Abstract
Thermogenesis, the production of heat energy, is an essential component of the homeostatic repertoire to maintain body temperature in mammals and birds during the challenge of low environmental temperature and plays a key role in elevating body temperature during the febrile response to infection. The primary sources of neurally regulated metabolic heat production are mitochondrial oxidation in brown adipose tissue, increases in heart rate and shivering in skeletal muscle. Thermogenesis is regulated in each of these tissues by parallel networks in the central nervous system, which respond to feedforward afferent signals from cutaneous and core body thermoreceptors and to feedback signals from brain thermosensitive neurons to activate the appropriate sympathetic and somatic efferents. This review summarizes the research leading to a model of the feedforward reflex pathway through which environmental cold stimulates thermogenesis and discusses the influence on this thermoregulatory network of the pyrogenic mediator, prostaglandin E2, to increase body temperature. The cold thermal afferent circuit from cutaneous thermal receptors ascends via second-order thermosensory neurons in the dorsal horn of the spinal cord to activate neurons in the lateral parabrachial nucleus, which drive GABAergic interneurons in the preoptic area to inhibit warm-sensitive, inhibitory output neurons of the preoptic area. The resulting disinhibition of thermogenesis-promoting neurons in the dorsomedial hypothalamus and possibly of sympathetic and somatic premotor neurons in the rostral ventromedial medulla, including the raphe pallidus, activates excitatory inputs to spinal sympathetic and somatic motor circuits to drive thermogenesis.
Thermogenesis, the production of heat energy, is an essential component of the homeostatic repertoire to maintain body temperature during the challenge of low environmental temperature. In mammals, alert consciousness and normally patterned motor activities only occur when the temperature of the central nervous system is approximately 36-39°C. A fall in cellular temperature reduces enzyme efficiency and diffusion capacity, reducing cellular energy availability and membrane ion fluxes. Below-normal brain temperatures are associated with reduced alertness and with behavioural and physiological disorganization, although without physical damage to the CNS or peripheral tissues. In hibernating mammals, for instance, reduction of body and brain temperature to approximately 5°C is associated with torpor and inactivity, but normal consciousness and behaviour resume with reversal of the hibernation process. Additionally, the heat generated during stimulated thermogenesis contributes to fever, a controlled elevation in body temperature that reduces pathogen viability and stimulates immune cell responses. Since energy consumption during thermogenesis can involve oxidation of lipid fuel molecules, regulation of thermogenesis in response to metabolic signals can also contribute to energy balance and regulation of body adipose stores.
Thermogenesis can occur to a greater or lesser extent in most tissues, since heat generation is a byproduct of the inefficiency of mitochondrial ATP production and of ATP utilization. However, CNS thermoregulatory networks can stimulate thermogenesis in response to a cold environment, to a fall in core body temperature or to the presence of pyrogenic cytokines primarily in three tissues: brown adipose tissue (BAT), heart and skeletal muscle. During the rapid, repeated skeletal muscle contractions of shivering and during increases in heart rate, thermogenesis arises primarily from the inefficiency of energy utilization in cross-bridge cycling and calcium ion sequestration and, to a lesser degree, from mitochondrial membrane proton leak in the course of ATP production from fuel substrate oxidation (Rall & Woledge, 1990; Jubrias et al. 2008). The muscle contractions of shivering result from rhythmic bursts of activity in the α-motoneurons innervating skeletal muscle fibres. The CNS network generating the cold-evoked bursts of α-motoneuron activity is not well understood, but includes transmission of cutaneous cold afferent signals through the lateral parabrachial nucleus (LPB; Nakamura & Morrison, 2008a), integration of thermoregulatory signals in the preoptic area (POA; Zhang et al. 1995) and activation of fusimotor neurons that is dependent on neurons in the rostral ventromedial medulla (RVLM; Tanaka et al. 2006; Brown et al. 2007). The increases in heart rate in response to cold or during fever are sympathetically mediated and involve a neural network that parallels the one controlling the sympathetic activation of BAT thermogenesis.
In contrast to the indirect nature of shivering thermogenesis in skeletal muscles that are normally used to produce movement and posture, non-shivering or adaptive thermogenesis in BAT is the specific metabolic function of this tissue and is accomplished by the heat-generating capacity of a significant facilitated proton leak across the extensive mitochondrial membranes of the brown adipocytes, which occurs because of the high expression of uncoupling protein 1 (UCP1) in BAT mitochondria (Cannon & Nedergaard, 2004). The level of BAT sympathetic nerve activity (SNA) and noradrenaline release and β3-adrenergic receptor binding to brown adipocytes determine the level of thermogenesis in BAT by regulating both the activity of lipases providing the immediate fuel molecules for BAT mitochondria and the level of expression of BAT mitochondrial UCP1 (Cannon & Nedergaard, 2004).
Brown adipose tissue has developed as an essential thermoregulatory effector in cold defense in rodents and other small mammals (Golozoubova et al. 2006), including infant humans, since their large surface area to body mass ratio suggests that basal metabolism alone would yield insufficient heat to maintain body temperature in cold environments. The potential significance of BAT thermogenesis in adult humans and other large mammals has been controversial, at least in part, because evidence for its existence has been lacking. However, several recent observations using positron emission tomographic scanning to assess tissue glucose uptake (reviewed by Nedergaard et al. 2007) have demonstrated a remarkable amount of brown adipose tissue in adult humans, and the locations of BAT depots in adult humans bear a striking similarity to those in rodents: a large BAT pad in the vicinity of the scapulae and shoulders and individual pads atop each sympathetic ganglion and surrounding the adrenal glands and kidneys. The curious localization of BAT pads, exemplified by those over each sympathetic ganglion, suggests that, in addition to the defense of core temperature in the cold, BAT may also serve an as yet undescribed function to maintain optimal neuronal and synaptic function in specific locations in situations of reduced core body temperature. Similar to its function in smaller mammals, adult human BAT activity (assessed by glucose uptake) is highly responsive to β-adrenergic agonists (Soderlund et al. 2007) and to environmental temperature (Christensen et al. 2006). The recent description of adult human BAT is expected to stimulate quantitative assessment of its significance in adult human thermoregulation and energy homeostasis.
