Abstract
How proteins assemble into sarcomeric arrays to form myofibrils is controversial. Immunostaining and transfections of cultures of cardiomyocytes from 10-day avian embryos led us to propose that assembly proceeded in three stages beginning with the formation of premyofibrils followed by nascent myofibrils and culminating in mature myofibrils. However, premyofibril and nascent myofibril arrays have not been detected in early cardiomyocytes examined in situ in the forming avian heart suggesting that the mechanism for myofibrillogenesis differs in cultured and uncultured cells. To address this question of in situ myofibrillogenesis, we applied non-enzymatic procedures and deconvolution imaging techniques to examine early heart forming regions in situ at 2- to 13-somite stages (beating begins at the 9-somite stage), a time span of about 23 hours. These approaches enabled us to detect the three myofibril stages in developing hearts supporting a three-step model of myofibrillogenesis in cardiomyocytes, whether they are present in situ, in organ cultures or in tissue culture. We have also discovered that before titin is organized the first muscle myosin filaments are about half the length of the 1.6µm filaments present in mature A-bands. This supports the proposal that titin may play a role in length determination of myosin filaments.
Keywords: Myofibrillogenesis, non-muscle myosin IIB, Myofibril, alpha-actinin, titin, myosin, A-bands, premyofibril, nascent myofibril, mature myofibril
Introduction
The first muscle to assemble in avian and mammalian embryos appears in cells destined to form the heart. Early in embryogenesis, muscle-specific structural proteins are expressed in precardiac mesoderm cells, and in a series of reactions, the proteins are assembled into a contractile apparatus composed of myofibril arrays that supply the force needed to direct blood throughout the forming embryo. The steps in this assembly process are not understood in detail, but in broad terms they involve filament formation, association of binding proteins with filaments, and integration of filaments into contractile subunits that are linked in series and in parallel to create myofibril arrays (Sanger et al., 2004; 2005; Stout et al., 2008). In vertebrates each contractile subunit or sarcomere, is about two microns at rest length, bounded by Z-bands that serve as embedding sites for thin filaments and titin molecules, both of which interact with thick filaments, that are aligned laterally in uniform elongated blocks termed A-bands. The thin and thick filaments consist of filament-forming proteins, actin and myosin, respectively, and proteins that bind to them: nebulins, tropomyosin and troponins bind actin; C-Protein, myomesin, and creatine kinase bind myosin. The giant molecule, titin, spans half the sarcomere from Z-band to the middle of the A-band. Proteins of the Z-band, the most prominent of which is α-actinin, provide a scaffold for integrating the sarcomeres with the plasma membrane and with the signaling molecules localized at this sarcomere-membrane juncture (Clark et al., 2002).
In the search to understand how these protein complexes are assembled into contractile units in cardiomyocytes, substantial effort has been focused on detecting the subcellular sites where individual proteins are initially expressed, and determining the initial localization of the proteins with respect to one another. The picture that has emerged from these studies is one of coordinate expression of the major sarcomeric proteins of the forming myofibril with temporally and spatially distinct association of particular proteins into sub-sarcomeric complexes that become integrated into full sarcomeres (Sanger et al., 2004; 2005; Wang et al., 2007 Stout et al., 2008). Resolving the temporal sequence of sarcomeric protein interactions has been a long-term challenge that has relied on antibody specificity and sensitivity, selection of myofibril-forming cells for observations, and microscopic resolution of the assembling complexes. In the selection of cells, avian material has been the most widely studied vertebrate model system for its ease of manipulation in situ, in explant cultures, and in cultures of dissociated embryonic cardiac tissue. The widespread similarity in composition and structure of myofibrils across vertebrate species suggests that the basic assembly process in avian cells is likely to be universal in other vertebrates (Sanger et al., 2005).
Currently there is a lack of consensus on how sarcomeric proteins assemble into myofibrils in avian cardiomyocytes (Sanger et al., 2005). In broad terms, there is disagreement on whether the two main sarcomeric subunits, A-bands and I-Z-I-bands, assemble in tandem on a temporary stress fiber-like template (Dlugosz et al., 1984); or whether they assemble independently of one another and subsequently interdigitate to create a sarcomere (Holtzer et al., 1997; Gregorio and Antin, 2000); or whether myofibrillogenesis proceeds as a transition through three categories of fibrils: premyofibrils containing non-muscle myosin II, nascent myofibrils containing both non-muscle myosin II and muscle specific myosin II, and mature myofibrils containing the muscle specific myosin II and no non-muscle myosin II (Rhee et al., 1994; Du et al., 2003; Wang et al., 2005b). The strongest data currently against the premyofibril model for myofibrillogenesis are the immunostaining results obtained from hearts of avian embryos fixed in situ at the eight- to twelve-somite stages (Ehler et al., 1999) in which no evidence for premyofibrils or even localized non-muscle myosin II was found in the eight-somite embryos, and the first sarcomeric band spacings of alpha-actinin were the adult length (about two microns) and not the shorter spacings reported for premyofibrils (Rhee et al., 1994; Du et al., 2003).
In order to resolve these conflicting views on myofibrillogenesis, we have used non-enzymatic fixation methods on two- to thirteen-somite quail hearts in situ (Manasek, 1968), and used deconvolution microscopy for better detection of details in the cardiomyocytes in the forming heart tissue. We also used the same methods on cultured cardiomyocytes isolated from 10-day old quail embryos to compare the two different preparations. We present evidence that premyofibrils are present in the forming heart at the 5-somite stage with minisarcomeres containing Z-bodies stained with sarcomeric alpha-actinin antibodies intercalated with bands of non-muscle myosin II. Nascent myofibrils containing periodic bands of non-muscle myosin II and overlapping arrays of muscle specific myosin adjacent to these fibers were detected by the 6-somite stage. By the 9-somite stage, when cardiac contractions first occurred in the intact heart, A-bands in mature myofibrils in the cardiomyocytes contained only the muscle specific myosin II. Deconvolution microscopy of the spreading edges of older cultured cardiomyocytes revealed premyofibrils whose spacings of sarcomeric alpha-actinin and non-muscle myosin IIB were identical to those measured in the first cardiomyocytes in situ. These results are consistent with a premyofibril model of myofibrillogenesis, whether the process occurs in situ, in cardiac explants (Du et al., 2003), or in tissue culture (Rhee et al., 1994; Dabiri et al., 1997).
Materials and methods
Preparation of embryos and isolated cardiomyocytes
Fertilized quail eggs (Coturnix, Japanese Quail) were purchased from CBT Farms (Chestertown, MD). Between 150 and 200 embryos were incubated in situ, i.e., inside the egg, and isolated at intervals covering the first 40 hrs. after fertilization. The embryos were staged by observation of the numbers of somite pairs and were fixed from the 2-somite (pairs) stage through the 13-somite stage, a time span of approximately 23 hours. Over this time, mesoderm condensed into paired heart rudiments (1–4 somite pairs) that fused into a single heart tube (5−7 somite pairs) that began beating (9-somite stage). The heart tube was examined in the 9 to 12 somite embryo. Eggs were carefully opened with sterile forceps, and the embryos, 2- to 13-somite stages (Hamburger and Hamilton, 1951), were excised and immediately transferred into 35 mm Petri dishes containing 3.5% formaldehyde solution (pH7. 4°C) in Mg 2+-free saline, buffered with 10 mM Hepes and 2 mM CaCl2 (MFH) (Shiraishi et al., 1993). The vitelline membranes were peeled immediately from the embryos while still in the fixative solution. The embryos were fixed at 4°C for 15 minutes, and then permeabilized with 0.2% Triton X-100 in MFH for 15 minutes at room temperature.
Cardiomyocytes also were isolated from 10-day-old quail embryos as previously described (Dabiri et al., 1999), and plated on cover slips in Petri dishes. The cells were cultured for two to four days before fixation, premeabilization and immunofluorescence (Dabiri et al., 1999).
Immunostaining procedure
Intact embryos were processed, several embryos at a time, in 35mm Petri dishes that were placed in humidity chambers (Dabiri et al., 1999). The embryo tissue was blocked for 20 minutes at room temperature with 5 % milk (BioRad Laboratories, Hercules, CA). After the milk was removed with a transfer pipette, 200 µl primary antibody, diluted with Tris-buffer saline containing 1 mM of CaCl2 (TBS, pH 7.4), and 5 % milk, was applied to the embryos with the dishes tilted at a 45-degree angle to allow the small volume to cover the embryos. After incubation at room temperature for 1 hour with primary antibodies, the embryos were rinsed several times in TBS, and incubated with secondary antibodies and DAPI for 30 minutes at room temperature. Fluorescently labeled phalloidin was applied for 30 minutes at room temperature.
The heart forming regions were dissected in different ways according to the particular developmental stage so that the heart regions could be mounted appropriately. The paired rudiments of the heart are formed at the 2-somite stage. At the 5-somite stage, the two rudiments start fusing to form the heart tube. The stained embryos of the 2 through 5 somite stages were flattened by bisecting the head fold, and then were mounted in Mowiol containing the anti-fade reagent, n-propyl gallate (Dabiri et al., 1999; Calbiochem, La Jolla, CA). The neurotubes beneath the forming heart tubes of older embryos (6 through 13 somites) were carefully excised before the embryos were mounted in Mowiol. The cover slip was placed gently on the embryo to avoid changing the shape of the forming hearts. Cardiomyocytes isolated from 10-day-old embryos and cultured on cover slips for 3 – 4 days were fixed, permeabilized and stained with the same procedures described above and mounted in Mowiol.
