long-term potentiation and contextual fear conditioning increase neuronal glutamate uptake. Nat Neurosci. 2002;5:155–161. doi: 10.1038/nn791. [DOI] [PubMed] [Google Scholar]
- 25.Pita-Almenar JD, Collado MS, Colbert CM, Eskin A. Different mechanism exist for the plasticity of glutamate reuptake during early long-term potentiation (LTP) and late LTP. J Neurosci. 2006;26:10461–10471. doi: 10.1523/JNEUROSCI.2579-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tzingounis AV, Wadiche JI. Glutamate transporters: Confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci. 2007;8:935–947. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]
- 27.Manabe T, Wyllie DJA, Perkel DJ, Nicoll RA. Modulation of Synaptic Transmission and Long-Term Potentiation: Effects on Paired Facilitation and EPSC Variance in the CA1 Region of the Hippocampus. J Neurophysiol. 1993;70:1451–1459. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- 28.Lûthi A, et al. Bi-directional modulation of AMPA receptor unitary conductance by synaptic activity. BMC Neurosci. 2004;5:44. doi: 10.1186/1471-2202-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayashi Y, et al. Driving AMPA receptors into synapses by LTP and CaMKII: Requirement for DluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- 30.Boehm J, et al. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–225. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- 32.Gao C, Sun X, Wolf ME. Activation of D1 dopamine receptors increases surface expression of AMPA receptors and facilitates their synaptic incorporation in cultured hippocampal neurons. J Neurochem. 2006;98:1664–1677. doi: 10.1111/j.1471-4159.2006.03999.x. [DOI] [PubMed] [Google Scholar]
- 33.Duffy SN, Nguyen PV. Postsynaptic application of a peptide inhibitor of cAMP-dependent protein kinase blocks expression of long-lasting synaptic potentiation in hippocampal neurons. J Neurosci. 2003;23:1142–1150. doi: 10.1523/JNEUROSCI.23-04-01142.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- 35.Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- 36.Toyoda H, Wu LJ, Zhao MG, Xu H, Zhuo M. Time-dependent postsynaptic AMPA GluR1 receptor recruitment in the cingulate synaptic potentiation. Dev Neurobiol. 2007;67:498–509. doi: 10.1002/dneu.20380. [DOI] [PubMed] [Google Scholar]
- 37.Matus A. Actin-based plasticity in dendritic spines. Science. 2000;290:754–758. doi: 10.1126/science.290.5492.754. [DOI] [PubMed] [Google Scholar]
- 38.Kim CH, Lisman JE. A role of actin filament in synaptic transmission and long-term potentiation. J Neurosci. 1999;19:4314–4324. doi: 10.1523/JNEUROSCI.19-11-04314.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krucker T, Siggins GR, Halpain S. Dynamic actin filaments are required for stable long-term potentiation (LTP) in area CA1 of the hippocampus. Proc Natl Acad Sci USA. 2000;97:6856–6861. doi: 10.1073/pnas.100139797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okamoto K, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci. 2004;7:1104–1112. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- 41.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen L, Liang F, Walensky LD, Huganir RL. Regulation of AMPA Receptor GluR1 Subunit Surface Expression by a 4.1N-Linked Actin Cytoskeletal Association. J Neurosci. 2000;20:7932–7940. doi: 10.1523/JNEUROSCI.20-21-07932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schulz TW, et al. Actin/alpha-actinin-dependent transport of AMPA receptors in dendritic spines: Role of the PDZ-LIM protein RIL. J Neurosci. 2004;24:8584–8594. doi: 10.1523/JNEUROSCI.2100-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kopec CD, Li B, Wei W, Boehm J, Malinow R. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J Neurosci. 2006;26:2000–2009. doi: 10.1523/JNEUROSCI.3918-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larson J, Xiao P, Lynch G. Reversal of LTP by theta frequency stimulation. Brain Res. 1993;600:97–102. doi: 10.1016/0006-8993(93)90406-d. [DOI] [PubMed] [Google Scholar]
- 46.O'Dell TJ, Kandel ER. Low-frequency stimulation erases LTP through an NMDA receptor-mediated activation of protein phosphatases. Learn Mem. 1994;1:129–139. [PubMed] [Google Scholar]
- 47.Bagal AA, Kao JP, Tang CM, Thompson SM. Long-term potentiation of exogenous glutamate responses at single dendritic spines. Proc Natl Acad Sci USA. 2005;102:14434–14439. doi: 10.1073/pnas.0501956102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andrásfalvy BK, Magee JC. Changes in AMPA receptor currents following LTP induction on rat CA1 pyramidal neurones. J Physiol. 2004;559:543–554. doi: 10.1113/jphysiol.2004.065219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rusakov DA, Kullmann DM. A tortuous and viscous route to understanding diffusion in the brain. Trends Neurosci. 1998;21:469–470. doi: 10.1016/s0166-2236(98)01309-5. [DOI] [PubMed] [Google Scholar]
- 50.Kullmann DM, et al. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: Where and why? Prog Biophys Mol Biol. 2005;87:33–46. doi: 10.1016/j.pbiomolbio.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.