This review describes our current understanding of the central thermoregulatory network through which cutaneous cold sensation or reductions in brain and core temperature elicit increases in BAT SNA and thermogenesis. The regulation of BAT thermogenesis in response to environmental temperature constitutes a feedforward reflex (Fig. 1), in that the stimulus of a cold environment in contact with skin thermal receptors is not, itself, affected by the evoked thermogenic response, but rather, BAT thermogenesis is initiated to counter a ‘predicted’ fall in body core temperature that would result from the exposure to the cold environment. In contrast, the control of BAT thermogenesis by temperature-sensitive neurons in the brain constitutes a negative feedback reflex in that the neurons activated by the increases in brain temperature resulting from stimulated BAT thermogenesis act, in turn, to inhibit sympathetic outflow to BAT.
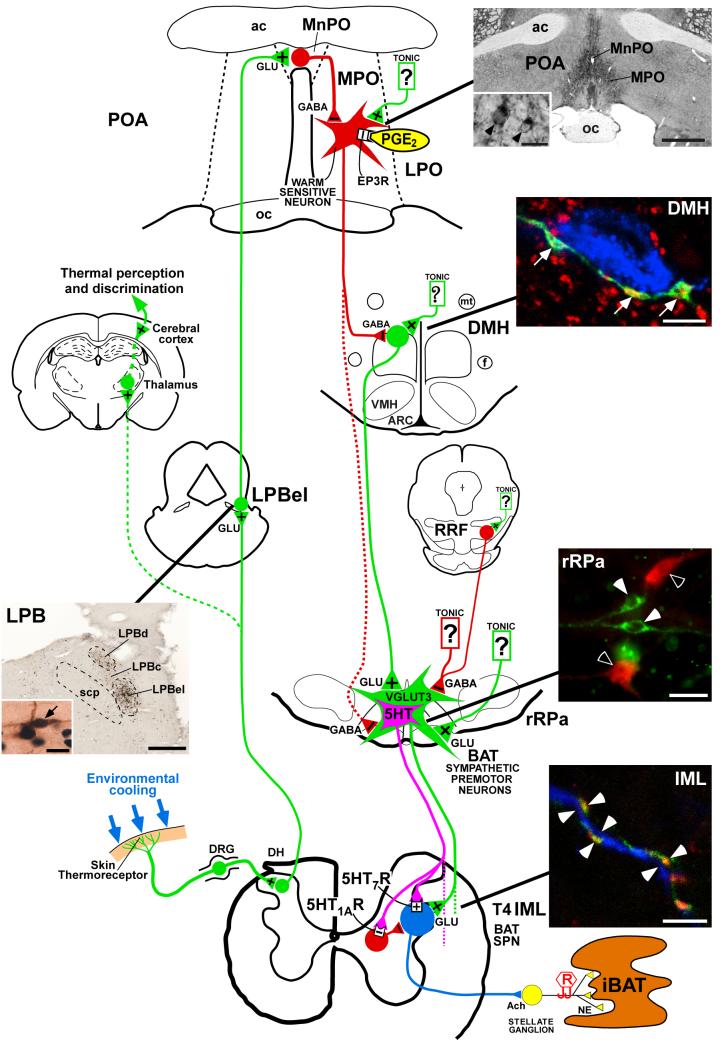
Figure 1. Schematic diagram summarizing the proposed model for the feedforward reflex circuit for cold-evoked brown adipose tissue thermogenesis.
Photomicrographs of the lateral parabrachial nucleus (LPB), the preoptic area (POA), the dorsomedial hypothalamus (DMH), the rostral raphe pallidus (rRPa) and spinal intermediolateral nucleus (IML) illustrate the anatomical substrates for key neurochemical and synaptic aspects of the proposed thermoregulatory circuit. In the LPB panel, (LPBel), central (LPBc) and dorsal (LPBd) subnuclei of the LPB contain neurons that are retrogradely labelled with a tracer (brown) from the median preoptic (MnPO) subregion of the POA. Retrogradely labelled neurons in the LPBel and LPBc, but not those in the LPBd, also express Fos (blue-black nuclei) following cold exposure of the animals (arrow, inset); scp, superior cerebellar peduncle; scale bars represent 0.5 mm and 15 μm (inset). Reproduced with permission from Nakamura & Morrison, 2008b). In the POA panel, immunohistochemistry for EP3 receptors (EP3R) shows the localization of these receptors in cell bodies (inset, arrowheads) and dendritic fibres of neurons that are distributed in the MnPO and medial preoptic (MPO) subregions of the POA; ac, anterior commissure; oc, optic chiasm; LPO, lateral preoptic area; PGE2, prostaglandin E2; GLU, glutamate; scale bars represent 1 mm and 20 μm (inset). Reproduced with permission from Nakamura et al. (1999). In the DMH panel, axon swellings of POA neurons (green) that are positive for a marker of GABAergic terminals (red) are closely apposed (arrows) to neurons that are retrogradely labelled with a tracer (blue) from the rRPa; ARC, arcuate nucleus; f, fornix; mt, mammillothalamic tract; VMH, ventromedial hypothalamic nucleus; RRF, retrorubral field; scale bar represents 5 μm. Reproduced with permission from Nakamura et al. (2005). The rRPa panel shows double immunofluorescence labelling for vesicular glutamate transporter 3 (VGLUT3)-positive (green, white arrowheads) and serotonin (5-HT)-positive neurons (red, open arrowheads); BAT, brown adipose tissue; scale bar represents 30 μm. The IML panel shows that axon swellings of rRPa neurons (green) that are positive for VGLUT3 (red) are closely associated (white arrowheads) with dendritic fibres positive (blue) for a marker of sympathetic preganglionic neurons (SPNs); Ach, acetylcholine; DH, dorsal horn; DRG, dorsal root ganglia; iBAT, interscapular BAT; NA, noradrenaline; R, recording electrode; scale bar represents 5 μm. Reproduced with permission from Nakamura et al. (2004a).