Antibodies
Monoclonal antibodies were purchased from the following sources: anti-sarcomeric α-actinin (IgG, A7811), Sigma (St. Louis, MO); anti-titin antibody (9D10; IgM, a region of titin near the A-I junction, Wang et al., 1991) and MF20 (IgG antibody against muscle myosin), Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA); anti-non-muscle myosin IIB (IgM, MAB1670), Chemicon (Temecula, CA, USA). Rhodamine and fluorescein phalloidin, probes specific for actin filaments, were purchased from Molecular Probes (Eugene, OR). Muscle myosin II antibody was obtained from Dr. Frank Pepe (University of Pennsylvania School of Medicine), and previously described in Sanger et al. (1986) referred here to as FAP Mab. DAPI was obtained from Sigma (St. Louis, MO). Fluorescently labeled secondary antibodies purchased from Jackson ImmunoResearch Laboratories (West Grove, PA) were selected to be non-cross reacting.
Deconvolution Microscopy
A DeltaVision Restoration microscopy system (Applied Precision, Issaquah, WA) was used to acquire images of the cardiomyocytes in Z-axis optical sections that were processed with deconvolution software. The microscope was an Olympus IX70 microscope and an Olympus 100X Plan Apo, 1.35 N.A. objective lens. For recording the labeled antibodies and phalloidin, the following filters were used: fluorescein with 490/528 nm; rhodamine with 555/617nm ; CY5 with 640/685 nm; DAPI with 360/457 nm. Some images were deconvolved using 10 cycles with SoftWoRx image analysis and all images were assembled for publication with Adobe Photoshop (San Jose, CA). The myosin filaments and A-bands were measured in undeconvolved images with SoftWoRx image analysis software, and analyzed with Excel software.
Results
Non-muscle myosin and α-actinin assemble in premyofibrils at an early stage of myofibrillogenesis
Fibrils positive for F-actin and the non-muscle isoform of myosin IIB and muscle specific alpha-actinin could be seen in cardiomyocytes as early as the five somite stage. The non-muscle myosin and alpha-actinin were arranged along the fibrils in small bands that did not overlap (Figure 1 A−G), and in many cases were alternating (Figure 1 H). The average separations between the alpha-actinin staining bodies were about 0.75µm, as opposed to the separation between alpha-actinin localized in Z-bands (1.8 to 2 µm) in mature myofibrils. The early fibrils are identical in staining and periodicities to the premyofibrils detected with the same antibodies at the edges of 10-day old cultured cardiomyocytes (Figure 1 E, F, G). We conclude that the detected fibers in the developing heart region in the embryo are premyofibrils (Rhee et al., 1994). These arrangements of non-muscle myosin IIB and sarcomeric α-actinin in both types of cardiomyocytes are identical to the premyofibrils detected in mesoderm explant cardiomyocytes (Du, et al 2003).
Figure 1. Arrangement of sarcomeric alpha-actinin.
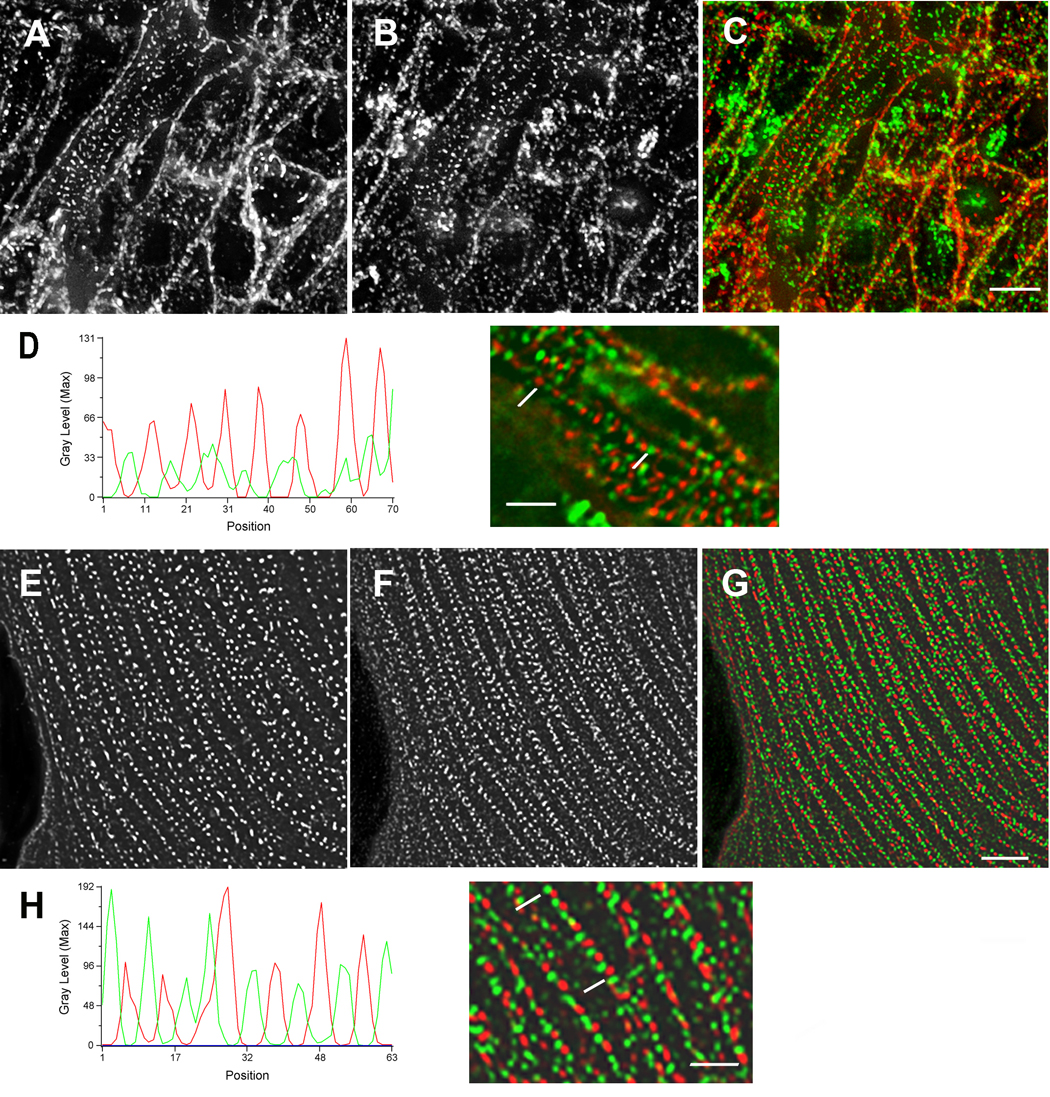
Arrangement of sarcomeric alpha-actinin (A, E, red in C, D, G, H) and non-muscle myosin IIB (B, F, green in C, D, G, H) early in myofibrillogenesis in cardiomyocytes fixed in situ (6-somite stage, HH stage 8+) (A–D), and in the spreading margin of cells cultured for three days after isolation from 10-day embryonic hearts (E–H). Graph and high magnification view of premyofibrils below each set illustrate the alternating pattern of the two proteins that can be resolved in cells in situ (D) and that is clearer in the flat cultured cardiomyocytes (H). The sizes and spacing of the two different types of bands are the same in the two types of cardiomyocytes. Bars = 5 µm A–C and E–G. Bars = 2 µm in D and H.
In 9-somite stage embryos scanned at 0.15µm intervals in the Z-axis, we detected premyofibrils and mature myofibrils at the same and different planes of the same cardiomyocytes (Figure 2, Figure 3). A plane near the middle of the cells exhibited sarcomeric α-actinin staining in the Z-bands, separated from each other by a distance of two microns, spacings identical to those found in mature myofibrils (Figure 2 A–C). Near the upper plane of the same cell, both sarcomeric α-actinin and non-muscle myosin IIB were present in small bands characteristic of premyofibrils (Figure 2 D–F). Premyofibrils were often associated with the cell edges (Figure 2 B, Figure 3), suggesting that the assembly of myofibrils is initiated in proximity to the cell surface in the intact heart, as detected in living, cultured cardiomyocytes expressing alpha-actinin-GFP (Dabiri et al., 1997). The absence of non-muscle myosin IIB staining of the area between the Z-bands (sites of the A-bands containing muscle myosin IIB) of the mature myofibrils (Figure 2 A–C, Figure 3 A–C) is an indication of the specificity of this non-muscle myosin antibody.
Figure 2. Premyofibrils and mature myofibrils.