Mechanisms for thermoreception
Cutaneous cool thermoreception
Temperature information that is detected by thermoreceptors located in surface and core body parts is transmitted to the POA, which is a thermoregulatory centre located in the rostral pole of the hypothalamus. Environmental temperature has direct and more rapid effects on skin temperature than on the temperatures within the body core. When environmental temperature is reduced, skin temperature rapidly falls, whereas brain and rectal temperatures are not affected or slightly increased in rats (Lomax et al. 1964; Bratincsak & Palkovits, 2005), cats (Forster & Ferguson, 1952) and dogs (Hellstrom & Hammel, 1967). Thus, feedforward thermal afferents from the skin provide the POA with signals to rapidly initiate cold-defensive thermogenic responses before environmental thermal challenges affect body core temperature.
The molecular mechanism of cutaneous thermoreception has been extensively investigated. Recent studies suggest that the transient receptor potential (TRP) family of cation channels mediates sensation across a broad physiological range of temperatures. Among the TRP family channels, TRPM8 mediates a dominant part of cold sensation, since TRPM8-deficient nerve fibres show profound loss of cold sensitivity and TRPM8-deficient mice exhibit a reduced ability to avoid innocuous cold temperatures (Fig. 2; Bautista et al. 2007; Colburn et al. 2007; Dhaka et al. 2007). The TRPM8 channel is activated by modest cooling (environmental temperatures <27°C; McKemy et al. 2002; Peier et al. 2002a) and is distributed in the cell bodies, axons and peripheral free nerve endings of a population of primary somatosensory neurons that have small-diameter cell bodies in the dorsal root and trigeminal ganglia (McKemy et al. 2002; Peier et al. 2002a; Bautista et al. 2007), consistent with a role for TRPM8 in cold-sensing through C fibres. The TRPM8 channel can also be activated by menthol or icillin (McKemy et al. 2002; Peier et al. 2002a), and application of menthol to the skin evokes warm-seeking behaviour as well as cold-defensive, physiological responses, including BAT and shivering thermogenesis and cutaneous vasoconstriction (Tajino et al. 2007). However, the ability of TRPM8-deficient mice to maintain body temperature in cold environments has not yet been tested, although their basal core body temperature in a thermoneutral environment is not different from that of wild-type mice (Bautista et al. 2007).
Figure 2. Mice deficient in TRPM8 exhibit profoundly diminished responses to cold.
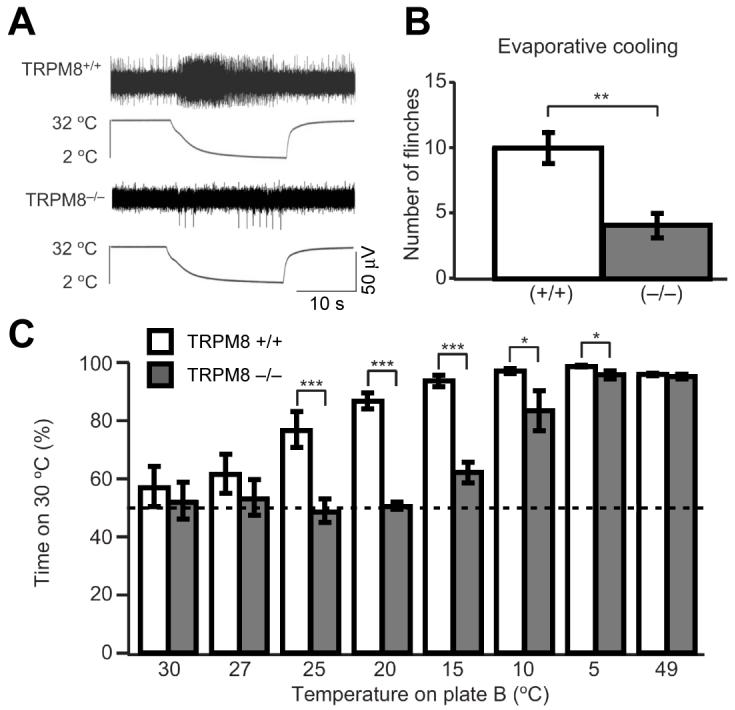
A, the discharge response of cutaneous C fibres to a cold ramp that is seen in wild-type mice (top) mostly disappears in TRPM8-deficient mice (bottom). B, licking and flinching in response to evaporative cooling was measured for 1 min following application of acetone to the hindpaw. The TRPM8-deficient mice displayed significantly decreased behaviour compared with wild-type littermates. C, wild-type and TRPM8-deficient littermates were allowed to choose between adjacent surfaces adjusted to 30°C versus a range of temperatures, as shown. The percentage of time spent at 30°C over a 5 min period is shown. The TRPM8-deficient mice show a clear, but not complete, deficit in their ability to discriminate between cold and warm surfaces. Graphs display means ± s.e.m.; *P < 0.05, **P < 0.01 and ***P < 0.001. Modified with permission from Bautista et al. (2007)
Another potential cold receptor TRP channel is TRPA1, which is activated by colder temperatures (environmental temperatures <17°C) than TRPM8 or by mustard oil and other pungent spice ingredients (Story et al. 2003; Bandell et al. 2004; Jordt et al. 2004). However, contribution of TRPA1 to cold sensation in vivo is still controversial. Kwan et al. (2006) showed that TRPA1-deficient mice displayed a reduced sensitivity to a cold temperature (0°C), while Bautista et al. (2006) found no such deficit in the response to even lower temperatures (environmental temperatures ∼-10°C) in TRPA1-deficient mice, and did not observe a delayed onset of shivering when these deficient mice were exposed to cold temperatures. A non-TRP channel-mediated cold-sensing mechanism in which cooling causes depolarization of cold-sensory neurons by closing a background K+ channel has also been proposed (Viana et al. 2002).