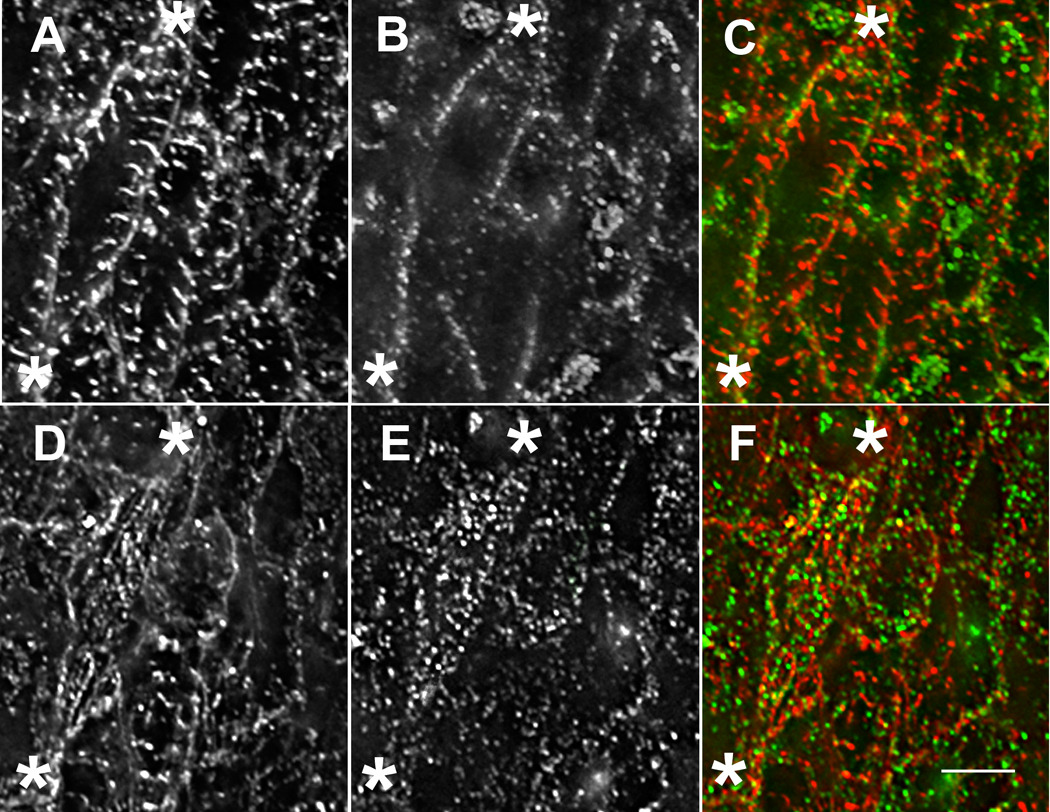
Premyofibrils and mature myofibrils in the same cardiomyocytes in situ (A–F) (9 somite stage, HH stage 9+). Two different focal planes of the same cardiomyocytes in situ stained with sarcomeric alpha-actinin antibody (A, D, red in C, F) and non-muscle myosin IIB antibody (B, E, green in C, F). In a plane near the middle of a cell marked at either end with asterisks (A–C), sarcomeric alpha-actinin is in Z-bands of myofibrils (A, red in C), and non-muscle myosin II is in small bands around the cell periphery (B, green in C). In a plane near the top of the same cell (asterisks, D–F), both sarcomeric alpha-actinin (D) and non-muscle myosin II (E) are in small bands suggesting that the assembly of myofibrils is initiated in proximity to the cell surface. Intermediate stages of assembly can be seen in the same cell. Bar = 5 µ.
Figure 3. Premyofibrils and Z-bands at same level of cell.
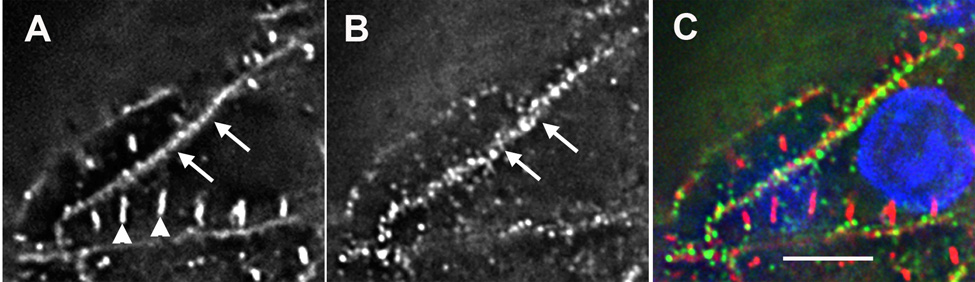
Premyofibrils and mature myofibril in the same plane of a cardiomyocyte fixed in situ. (9 somite stage, HH stage 9+) stained with DAPI (blue in C) and antibodies to sarcomeric alpha-actinin (A and red in C) and non-muscle myosin IIB (B and green in C). (A) Sarcomeric alpha-actinin is present in both Z-bands (arrowheads) of a mature myofibril and z-bodies of a premyofibril (arrows) along the boundary of the same cell. (B) Non-muscle myosin IIB is localized with sarcomeric alpha-actinin in the premyofibril (arrows). (C) Non-muscle myosin IIB is not localized in bands between the Z-bands as expected. Bar = 5 µ.
In 9-somite stage embryos, individual cardiomyocytes have both premyofibrils and mature myofibrils (Figure 2, Figure 3), although there is a difference in the distribution of the two structures within the cells. Mature myofibrils show Z-bands spaced 2µm apart with no intervening bands of non-muscle myosin IIB (Figures 2 A–C). The absence of non-muscle myosin IB antibody staining between the Z-bands, where muscle specific myosin IIB is present in A-bands (Figure 3) is an indication of the specificity of this non-muscle myosin antibody. Images collected at 0.15µm intervals in the Z-axis show mature myofibrils concentrated primarily in the middle planes of the cardiomyocytes (Figures 2 A–C), whereas premyofibrils, with small adjacent bands of sarcomeric α-actinin and non-muscle myosin IIB, were concentrated in the upper plane of the same cell (Figure 2 D–F). Premyofibrils were also localized along the cell edges (Figures 2 B, Figure 3) suggesting that the assembly of myofibrils is initiated in proximity to the cell surface in the intact heart, as detected in living, cultured cardiomyocytes expressing alpha-actinin-GFP (Dabiri et al., 1997).
As muscle myosin II becomes organized into A-bands, non-muscle myosin II is reduced
As development progressed muscle myosin II organization changed from bundles that varied in length up to 25 microns (Figure 4 A; 6 somites) to increasing numbers of A-bands (Figure 4 D, G; 7 and 12 somites). The muscle myosin II bundles were closely associated with non-muscle myosin IIB, and in several places the two myosins appeared to be in the same fibril (Figure 4 A–C; Figure 5 arrows A–C). As A-bands began to form, bands of non-muscle myosin were often adjacent to the A-bands, but were never colocalized with A-bands (Figure 4 D–F). Concurrent with the formation of mature myofibrils was a decrease in non-muscle myosin IIB (Figure 4 B. E, H). Even in later stages (12 somites), however, the two myosin isoforms had nonuniform distributions in cardiomyocytes with the non-muscle isoform more abundant in the planes closest to the surface (Figure 5 B vs. E) where the muscle myosin was in bundles of varying lengths (Figure 5 A). In several cases the two myosins appeared to be in the same fibrils (Figure 5 C). In planes near the middle of the cells where most muscle myosin was organized in A-bands (Figure 5 D), there were few non-muscle myosin IIB fibers (Figure 5 E).
Figure 4. Assembly of A-bands in cardiomyocytes in situ.
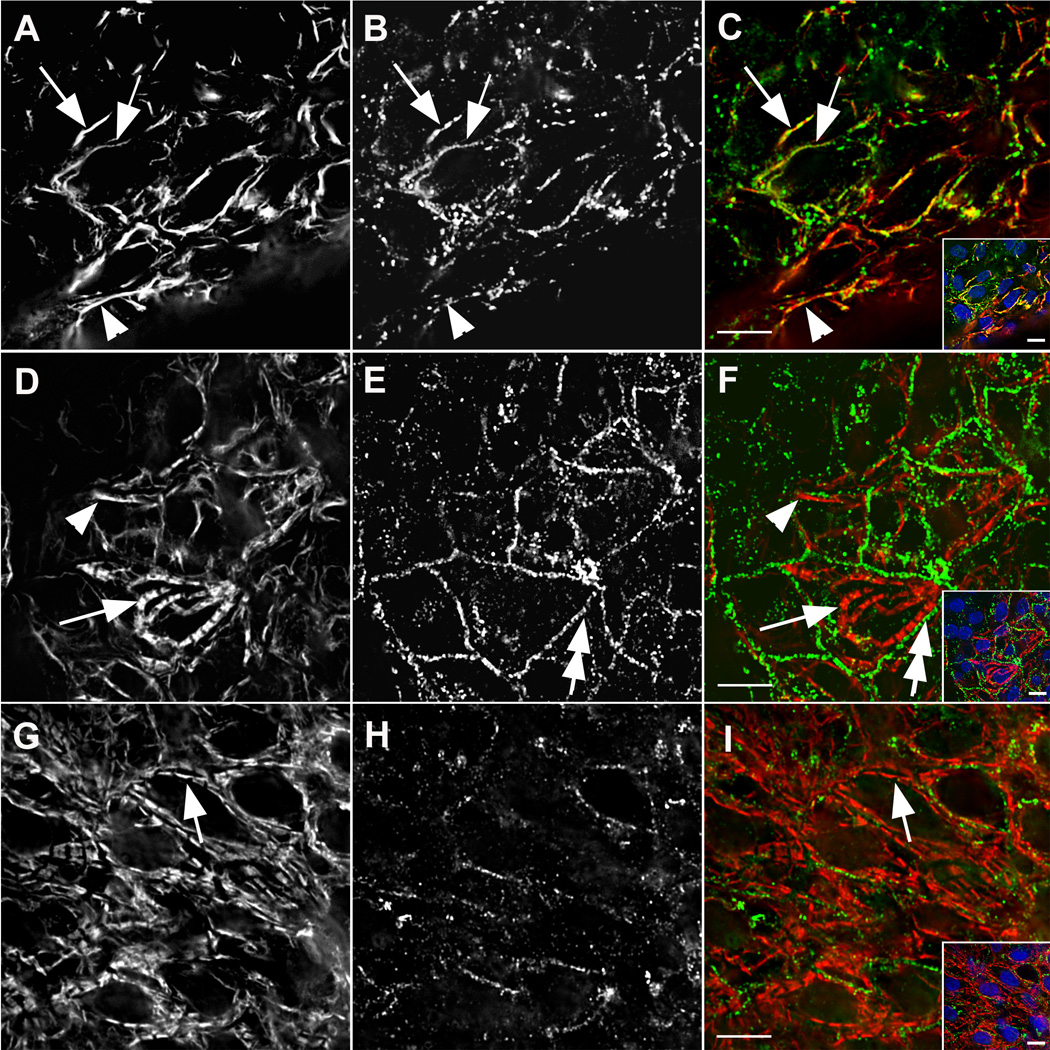
Assembly of A-bands in cardiomyocytes in situ. The embryos were stained with antibodies against muscle myosin II and non-muscle myosin IIB at three developmental stages. (A–C) Early (6-somite), (D–F) middle (7-somite), and (G–I) late (12-somite) stages of myofibril assembly showed decreasing association of muscle-specific myosin II (A, D, G, red in C, F, I) with non-muscle myosin IIB (B, E, H, green in C, F, I). Before muscle myosin II was organized in A-bands (arrows D, F and G, I), it was present in bundles that varied in length (arrowheads A, C and D, F). Non-muscle myosin IIB was in small bands that often appeared to be coincident with fibrils of muscle-specific myosin in early stages (arrows, A–C); adjacent to myofibrils in middle stages (double arrows, E, F); and finally absent among mature myofibrils (G–H). Inserts show low magnification views of the cells and nuclei stained with DAPI (blue). Bars = 10 µm.
Figure 5. Intracellular asynchrony of myofibrillogenesis.
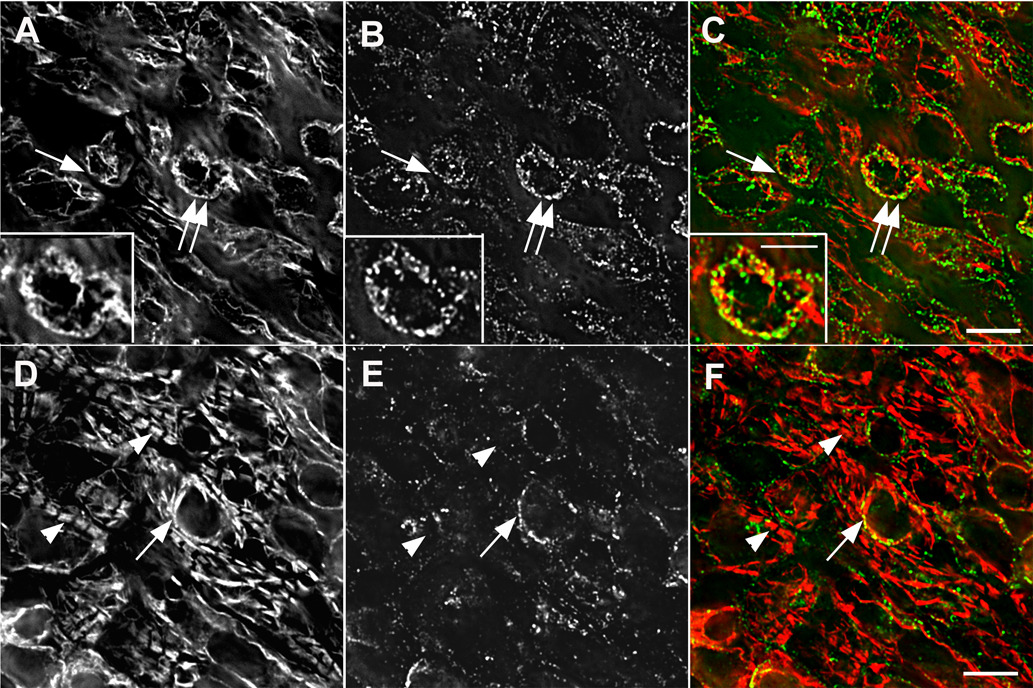
Intracellular asynchrony of myofibrillogenesis in situ with nascent and mature myofibrils in the same cells (12-somite stage, HH stage 10+). Two focal planes (A–C and D–F) of the same cardiomyocytes in situ stained with the two different myosin II antibodies: muscle-specific myosin II (A, D red in C, F); non-muscle myosin IIB (B, D green in C, F). There was a greater amount of non-muscle myosin IIB in the plane just beneath the upper layer of tissue (B, C) than in a lower plane (E, F), whereas organized A-bands of muscle-specific myosin II were numerous in the lower plane (D, F) but few A-bands were present in the upper plane (A, C). In some cells the two myosins were in the same fibrils (arrows, A–C: double arrows point to area enlarged in insets), but they were not precisely colocalized (insets A–F). Non-muscle myosin IIB was not associated with the muscle myosin II in the A-bands, i.e., mature myofibril (arrowheads D, F). Bars in C, F = 10 µm. Bar in inset = 5 µm.
Dividing cardiomyocytes in situ and in tissue culture
Entry of cardiomyocytes into cell division leads to the loss of mature myofibrils whether in situ or in tissue culture (Kaneko et al., 1984; Conrad et al., 1991; 1995; Ahuja et al., 2004). In this study, the dividing quail cardiomyocytes, fixed in situ (Figure 6) and in culture (Figure 7), also show a loss of premyofibrils and mature myofibrils. The dividing cardiomyocytes fixed in situ also lacked the overlapping muscle myosin filaments (a characteristic of nascent myofibrils) common in the surrounding interphase cardiomyocytes (Figures 5 A–F; Figure 6). The punctate staining of the non-muscle myosin IIB and sarcomeric alpha-actinin in the cleavage furrows of cardiomyocytes (Figure 6; Figure 7A and B) resembles patterns of these proteins in cleavage furrows of live non-muscle cells (Sanger et al., 1989; 1998; 2000). It also resembles the structure of cardiomyocyte premyofibrils.
Figure 6. Division in situ.
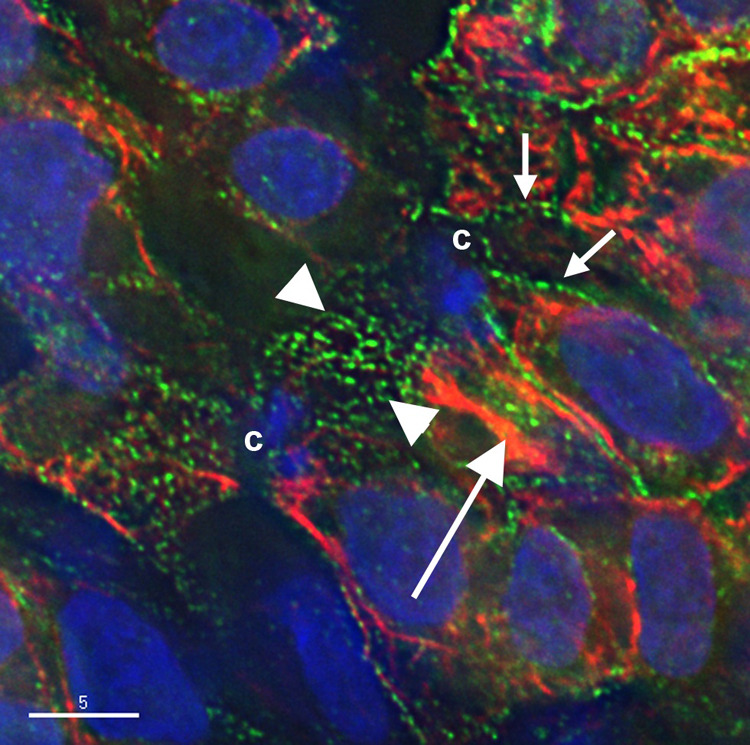
Cardiomyocyte in an embryo fixed in situ undergoing cytokinesis surrounded by interphase cells (12-somite stage, HH stage 10+). DAPI stains the nuclei of the interphase cells and the condensed chromatin (c) of the dividing cardiomyocyte. The cells have been stained with non-muscle myosin IIB (green) and muscle myosin II (red). Non-muscle myosin IIB is in a punctate arrangement in the cleavage furrow (between the arrowheads) and in fibrils in the interphase cells (small arrows). The muscle myosin II in the interphase cells is in an overlapping pattern (large arrow), but is not organized in the dividing cell. Bar = 5 µm.
Figure 7. Division in culture.
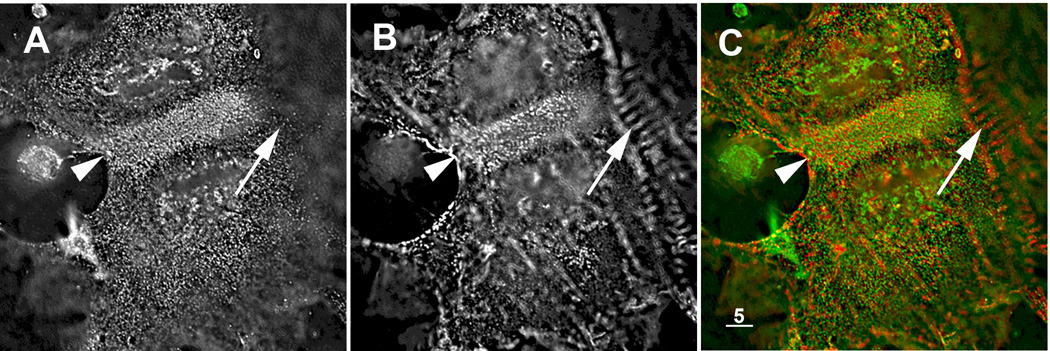
Dividing cardiomyocyte in tissue culture from 10-day quail embryo hearts co-stained with antibodies to non-muscle myosin IIB (A, green in C) and sarcomeric alpha-actinin (B, red in C). Both proteins are arranged in a punctate pattern in the cleavage furrow (arrowhead). As expected, there is no non-muscle myosin IIB staining between the Z-bands of the mature myofibril (arrows in A to C) in an adjacent interphase cardiomyocyte. Bar = 5 µm.