Cutaneous warm thermoreception
The TRP family also has warm-sensitive channels, TRPV3 and TRPV4, which are activated by innocuous warm temperatures with a environmental temperature threshold of 33-39 and 25-34°C, respectively (Guler et al. 2002; Peier et al. 2002b; Smith et al. 2002; Watanabe et al. 2002; Xu et al. 2002). The TRPV3 channel is increasingly activated by repeated heating (Peier et al. 2002b;Xu et al. 2002) and, intriguingly, shows different sensitivity to the direction of temperature change, resulting in hysteresis across thermal activation-deactivation cycles (Xu et al. 2002), similar to thermosensitive responses exhibited by primary warm afferent fibres (Hensel et al. 1960; Iriuchijima & Zotterman, 1960; Hellon et al. 1975). The TRPV3 channel is also activated by camphor (Moqrich et al. 2005), which sensitizes cutaneous warm sensation, or by flavours derived from oregano, thyme and clove (Xu et al. 2006), which elicit a warm sensation when applied to the tongue. Expression of either TRPV3 or TRPV4 is prominent in keratinocytes in skin epidermis, but low in somatosensory ganglia (Guler et al. 2002; Peier et al. 2002b;Xu et al. 2006). Although it is still unknown how thermal information detected by these TRP channels in keratinocytes is transmitted to sensory nerves, factors released from stimulated keratinocytes, such as interleukin-1β (Xu et al. 2006), might diffuse to adjacent sensory nerves. Mice lacking either TRPV3 or TRPV4 show altered behaviours in discriminating innocuous warm temperatures compared with wild-type mice (Lee et al. 2005; Moqrich et al. 2005). However, TRPV4-deficient mice exhibit intact circadian changes in body temperature and can defend their core temperature in a cold (4°C) or hot environment (35°C; Liedtke & Friedman, 2003; Lee et al. 2005). The effect of TRPV3 deficiency on thermoregulatory responses to environmental thermal challenges remains to be investigated.
Thermoregulatory role of peripheral TRPV1
Intriguing effects on body temperature control have been reported for agonists and antagonists of TRPV1, a TRP channel that can be activated by a noxious range of heat (>43°C), by protons (pH ≤ 5.9) or by capsaicin (Caterina et al. 1997; Tominaga et al. 1998). Peripheral or central administration of capsaicin induces hypothermia (Jancso-Gabor et al. 1970; Hori, 1984), an effect beneficial to people living in hot climates, but is ineffective in TRPV1-deficient mice (Caterina et al. 2000). Furthermore, administration of potent TRPV1 antagonists induces hyperthermia (Swanson et al. 2005; Gavva et al. 2007) by both increasing metabolism and reducing heat loss from the body surface, but not by evoking warm-seeking behaviour (Steiner et al. 2007), and this hyperthermic effect is probably exerted by antagonizing TRPV1 channels located within abdominal viscera (Steiner et al. 2007). Therefore, tonic activation of peripheral TRPV1 channels, effected by non-thermal stimuli at body temperatures below the threshold for TRPV1 activation, could provide afferent signals to lower body temperature; however, TRPV1-deficient mice exhibit no obvious deficit in body temperature control (Szelenyi et al. 2004; Iida et al. 2005). Further investigations would be required to elucidate the thermoregulatory role of TRPV1.
Thermoreception in the body core
In addition to cutaneous thermoreception, thermoreceptive mechanisms exist in body core structures, including the brain, spinal cord and abdomen. The POA contains abundant neurons whose activity is affected by local brain temperature (Nakayama et al. 1961, 1963) and these are described below. Cold and warm receptors are included among the splanchnic and vagus nerve afferent fibres distributed in the abdomen, and their responses to temperature changes are similar to those of cutaneous thermoreceptors (Riedel, 1976; Gupta et al. 1979). However, how abdominal thermal information is transmitted to the POA is mostly unknown. Temperature changes in the spinal cord can affect the activity of thermoregulatory neurons in the POA (Guieu & Hardy, 1970). Although this finding implies the existence of thermoreceptive mechanisms inherent to the spinal cord, such as thermosensation by spinal neurons, TRP channels that are located in the central endings of primary somatosensory fibres in the spinal dorsal horn (Tominaga et al. 1998; Bautista et al. 2007) are also likely to sense spinal temperature, and the spinal thermal signals could be integrated with cutaneous thermal signals at the spinal cord level. Temperatures in deep body core structures, including the brain, are not as immediately susceptible to changes in environmental temperature as are skin temperatures (Forster & Ferguson, 1952; Lomax et al. 1964; Hellstrom & Hammel, 1967; Bratincsak & Palkovits, 2005). Thus, rather than responding directly to changes in environmental temperature, core body thermosensation would be expected to play a role: (a) in setting the basal tone of thermoregulatory effector efferents; (b) in enhancing thermoregulatory responses in situations of extreme thermal environments when the feedforward thermoregulatory responses driven by changes in skin temperature have proven inadequate to prevent changes in brain or body core temperature; and (c) in responding to challenges to thermal homeostasis involving changes in temperature within the body, such as exercise, intake of cold fluids or haemorrhage. Thus, it is important to understand how thermosensory signals from the skin and from the body core are integrated.