Short filaments of muscle myosin present during the early stages of myofibrillogenesis are not associated with titin
When very early embryos at the two somite stage (HH stage 7+) were labeled with either of two antibodies directed against muscle myosin II heavy chains, rodlets of muscle myosin II were detected scattered around the nuclei of these first cardiomyocytes (Figure 8 A,C and D,F). The anti-myosin FAP Mab stained these rodlets with a less intense central region (Figure 8 D), the same type of muscle myosin II staining that is visible in the center of the A-bands of mature myofibrils in cardiomyocytes fixed in situ (Figure 10 G). The MF 20 anti-myosin antibody stained the rodlets in a continuous pattern as it does A-bands of mature myofibrils (not shown). The rodlets had an average length of 0.76 microns (0.76±0.07, n=65) as compared to A-bands in mature myofibrils, stained by either of the same myosin II antibodies whose average lengths were 1.6 microns (1.60±0.08; n=65) (Figure 6). Costaining with the 9D10 titin antibody (A-I junction epitope) showed only dots of titin reactivity in the cardiomyocytes fixed at the two somite stage in situ (Figure 8 B, green in C).
Figure 8. Titin and muscle myosin II filaments.
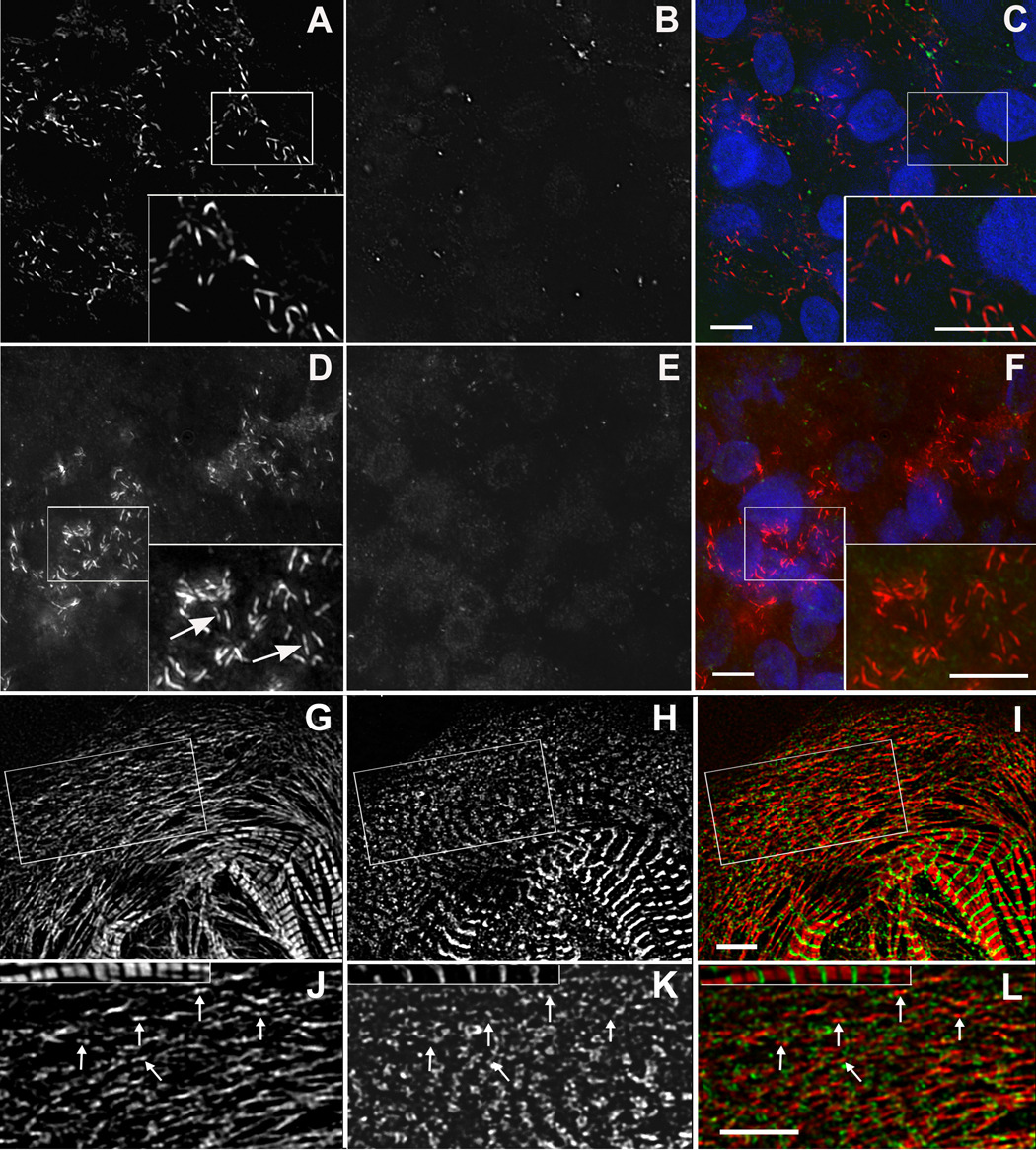
Titin and muscle myosin II filaments. Embryos at two developmental stages (2 somites A–F; 5 somites G–I) stained with either of two different muscle myosin II antibodies (MF20, A and red in C; Pepe myosin II antibody, D, G, and red in F, I) and titin (9D10, A–I junction epitope, B, E, H and green in C, F, I). At the 2 somite stage short rodlets of muscle myosin ( A, D, and red in C, F) were scattered in the cytoplasm, and titin staining was present as a few dots in some cells (B and green in C). The myosin rodlets were labeled uniformly with MF20 antibody (inset in A and C), whereas the staining with the Pepe myosin antibody revealed a bare zone in the middle of the rodlets (inset in D [arrows] and F). Similar myosin rodlets were present along with small aggregates of titin in the spreading margins of a cardiomyocyte in culture for three days after isolation from 10-day embryonic hearts (G–I). Box in G–I is shown enlarged in J–L with a myofibril from the cell at the same enlargement. Many of the myosin rodlets had a bare midzone (J arrows). The titin had no regular pattern of localization with respect to the myosin rodlets (K, L arrows). Bars = 5 µm.
Figure 10. Titin (9D10 antibody) and muscle myosin.
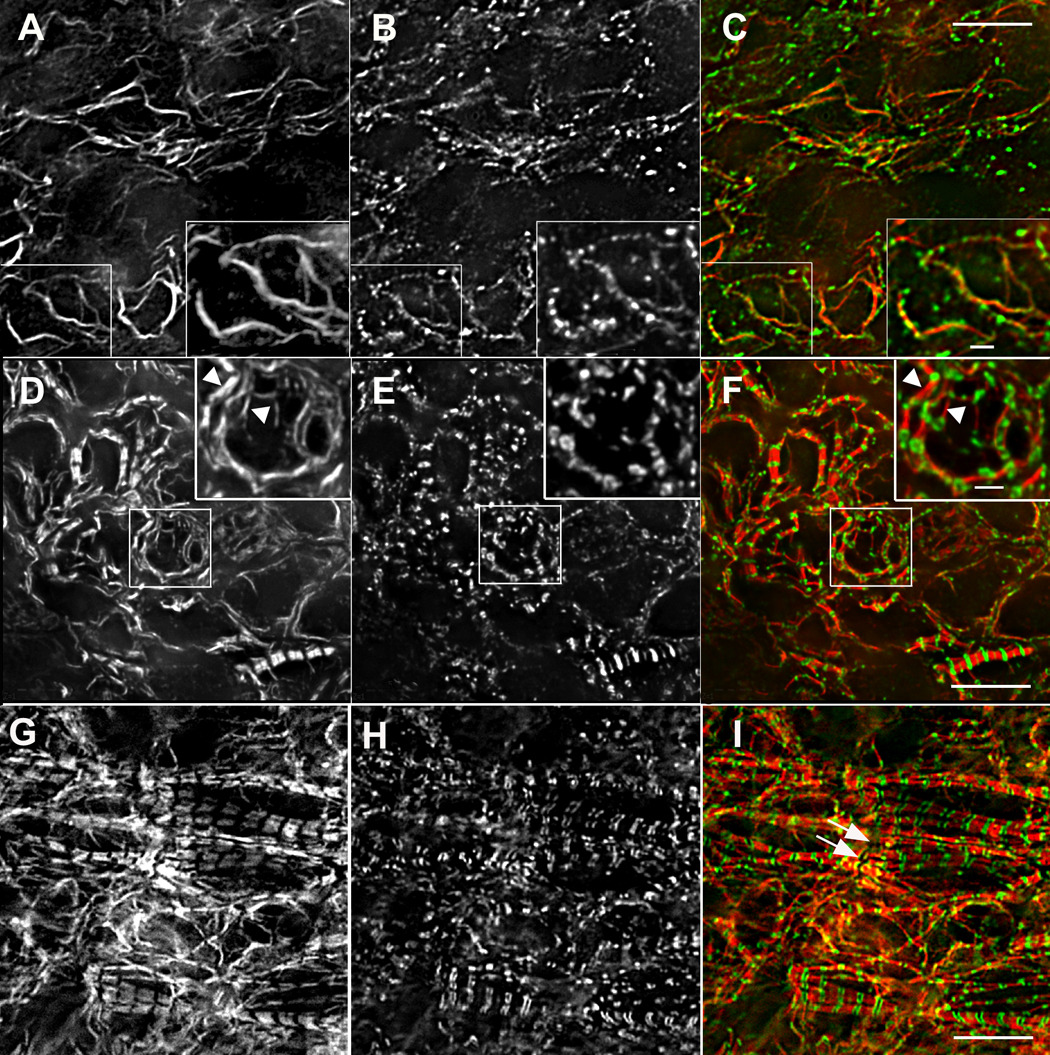
Titin (9D10 antibody) and muscle myosin (Pepe antibody) localization during A-band assembly. Early (A–C), middle (D–F), and late (G–I) stages of A-band assembly show muscle specific myosin (A, D, G, red in C, F, I) progressing from long bundles of myosin filaments (A) to emerging A-bands (D) to ordered, aligned A-bands in myofibrils (G). Regions in small boxes A–F are shown at higher magnification in larger insets A–F. Before A-bands formed, muscle myosin was present in bundles of varying lengths (A, and red in C), and the 9D10 titin epitope was in small puncta (B, and green in C) that sometimes appeared as beads along the myosin bundles (large inset A–C). (5 somite stage) At later stages when A-bands began to form, the titin 9D10 epitope localized at the ends of the assembling A-bands (arrowheads in D–I). Bars in large insets A–F = 2µm. All other bars = 10µm.