Central afferent pathway for thermosensation
Dorsal horn lamina I neurons
Thermal information detected by cutaneous thermoreceptors is transmitted through primary somatosensory fibres to the spinal or trigeminal dorsal horns (Fig. 1), in which lamina I neurons receive most cutaneous thermal signals (Craig, 2002). The best-known thermal somatosensory ascending pathway from lamina I neurons is the spinothalamocortical pathway, in which lamina I neurons directly synapse on neurons in the thalamus that project to the primary somatosensory cortex, leading to perception and discrimination of cutaneous temperature (Craig et al. 1994; Craig, 2002). Craig and colleagues have described three main classes of spinothalamic and trigeminothalamic lamina I neurons that were categorized by their responses to cutaneous thermal and mechanical stimuli: nociceptive-specific cells responding to noxious mechanical and heat stimuli; polymodal nociceptive cells responding to noxious mechanical, heat and cold stimuli; and thermoreceptive-specific cells responding linearly to graded, innocuous cooling or warming stimuli and not being activated further in the noxious temperature range (Andrew & Craig, 2001; Craig et al. 2001). Considering that cutaneous thermal stimuli that trigger thermoregulatory responses are mostly in the innocuous range, thermoreceptive-specific lamina I neurons probably convey the dominant signals leading to body temperature control. The question of whether the spinothalamocortical pathway contributes to the thermoregulatory responses to defend body temperature during changes in environmental temperature and, if not, what mechanism conveys cutaneous thermal information to the thermoregulatory centre in the POA, has only recently been answered.
Thermoregulatory afferent pathway to the POA
Neuroanatomical and physiological experiments to answer these questions identified a ‘missing link’ in the central thermal afferent pathway connecting second-order thermal sensory neurons in the spinal dorsal horn to the thermoregulatory integration site in the POA. In functional neuronal tracing experiments, neurons retrogradely labelled following tracer injections into the POA and activated (Fos expression) following cold exposure (4°C) of the animals were found to be densely clustered in the external lateral (LPBel) subnucleus of the mesencephalic LPB with an extension into the central subnucleus (LPBc; Fig. 1, LPB inset; Nakamura & Morrison, 2008a). The greatest numbers of double-labelled LPB neurons were found when the tracer injection was centred on the mid-line subregion of the POA, including the median preoptic nucleus (MnPO), suggesting that neurons in the LPBel and LPBc transmit cool thermosensory signals mainly to the MnPO rather than more lateral POA subnuclei, such as the medial (MPO) or lateral POA (LPO; see Fig. 1, POA inset, for anatomical definitions of the POA subnuclei). The LPB receives numerous projections from the dorsal horn (Cechetto et al. 1985; Bernard et al. 1995; Feil & Herbert, 1995). Indeed, axonal swellings of dorsal horn neurons were found to be closely associated with postsynaptic structures of POA-projecting LPBel neurons (Nakamura & Morrison, 2008a), providing further support for the view that POA-projecting LPBel neurons are activated by direct somatosensory inputs from dorsal horn lamina I neurons.
Physiologically, the LPB-mediated thermosensory pathway to the POA comprises an essential link in the feedforward pathway through which thermal homeostasis is maintained during environmental cold challenges. Either inhibition of local neurons in the LPBel or blockade of their glutamate receptors completely suppresses skin cooling-evoked cold-defense responses, including BAT and shivering thermogenesis and increases in metabolism and heart rate (Fig. 3; Nakamura & Morrison, 2008a). Thus, activation of LPBel neurons, probably by glutamatergic inputs from lamina I neurons driven by cutaneous cool signals, is essential for transmission of the cold thermal afferent stimulus to initiate cold defense responses (Fig. 1). Consistent with this notion, rats that have bilateral lesion of the LPB fail to maintain body temperature in a cool environment (17°C; Kobayashi & Osaka, 2003). Glutamatergic stimulation of LPBel neurons with NMDA can evoke increases in BAT thermogenesis, metabolism and heart rate that mimic skin cooling-evoked physiological responses. Such responses triggered by LPBel stimulation are blocked by antagonizing glutamate receptors in the MnPO (Nakamura & Morrison, 2008a), suggesting that cutaneous cool signalling to the POA is mediated by glutamatergic inputs from LPBel neurons to the MnPO (Fig. 1). The idea that the MnPO receives glutamatergic inputs for eliciting cold-defensive responses to environmental cooling is also supported by the finding that glutamatergic stimulation of MnPO neurons evokes thermogenic, metabolic and tachycardic responses similar to cold-defensive responses (Nakamura & Morrison, 2008b). Whether different thermoregulatory effector tissues are activated by different populations of MnPO neurons or whether a homogeneous population of cool afferent-responsive MnPO neurons influences effector-specific populations of MPO neurons is unknown.
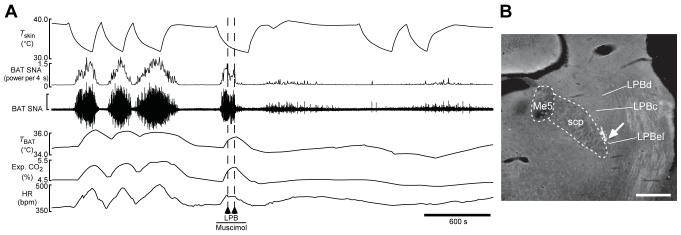
Figure 3. Inhibition of neuronal activation in the lateral parabrachial nucleus (LPB) blocks skin cooling-evoked thermogenic, metabolic and cardiac responses.
A, increases in brown adipose tissue sympathetic nerve activity (BAT SNA), BAT temperature (TBAT), expired (Exp.) CO2 and heart rate (HR) that were evoked by reducing temperature of rat trunk skin (Tskin) were no longer observed after bilateral nanoinjections (dashed lines) of muscimol into the external lateral part of the LPB (LPBel); vertical scale bar represents 100 μV for BAT SNA. B, representative view of a nanoinjection site in the LPBel as identified with fluorescent beads (arrow). Abbreviations: LPBc, central part of the LPB; LPBd, dorsal part of the LPB; and Me5, mesencephalic trigeminal nucleus; scale bar represents 0.5 mm. Modified with permission from Nakamura & Morrison (2008a).
In addition to thermosensory signals coming from the skin through the spinal cord, the LPB receives massive visceral afferent information, via the nucleus of the solitary tract, related to gastric tension, satiety, taste, thirst, blood pressure and temperature (Saper, 2002; Geerling & Loewy, 2008). The LPB subregions that receive these visceral projections from the nucleus of the solitary tract are located primarily caudal to the mesencephalic LPBel that receives thermal somatosensory projections from the dorsal horn (Herbert et al. 1990; Nakamura & Morrison, 2008a). It is possible, however, that POA-projecting, cool-responsive neurons in the LPBel receive visceral afferent signals through their caudally extending dendritic arbors or through interneurons connecting caudal and rostral LPB subregions. Further studies are needed to determine whether the thermal somatosensory responses of LPBel neurons in the MPO modified by other modalities to provide an integrated signal to the POA, a central site controlling a variety of homeostatic functions.
Recordings from single LPB cells in vivo revealed that the firing rate of most neurons in the LPBel that were antidromically identified as projecting to the MnPO increased markedly in response to skin cooling and then returned to the basal level following skin rewarming; responses that paralleled the skin cooling-evoked changes in BAT SNA (Nakamura & Morrison, 2008a). The firing rate increase of these cooling-responsive LPBel neurons was linearly related to the skin temperature in the range from 36.6 to 34.4 °C (a range just below the normal body temperature of rats) and was followed by a sustained elevation in firing during further skin cooling. Although skin temperature was still low, LPBel neuronal discharge rapidly returned to basal levels shortly after skin rewarming was initiated (Nakamura & Morrison, 2008a). This differential sensitivity to the direction of skin temperature change might be ascribed to properties of cutaneous thermoreceptors, since a similar response hysteresis to skin cooling and rewarming has been observed in neurons in the thermosensory pathway, including cool-sensory primary fibres (Hensel et al. 1960; Iriuchijima & Zotterman, 1960) as well as thermoreceptive-specific lamina I neurons mediating cool signals (Craig et al. 2001). Most cool-sensitive LPBel neurons are not activated in response to noxious mechanical stimulus such as tail pinch (Nakamura & Morrison, 2008a), providing further support for the idea that LPBel neurons involved in thermoregulatory afferent signalling receive thermal information predominantly from thermoreceptive-specific lamina I neurons rather than other lamina I neurons mediating nociception.
Preliminary data suggest that cutaneous warm signals are also sent to the POA through an LPB-mediated thermal afferent pathway, which involves neurons in a different subnucleus of the LPB. Many POA-projecting neurons that are activated by innocuous heat exposure (environmental temperature of 36°C) are distributed in the dorsal subnucleus of the LPB, which also contains POA-projecting neurons whose firing activity is markedly increased by skin warming (Nakamura & Morrison, 2007b). Although the functional contribution of these warm-responsive LPB neurons in thermoregulatory responses is currently unknown, it seems reasonable to assume that cool and warm thermosensory signals, transmitted from the skin through separate LPB pathways, would have opposite effects on POA neurons controlling thermogenesis.
Spinothalamocortical pathway
The spinothalamo-cortical pathway for thermal perception and localization does not contribute to the feedforward mechanism for triggering involuntary thermoregulatory responses to environmental cold challenges. Rats with lesions of the thalamic regions that receive thermal somatosensory signals from the spinal cord exhibit no deficits in sympathetic thermogenic responses to skin cooling, but lack the skin cooling-evoked changes in primary somatosensory cortical EEG activity that are observed in intact animals, indicating that autonomic cold-defensive responses do not require a thalamic relay (Nakamura & Morrison, 2008a). Consistently, even after removal of the neocortex, dorsal hippocampus and most of the striatum, animals still maintain the ability to initiate a metabolic increase in response to skin cooling (Osaka, 2004b). Therefore, there are at least two thermosensory pathways ascending from the dorsal horn: the spinothalamocortical pathway, leading to perception and discrimination of cutaneous thermal sensation; and the spinoparabrachiopreoptic pathway, transmitting feedforward information on cutaneous temperature to the POA that is required for defending body temperature against changes in environmental temperature (Fig. 1). Anatomical studies suggest that dorsal horn lamina I neurons can provide cutaneous thermal signals to both the thalamus and LPB through their axon collaterals (Hylden et al. 1989; Li et al. 2006). Separate sensory afferent pathways for thermal perception and for thermoregulation may allow thermosensory signals going through the thalamus to the neocortex to be modulated by sensory signals of a different modality, such as vision or audition (Driver & Noesselt, 2008), while those to the POA via the LPB could be integrated with information on the status of other homeostatic variables, such as body fluid osmolarity and sodium and water balance (De Castro e Silva et al. 2006; Geerling & Loewy, 2006; De Luca et al. 2007) or food intake and fuel availability (Wang et al. 1999; Yamamoto & Sawa, 2000; Wilson et al. 2003). Additionally, the spinothalamocortical pathway may play a role in the appropriate induction of behavioural thermoregulatory responses, such as seeking a warm or cold environment.
Preoptic area mechanisms in thermoregulation
Response to thermal afferents from the skin
Based on the aforementioned findings, the POA receives feedforward cool signals from the skin that are probably mediated by glutamatergic inputs from cool-responsive LPBel neurons. The fact that the POA subregion receiving thermosensory cold signals is confined to the MnPO is suggested by the findings that the projections from LPBel neurons activated by skin cooling terminate mainly in a median part of the POA (Nakamura & Morrison, 2008a) and that glutamatergic stimulation or disinhibition of the MnPO with nanoinjections of NMDA or bicuculline, respectively, evokes physiological responses mimicking cold-defensive responses, while the same stimulation of the MPO or LPO does not (Nakamura & Morrison, 2008b). Similarly, disinhibition of neurons in the MnPO elicits shivering (K. Nakamura & S. F. Morrison, unpublished observations). Furthermore, inhibition of MnPO neurons completely blocks the activation of BAT thermogenesis and increases in metabolism and heart rate that are all evoked by skin cooling (Nakamura & Morrison, 2008b), indicating that activation of MnPO neurons is an essential process in the central mechanism for eliciting cold-defensive responses to environmental cold challenges (Fig. 1).