The discovery of short myosin filaments in cardiomyocytes fixed in situ led us to examine cultured embryonic cardiomyocytes to determine if similar filaments were present in the spreading edges of these cells. Similar myosin rodlets were present together with titin in the spreading margins of cardiomyocytes cultured for three days after isolation from a 10-day embryonic heart (Figure 100 G–L). In the cultured cardiomyocytes, small aggregates of titin were intermingled with the myosin rodlets in the spreading margins but had no regular pattern of localization with respect to the myosin (arrows J–L). In mature myofibrils in these cells the titin antibody (9D10) was localized in its expected doublet pattern at the edges of the A-bands (Figure 5 G–I and inset J–K). The rodlets had an average length of 0.66 microns (0.66±0.11, n=305) as compared to A-bands in mature myofibrils, stained by either of the same myosin II antibodies whose average lengths were 1.46 microns (1.45±0.07; n=295) (Figure 9).
Figure 9. Lengths of Muscle Myosin Filaments.
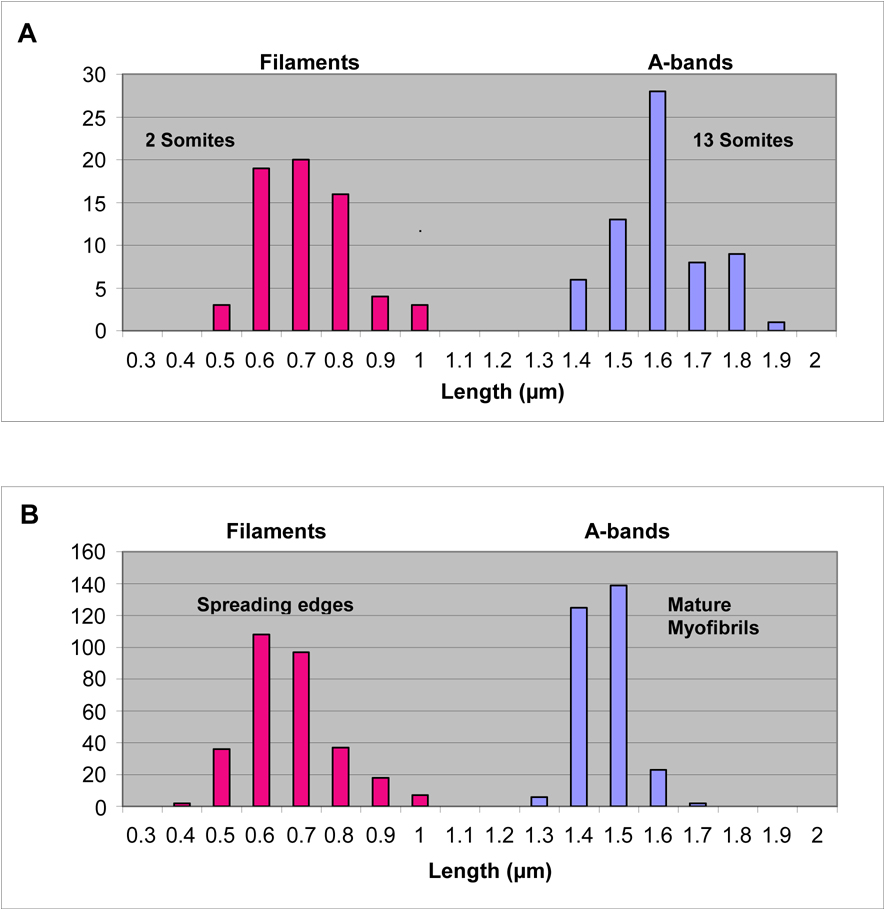
(A) The distribution of lengths of muscle myosin “filaments” or rodlets (2 somite stage) and A-bands (13 somite stage) in cardiomyocytes fixed in situ. The myosin “filaments” at the 2 somite stage with an average length of 0.8 µm are much shorter than the 1.6 µm average myosin filament lengths measured in A-bands. (B) The distribution of lengths of muscle myosin “filaments” in the spreading edges of cultured cardiomyocytes and in A-bands in the mature myofibrils in the same cells. The myosin “filaments” or rodlets at the spreading edges with an average length of about 0.7 µm are much shorter than the 1.5 µm lengths measured in the A-bands.
Association of titin with myosin during A-band formation in situ
Before A-bands had formed and muscle myosin was arrayed in bundles of varying lengths (Figure 4 A and Figure 10 A), titin was localized in small, closely spaced puncta (Figure 10 B) that often were aligned along the myosin bundles, but in other places had no obvious association with the myosin (Figure 10 C). When 1.6 µm A-bands began to assemble, the titin antibody was localized at the ends of each narrow A-band (Figure 10 D–F). In cardiomyocytes with aligned A-bands, the titin was present in spacings and patterns typical of mature myofibrils (Figure 10H) stained with a 9D10 titin antibody (Rhee et al., 1997).
DISCUSSION
The aim of this study was to determine if the distribution of sarcomere proteins in quail cardiomyocytes undergoing myofibrillogenesis in situ was comparable to what we have observed in cardiomyocytes cultured from 10-day avian embryos (Rhee et al., 1994) and in cardiomyocytes formed in precardiac mesoderm explant cultures (Du et al., 2003). Results from those previous studies led us to propose that myofibril assembly occurs in a sequence of steps that begins with the formation of premyofibrils composed of mini-sarcomeres of non-muscle myosin II and muscle isoforms of actin, actin associated proteins and α-actinin (Sanger and Sanger, 2001b; Sanger et al., 2005; Stout et al., 2008). In this model, premyofibrils develop into nascent myofibrils through the incorporation of muscle specific isoforms of myosin and other proteins that stabilize and organize the sarcomeric units. Subsequently non-muscle myosin II is lost, additional sarcomeric proteins are incorporated, and A-bands and Z-bands acquire the highly ordered configuration characteristic of mature myofibrils (Wang et al., 2007,Stout et al., 2008).
Although cardiomyocytes isolated from 10-day avian embryos have myofibrils at the time of culturing, they can assemble additional myofibrils (Dabiri et al., 1997), and when a cell enters mitosis myofibrils disassemble (Conrad et al., 1991), and then reassemble in the daughter cells (Sanger and Sanger, 2001a). Nevertheless, the two-dimensional environment in culture is different from the properties that cardiomyocytes encounter in situ, and it has been suggested that myofibrillogenesis in cells in the forming heart may follow a different pathway from that in single cells in culture (Gregorio and Antin, 2000; Rudy et al., 2001).
Two studies of chick myofibrillogenesis in situ do not support a premyofibril model. In a light and electron microscopic investigation by Tokuyasu and Maher (1987a, b) and another based on confocal microscopy analysis (Ehler et al., 1999), no evidence was found of fibrils that resembled premyofibrils described by Rhee et al. (1994). Rather, the first organized arrays of muscle proteins detected were dense body structures containing actin, α-actinin and titin separated from one another by two microns, i.e. the distance between Z-bands of fully formed sarcomeres (Tokuyasu and Mahler, 1987a, b; Ehler et al., 1999). In addition, in the confocal study non-muscle myosin II was seen to be diffuse in the cytoplasm and not associated with the earliest detected actin fibers in fixed embryos (Ehler et al., 1999). Non-muscle myosin II also was reported to be absent in cultured cardiomyocytes isolated from 10-day old chick embryos (Lu et al., 1992); however, an anti-platelet non-muscle myosin IIA isoform antibody was used in that study, whereas avian cardiomyocytes possess only the IIB isoform of non-muscle myosin, which is the myosin used for cytokinesis in these cells (Conrad et al., 1991; 1995). In addition, we have detected bands of non-muscle myosin IIB in association with z-bodies and actin in avian cultured cardiomyocytes (Rhee et al., 1994) and (Figure 1).
The recent development of deconvolution microscope systems, that combine wide-field microscopy, precise Z-axis sectioning and application of deconvolution algorithms to the image stacks, has made it possible to improve the detectability of fluorescently labeled structures in a fluorescent layer of sample that is less than 50µm (Swedlow et al., 2002). To try to maximize structural details we also avoided enzymes, i.e., hyaluronidases, used in previous studies to help antibodies penetrate the cell matrix surrounding cardiomyocytes (Tokuyasu and Maher, 1987a, b; Ehler et al., 1999), and instead permeabilized with detergents (Shiraishi et al., 1993). We used the same microscopy technique with cultured cardiomyocytes, isolated from 10-day old quail embryos, to compare the two different preparations and found similar distributions of muscle alpha-actinin and non-muscle myosin II in fibrils in the two kinds of cardiomyocytes (Figure 1). The images obtained in this study with deconvolution microscopy were significantly better than our confocal images of cardiomyocytes in precardiac mesoderm organ cultures (Du et al., 2003), revealing aspects of myofibrillogenesis not previously detected in thicker specimens, but conforming to those seen in the flatter, cultured embryonic cardiomyocytes.