In a thermoneutral environment, when cold-defensive responses are not needed, inhibitory projection neurons in the MPO are postulated to tonically inhibit neurons in caudal brain regions, such as the dorsomedial hypothalamus (DMH) and rostral ventromedial medulla, including the rostral raphe pallidus nucleus (rRPa), whose activation leads to stimulation of cold-defense responses (Fig. 1). That such a disinhibitory mechanism is a fundamental aspect of the control of thermoregulatory effectors for cold defense is based on the findings that: (a) a coronal transection just caudal to the POA evokes BAT thermogenesis (Fig. 4B;Chen et al. 1998); (b) lesion of the MPO, but not of the ventral LPO, evokes hyperthermia by increasing metabolism and by stimulating shivering thermogenesis and heat conservation through cutaneous vasoconstriction (Szymusiak & Satinoff, 1982); and (c) inhibition of neurons in the MPO, but not those in the MnPO or LPO, increases body core temperature, EMG activity (shivering), metabolism and heart rate (Osaka, 2004a;Zaretsky et al. 2006). Furthermore, BAT and shivering thermogenesis as well as increases in metabolism and heart rate that are evoked by skin cooling are blocked by antagonizing GABAA receptors in the MPO (Osaka, 2004a; Nakamura & Morrison, 2007a). Thus, skin cooling-evoked responses are postulated to require a local circuit in the POA in which cutaneous cool signals that are received by MnPO neurons provide a GABA input to the inhibitory projection neurons in the MPO to reduce their tonic activity, resulting in disinhibition of neurons in caudal brain regions whose excitation stimulates thermoregulatory effectors for cold defense (Fig. 1). Consistent with this hypothesis, increases in BAT thermogenesis, metabolism and heart rate that are evoked by stimulation of MnPO neurons are all reversed completely by antagonizing GABAA receptors in the MPO (Nakamura & Morrison, 2008b). The existence of GABAergic interneurons in the MnPO that innervate the MPO projection neurons, although not yet directly demonstrated, is supported by the anatomical observations that: (a) some MnPO neurons innervate the MPO (Uschakov et al. 2007); (b) the MnPO contains many GABAergic neurons (Nakamura et al. 2002; Gong et al. 2004); and (c) many neurons in the MnPO, rather than the MPO or LPO, are activated (express Fos protein) in response to reduced environmental temperature (Bratincsak & Palkovits, 2004). Furthermore, microdialysis determinations of extracellular GABA in the POA of freely moving rats were elevated during cold exposure and reduced during heat exposure (Ishiwata et al. 2005).
Figure 4. The preoptic area (POA) contains warm-sensitive neurons and maintains a tonic inhibition of neurons in caudal thermogenic brain areas.
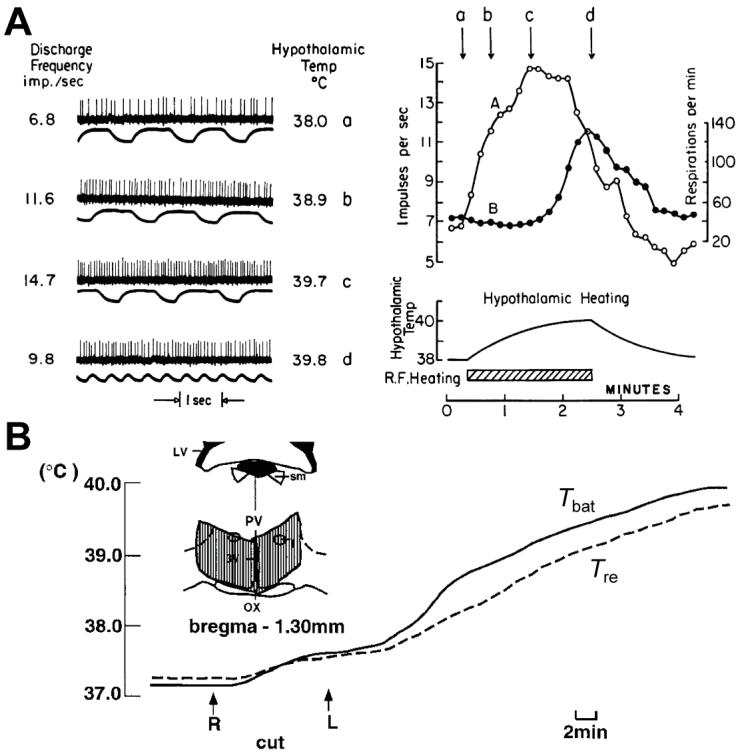
A, Nakayama and colleagues reported the first unit recording from warm-sensitive neurons in the POA and anterior hypothalamus of anaesthetized cats. Discharge frequency of this POA neuron and respiration rate increased in response to local heating by radio frequency current. Reproduced from the classic paper of Nakayama et al. (1963) with permission. B, Effects of knife cuts at the level immediately caudal to the POA on the interscapular BAT temperature (Tbat) and rectal temperature (Tre, core temperature). Knife cuts applied on the right (R) and left (L) sides increased BAT thermogenesis. Inset shows the extent of the knife cuts (hatched area). Abbreviations: 3V, third ventricle; LV, lateral ventricle; OX, optic chiasm; PV, paraventricular thalamic nuclei; and sm, stria medullaris thalami. Modified with permission from Chen et al. (1998).