Many older histological and electron microscopical studies have pointed out that myofibril assembly is associated with the cell surfaces (reviewed in Sanger et al., 2004). More recently, fluorescent probes in live and fixed cells have demonstrated that the earliest precursors of myofibrils are found at the edges of cultured cardiomyocytes (Rhee et al., 1994; Dabiri et al., 1997), and at the periphery of precardiac mesoderm explants and embryonic hearts (Du et al. 2003; Imanaka-Yoshida et al., 1998; Rudy et al., 2001; Ehler et al., 1999). Our data on the cardiomyocytes fixed in situ are consistent with these observations. Beginning at the 4–6-somite stage, premyofibrils, with small alternating bands of muscle α-actinin and non-muscle myosin IIB, were detected adjacent to the cell membrane (Figure 1 A–C). Similar fibrils can be seen at the spreading edges of very flat cultured cardiomyocytes (Figure 1 E–G). Probes for the two isoforms of myosin II and for sarcomeric α-actinin showed that premyofibrils in cardiomyocytes fixed in situ were concentrated in image planes closest to the top of cells (Figure 2 D–F, Figure 5 A–C), whereas, mature myofibrils were concentrated in the center sections (Figure 2 A–C, Figure 5 D–F).
Myosin molecules in A-bands of mature myofibrils in vertebrates are organized into uniformly straight bipolar thick filaments that are 1.6µm in length (Huxley, 1963). Before A-bands were seen in cardiomyocytes fixed in situ, however, muscle myosin II was distributed in fibrils that were often curved and varied in length up to 25µm (Figure 4 A–F) suggesting an overlapping arrangement of filaments within the fibril. Patterns of overlapping muscle myosin filaments have been seen in electron micrographs of developing myotubes from a limb bud of a larval newt (Kelly, 1969), and in immunostained nascent myofibrils in cultured embryonic cardiomyocytes (Rhee et al., 1994), and in precardiac explants (Du et al., 2003). Non-muscle myosin II often appeared localized with muscle myosin II in the same fibrils (Figure 4 A–C, arrows; Figure 5 A–C, arrows and inset), but it was not possible to determine whether small bands of the two myosins were actually colocalized or segregated in separate overlapping arrays.
An unexpected arrangement of muscle myosin II was seen at an even earlier stage (2–3 somites) in heart formation where scattered myosin rodlets about 0.76µm in length were present in the cells (Figure 8 A–F), significantly shorter than the 1.6 µm length expected for myosin filaments in a vertebrate muscle cell. Similar short myosin filaments also were seen proximal to the spreading edge of cultured cardiomyocytes (Figure 8, G–L). One of the two anti-myosin antibodies (FAP) stained these rodlets with a less intense central region (Figure 8 D, J), the same type of muscle myosin II staining that is visible in the center of the A-bands of mature myofibrils in cardiomyocytes fixed in situ and stained with the same antibody (Figure 10 G). The central part of the A-band, called the pseudo-H zone, represents the area of the thick filament lacking cross-bridges (Huxley, 1963). We assume that these rodlets are either single myosin filaments or several filaments that are aligned side by side to yield the pseudo H-zones in the central region (Figure 8 D).
These short muscle myosin filaments have not been reported before in the literature on vertebrate myofibrillogenesis (reviewed in Sanger et al., 2004; 2005). Since the initial electron microscopic studies of vertebrate myofibrillogenesis (Allen and Pepe, 1965; Auber, 1969; Kelly, 1969), investigators have reported that thick filaments appear at exactly 1.6 µm. More recent electron microscopic and immunofluorescence studies have reported similar observations (reviewed in Holtzer et al., 1997). We did not detect shorter myosin filaments in previous studies either (Rhee et al., 1994; Du et al., 2003). It is only with wide-field deconvolution microscopy that we detected these structures in cardiomyocytes both in cultured and in situ cardiomyocytes. In the present study the short muscle myosin II filaments appeared very early before there were myofibrils or groups of myosin filaments in the cells. In the same cells, titin, as judged by reactivity with the titin antibody 9D10 (A/I junction titin epitope), could only be detected as a few dots (Figure 8 B, E). If the short myosin filaments were present at later stages, we could not detect them in the crowded matrix of nascent myofibrillar structures. Similar short myosin filaments were seen proximal to the spreading edge of cultured cardiomyocytes (Figure 8, G–L), but in these cells numerous small puncta of titin staining were in the same area as the myosin rodlets. We never observed A-bands that were shorter than 1.6 µm. In invertebrate myofibrillogenesis, uniform A-bands increase in length over a period of days until they reach their final lengths (Aronson et al., 1961) of up to 20 µm (Del Castillo et al., 1972).
When the muscle myosin II filaments were in long fibrils before A-bands formed (Figure 10, A–C) the 9D10 antibody staining was distributed is small bands along the fibrils. Beginning at the 7-somite stage the typical pattern of titin at the A/I junction of sarcomeres was seen in a subset of myofibrils (mature myofibrils with 1.6um A-bands) in cells in which premyofibrils (no muscle myosin II) and nascent myofibrils (overlapping muscle myosin II filaments) were also present. By the 12-somite stage, the cells were filled with mature myofibrils with well-defined 1.6 µm A-bands. These observations indicate that the assembly of mature A-bands is preceded by the formation of short arrays of muscle myosin filaments that are unassociated with titin. Increased muscle myosin II staining in overlapping arrays and A-bands is accompanied by increased levels and organization of titin staining in association with the myosin. This is consistent with the proposal that titin may play a role in length determination of myosin filaments in A-bands (Wang and Wright, 1988; Whiting et al., 1989).
Conclusions
The results in this report support the view that myofibrillogenesis follows a common process that is independent of where the avian cardiomyocyte is studied, i.e., in intact embryonic hearts, in precardiac transplants or in tissue culture: premyofibrils to nascent myofibrils to mature myofibrils. Support for this premyofibril model has also been reported in cultured adult rat cardiomyocytes (LoRusso et al., 1997) and in cultured skeletal muscle cells (Sanger et al., 2002; Siebrands et al., 2004; Golson et al., 2004; Wang et al., 2005 a, b). Recent data from our laboratory demonstrate that there is a dynamic exchange of proteins between myofibrils and a sarcoplasmic pool during myofibrillogenesis (Wang et al., 2005a; 2007; 2008; Stout, 2008). Such an exchange would provide the mechanism for the remodeling that is needed as premyofibrils progress to mature myofibrils (Dabiri et al., 1997, Wang et al., 2005b). Evidence is presented for the first time that in the absence of titin, thick filaments assume a much shorter length than that detected in mature myofibrils. This supports the hypothesis that titin may play a role in determining the final lengths of the thick filaments aligned into uniform A-bands.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahuja P, Perriard E, Jean-Claude Perriard JC, Elisabeth Ehler E. Sequential myofibrillar breakdown accompanies mitotic division of mammalian cardiomyocytes. J. Cell Sci. 2004;117:3295–3306. doi: 10.1242/jcs.01159. [DOI] [PubMed] [Google Scholar]
- Allen ER, Pepe FA. Ultrastructure of developing muscle cells in the chick embryo. Am. J. Anat. 1965;116:115–148. doi: 10.1002/aja.1001160107. [DOI] [PubMed] [Google Scholar]
- Aronson J. Sarcomere size in developing muscles of a tarsonemid mite. J. Biophys. Biochem. Cytol. 1961;11:147–156. doi: 10.1083/jcb.11.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auber J. La myofibrillogenese du muscle strie II. Vertebres. J. Microscopie. 1969;8:367–390. [Google Scholar]
- Clark KA, McElhinny AS, Beckerle MC, Gregorio CC. Striated muscle cytoarchitecture: an intricate web of form and function. Ann. Rev. Cell Dev. Biol. 2002;18:637–706. doi: 10.1146/annurev.cellbio.18.012502.105840. [DOI] [PubMed] [Google Scholar]
- Conrad AH, Clark WA, Conrad GW. Subcellular compartmentalization of myosin isoforms in embryonic chick ventricle myocytes during cytokinesis. Cell Motil. Cytoskeleton. 1991;19:189–206. doi: 10.1002/cm.970190307. [DOI] [PubMed] [Google Scholar]
- Conrad AH, Jaffredo T, Conrad GW. Differential localization of cytoplasmic myosin II isoforms A and B in avian interphase and dividing embryonic and immortalized cardiomyocytes and other cell types in vitro. Cell Motil. Cytoskeleton. 1995;31:93–112. doi: 10.1002/cm.970310203. [DOI] [PubMed] [Google Scholar]
- Dabiri GA, Turnacioglu KK, Sanger JM, Sanger JW. Myofibrillogenesis visualized in living embryonic cardiomyocytes. Proc. Natl. Acad. Sci. USA. 1997;94:9493–9498. doi: 10.1073/pnas.94.17.9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabiri GA, Ayoob JC, Turnacioglu KK, Sanger JM, Sanger JW. Use of green fluorescent proteins linked to cytoskeletal proteins to analyze myofibrillogenesis in living cells. Methods Enzymol. 1999;302:171–186. doi: 10.1016/s0076-6879(99)02017-0. [DOI] [PubMed] [Google Scholar]
- DelCastillo J, Anderson M, Smith DS. Proventriculus of a marine annelid: muscle preparation with the longest sarcomere. Proc. Natl. Acad. Sci. USA. 1972;69:1669–1672. doi: 10.1073/pnas.69.7.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du A, Sanger JM, Linask KK, Sanger JW. Myofibrillogenesis in the first cardiomyocytes formed from isolated quail precardiac mesoderm. Dev. Bio. 2003;257:382–394. doi: 10.1016/s0012-1606(03)00104-0. [DOI] [PubMed] [Google Scholar]
- Dlugosz AA, Antin PB, Nachmias VT, Holtzer H. The relationship between stress fiber-like structures and nascent myofibrils in cultured cardiac myocytes. J Cell Biol. 1984;99:2268–2278. doi: 10.1083/jcb.99.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehler E, Rothen BM, Haemmerle SP, Komiyama M, Perriard JC. Myofibrillogenesis in the developing chicken heart: assembly of Z-disk, M-line and the thick filaments. J. Cell Sci. 1999;112:1529–1539. doi: 10.1242/jcs.112.10.1529. [DOI] [PubMed] [Google Scholar]
- Golson ML, Sanger JM, Sanger JW. Inhibitor arrest myofibrillogenesis in skeletal muscle cells at early stages of assembly. Cell Motil. Cytoskeleton. 2004;59:1–16. doi: 10.1002/cm.20017. [DOI] [PubMed] [Google Scholar]
- Gregorio CC, Antin PB. To the heart of myofibril assembly. Trends Cell Biol. 2000;10:355–362. doi: 10.1016/s0962-8924(00)01793-1. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryos. J. Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Holtzer H, Hijikata T, Zhang ZQ, Holtzer S, Protasi SF, Franzini-Armstrong C, Sweeney HL. Independent assembly of 1.6µm long bipolar MHC filaments and I-Z-I bodies. Cell Struct. Funct. 1997;22:83–93. doi: 10.1247/csf.22.83. [DOI] [PubMed] [Google Scholar]
- Huxley HE. Electron microscope studies on the structure of natural and synthetic protein filaments from striated muscle. J Mol Biol. 1963;7:281–308. doi: 10.1016/s0022-2836(63)80008-x. [DOI] [PubMed] [Google Scholar]
- Imanaka-Yoshida K, Knudsen KA, Linask KL. N-cadherin id required for the differentiation and initial myofibrillogenesis of chick cardiomyocytes. Cell Motil. Cytoskeleton. 1998;39:52–62. doi: 10.1002/(SICI)1097-0169(1998)39:1<52::AID-CM5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Kaneko H, Okamoto M, Goshima K. Structural changes of myofibrils during mitosis of newt myocardial cells in culture. Exp. Cell Res. 1984;153:483–498. doi: 10.1016/0014-4827(84)90615-3. [DOI] [PubMed] [Google Scholar]
- Kelly DE. Myofibrillogenesis and Z-band differentiation. Anat. Rec. 1969;163:403–426. doi: 10.1002/ar.1091630305. [DOI] [PubMed] [Google Scholar]
- LoRusso SM, Rhee D, Sanger JM, Sanger JW. Premyofibrils in spreading adult cardiomyocytes in tissue culture: evidence for reexpression of the embryonic program for myofibrillogenesis in adult cells. Cell Motil. Cytoskeleton. 1997;37:183–198. doi: 10.1002/(SICI)1097-0169(1997)37:3<183::AID-CM1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Lu M-H, Dilullo C, Schultheiss T, Holtzer J, Murray JM, Choi J, Fischman DA, Holtzer H. The vinculin/sarcomeric-alpha-actinin/alpha-actin nexus in cultured cardiac myocytes. J. Cell Biol. 1992;117:1007–1022. doi: 10.1083/jcb.117.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manasek FJ. Embryonic development of the heart. I. A light and electron microscopic study of myocardial development in the early chick embryo. J. Morphol. 1968;125:329–366. doi: 10.1002/jmor.1051250306. [DOI] [PubMed] [Google Scholar]
- Rhee D, Sanger JM, Sanger JW. The premyofibril: evidence for its role in myofibrillogenesis. Cell Motil. Cytoskeleton. 1994;28:1–24. doi: 10.1002/cm.970280102. [DOI] [PubMed] [Google Scholar]
- Rudy DE, Yatskievych TA, Antin PB, Gregorio CC. Assembly of thick, and thin, titin filaments in chick precardiac explants. Dev. Dyn. 2001;221:61–71. doi: 10.1002/dvdy.1125. [DOI] [PubMed] [Google Scholar]
- Sanger JM, Mittal B, Pochapin M, Sanger JW. Myofibrillogenesis in living cells microinjected with fluorescently labeled alpha-actinin. J. Cell Biol. 1986;102:2053–2066. doi: 10.1083/jcb.102.6.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger JM, Mittal B, Dome JS, Sanger JW. Analysis of cell division using fluorescently labeled actin and myosin in living PtK2 cells. Cell Motil. Cytoskeleton. 1989;14:201–219. doi: 10.1002/cm.970140207. [DOI] [PubMed] [Google Scholar]
- Sanger JM, JSDome JS, JWSanger JW. Unusual cleavage furrows in vertebrate tissue culture cells: insights into the mechanisms of cytokinesis. Cell Motil. Cytoskeleton. 1998;39:95–106. doi: 10.1002/(SICI)1097-0169(1998)39:2<95::AID-CM1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Sanger JM, Sanger JW. Assembly Of cytoskeletal proteins into cleavage furrows of tissue culture cells. Microscopy Research and Technique. 2000;49:190–201. doi: 10.1002/(SICI)1097-0029(20000415)49:2<190::AID-JEMT12>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Sanger JW, Chowrashi P, Shaner NC, Spalthoff S, Wang J, Freeman N, Sanger JM. Myofibrillogenesis in skeletal muscle cells. Clin Ortho. Related Res. 2002;403S:S153–S162. doi: 10.1097/00003086-200210001-00018. [DOI] [PubMed] [Google Scholar]
- Sanger JW, Sanger JM. Green fluorescent proteins improve myofibril research. Biophotonics International. 2001a;8:44–46. [Google Scholar]
- Sanger JW, Sanger JM. Myofibrillogenesis in cardiac muscle. In: Dube D, editor. Myofibrillogenesis. New York: Springer Verlag; 2001b. pp. 3–20. [Google Scholar]
- Sanger JW, Sanger JM, Franzini-Armstrong C. Assembly of the skeletal muscle cell. In: Engel AG, Franzini-Armstrong C, editors. Myology. 3rd Edition. New York: McGraw-Hill; 2004. pp. 45–65. [Google Scholar]
- Sanger JW, Kang S, Siebrands C, Freeman N, Du A, Wang J, Stout AL, Sanger JM. How to build a myofibril. J. Muscle Res. Cell Motility. 2006 doi: 10.1007/s10974-005-9016-7. (In Press) [DOI] [PubMed] [Google Scholar]
- Schultheiss T, Lin Z, Lu MH, Murray J, Fischman DA, Weber K, Masaki T, Imamura M, Holtzer H. Differential distribution of subsets of myofibrillar proteins in cardiac nonstriated and striated myofibrils. J. Cell Biol. 1990;110:1159–1172. doi: 10.1083/jcb.110.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi I, Takamatsu T, Fujita S. 3-D observation of N-cadherin expression during cardiac myofibrillogenesis of the chick embryo using a confocal laser scanning microscope. Anat. Embryol. 1993;102:115–120. doi: 10.1007/BF00171742. [DOI] [PubMed] [Google Scholar]
- Siebrands CC, Sanger JM, Sanger JW. Myofibrillogenesis in skeletal muscle cells in the presence of taxol. Cell Motil. Cytoskel. 2004;58:39–52. doi: 10.1002/cm.10177. [DOI] [PubMed] [Google Scholar]
- Soteriou A, Gamage M, Trinick J. A survey of interactions made by the giant protein titin. J. Cell Sci. 1993;104:119–123. doi: 10.1242/jcs.104.1.119. [DOI] [PubMed] [Google Scholar]
- Stout AL, Wang J, Sanger JM, Sanger JW. Tracking changes in Z-band organization during myofibrillogenesis with FRET imaging. Cell Motil. Cytoskeleton. 2008 doi: 10.1002/cm.20265. (In Press) [DOI] [PubMed] [Google Scholar]
- Swedlow JR, Platani M. Live cell imaging using wide-field microscopy and deconvolution. Cell Struct. Funct. 2002;27(5):335–341. doi: 10.1247/csf.27.335. [DOI] [PubMed] [Google Scholar]
- Tokuyasu KT, Maher PA. Immunocytochemical studies of cardiac myofibrillogenesis in early chick embryos. J. Cell Biol. 1987a;105:2781–2793. doi: 10.1083/jcb.105.6.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu KT, Maher PA. Immunocytochemical studies of cardiac myofibrillogenesis in early chick embryos. II. Generation of alpha-actinin dots within titin spots at the time of the first myofibril formation. J. Cell Biol. 1987b;105:2795–2801. doi: 10.1083/jcb.105.6.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Wright J. Architecture of the sarcomere matrix of skeletal muscle: immunoelectron microscopic evidence that suggests a set of parallel inextensible nebulin filaments anchored at the Z line. J. Cell Biol. 1988;107:2199–2212. doi: 10.1083/jcb.107.6.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Shaner N, Mittal B, Zhou Q, Chen J, Sanger JM, Sanger JW. Dynamics of Z-band based proteins in developing skeletal muscle cells. Cell Motil. Cytoskel. 2005a;61:34–48. doi: 10.1002/cm.20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sanger JM, Sanger JW. Differential effects of latrunculin-A on myofibrils in cultures of skeletal muscle cells: Insights into mechanisms of myofibrillogenesis. Cell Motil. Cytoskeleton. 2005b;62:35–47. doi: 10.1002/cm.20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sanger JM, Kang S, Thurston H, Abbott LZ, Dube DK, Sanger JW. Ectopic expression and dynamics of TPM1α and TPM1κ in myofibrils of avian myotubes. Cell Motil. Cytoskeleton. 2007;64:767–776. doi: 10.1002/cm.20221. [DOI] [PubMed] [Google Scholar]
- Wang J, Thurston H, Essandoh E, Otoo M, Han M, Rajan A, Dube S, Zajdel RW, Sanger JM, Linask KK, Dube DK, Sanger JW. Tropomyosin expression and dynamics in developing avian embryonic muscles. Cell Motil. Cytoskeleton. 2008 doi: 10.1002/cm.20267. (In Press) [DOI] [PubMed] [Google Scholar]
- Whiting A, Wardale J, Trinick J. Does titin regulate the length of muscle thick filaments? J. Mol. Biol. 1989;205:263–268. doi: 10.1016/0022-2836(89)90381-1. [DOI] [PubMed] [Google Scholar]