Response to local brain temperature
Not only skin cooling but also cooling the local environment of POA neurons evokes sympathetic thermogenesis in BAT as well as shivering thermogenesis (Hammel et al. 1960; Imai-Matsumura et al. 1984). Nakayama and colleagues made important progress by obtaining the first single-cell recording from thermosensitive neurons that are abundantly distributed in the POA (Nakayama et al. 1961, 1963), most of which are activated by warm temperatures (warm-sensitive neurons; Boulant & Dean, 1986; Fig. 4A). Warm-sensitive POA neurons are tonically active at thermoneutral temperatures (Nakayama et al. 1961, 1963), and the POA contains warm-sensitive neurons whose tonic discharge is reduced by skin cooling and also whose thermosensitivity to preoptic temperature is increased when the skin is cooled (Boulant & Hardy, 1974). We therefore propose (Fig. 1) that tonically active, warm-sensitive neurons located in the MPO are inhibited by reductions in local temperature and by inputs from cool-sensitive cutaneous thermoreceptors relayed via GABAergic MnPO neurons and that they function as MPO projection neurons to inhibit thermogenic neurons in the DMH (Nakamura et al. 2005) and in rRPa (Nakamura et al. 2002). However, whether warm-sensitive neurons project axons outside of the POA remains unknown.
The neurophysiological mechanism underlying the thermosensitivity of warm-sensitive neurons in the POA continues to be investigated. The suggestion that a heat-induced membrane depolarization allows warm-sensitive neurons to reach their discharge threshold potential and then determines their discharge frequency (Kobayashi et al. 2006) contrasts with the concept that a temperature-dependent (i.e. warming-dependent) facilitation of the rate of rise of a depolarizing (pacemaker) prepotential in warm-sensitive neurons shortens the intervals between action potentials and thereby increases the firing rate (Boulant, 2006). In the latter case, a transient, outward hyperpolarizing K+ current (A-type potassium current) helps maintain a hyperpolarized membrane for a brief time after an action potential, and the heat-induced increase in the inactivation rate of the A-type potassium current allows the prepotential to depolarize at a faster rate (Boulant, 2006). Ion channels that could contribute to the thermosensitivity of warm-sensitive neurons, such as hyperpolarization-activated cyclic nucleotide-gated channels, background potassium leak channels and TRPV4, are distributed in the POA and these, except for TRPV4, are localized in the cell bodies of many POA neurons, but are also distributed ubiquitously in the brain (Guler et al. 2002; Wechselberger et al. 2006). Identification of the molecule(s) responsible for the thermosensitivity of POA neurons awaits further investigation, and the identification of specific anatomical markers for thermosensitive neurons would be a major discovery in this field.
Prostaglandin EP3 receptor-containing neurons in the POA in fever
Neurons in the POA are critically involved in the elaboration of fever as well as in thermoregulatory responses. Fever is a controlled elevation in body temperature that plays a significant role in the acute phase reaction stimulated by endogenous pyrogens released during infection, providing an optimal hyperthermic environment for mounting host defenses against invading bacteria and viruses while reducing pathogen viability. Prostaglanin E2 (PGE2), which is synthesized in the brain vasculature in response to immune signals (Elmquist et al. 1997; Matsumura et al. 1998; Yamagata et al. 2001), acts as a powerful endogenous pyrogenic mediator in the POA. An earlier study in which PGE1 was injected into various sites throughout subcortical brain regions indicated that the POA is the sole region that can sense the E-series of prostaglandins to produce fever (Stitt, 1973; Williams et al. 1977). In a later study, detailed prostaglandin-sensitive sites within the POA were located by nanoinjecting a small dose of PGE2 (1 ng in 10 nl vehicle) and showed that such sites are in the MPO and MnPO (Scammell et al. 1996). In these POA subregions, the EP3 subtype of PGE receptor is localized on many neuronal somata and dendrites (Fig. 1, POA diagram; Nakamura et al. 1999, 2000). Although mRNA expression for the EP1 and EP4 subtypes is also detected in the POA (Oka et al. 2000), analyses of mice lacking each of the known PGE receptor subtypes showed that only EP3 receptor-deficient mice completely failed to show a febrile response to PGE2, interleukin-1β or endotoxin (Ushikubi et al. 1998), and EP1 receptor-deficient mice showed a partial attenuation of endotoxin-induced fever (Oka et al. 2003). Furthermore, genetic deletion of the EP3 receptor specifically in neurons distributed in the MnPO and MPO suppressed most of the febrile response to PGE2 or endotoxin (Lazarus et al. 2007). These lines of evidence indicate that the EP3 receptors in somatodendritic portions of POA neurons are the principal target site of PGE2 for its pyrogenic action and that activation of these receptors by PGE2 triggers the neuronal processes for fever induction.
The neuronal population expressing EP3 receptors in the POA provides direct projections to both the DMH and rRPa (Nakamura et al. 2002, 2005) and, as evidenced by pseudorabies viral tracing, EP3 receptor-expressing POA neurons multisynaptically innervate BAT (Yoshida et al. 2003). Since the majority of EP3-expressing POA neurons are GABAergic (Nakamura et al. 2002), the finding that antagonizing GABAA receptorsinthe DMH or rRPa evokes fever-like responses, including BAT thermogenesis and tachycardia (Morrison, 1999; Morrison et al. 1999; Zaretskaia et al. 2002; Cao et al. 2004), suggests that disinhibition of BAT and cardiac sympathoexcitatory neurons in these sites could contribute to febrile thermogenesis. Coronal transection just caudal to the POA evokes BAT thermogenesis (Fig. 4B; Chen et al. 1998), and inhibition of POA neurons with a muscimol nanoinjection elicits hyperthermic, cardiovascular and neuroendocrine responses similar to those evoked by a PGE2 nanoinjection into the same site (Zaretsky et al. 2006). Binding of PGE2 to EP3 receptors can inhibit neuronal activity by coupling to inhibitory GTP-binding proteins (Narumiya et al. 1999), and the tonic activity of most warm-sensitive neurons in the POA is inhibited by the E-series of prostaglandins (Schoener & Wang, 1976; Ranels & Griffin, 2003). Nonetheless, some spliced variants of EP3 receptors can also couple partially to stimulatory GTP-binding proteins (Negishi et al. 1995) and some warm-sensitive neurons in the POA are activated by PGE2 (Matsuda et al. 1992). Together, these data suggest a model (Fig. 1) in which EP3-expressing POA neurons, potentially the population of warm-sensitive POA neurons described above, normally maintain a tonic GABAergic inhibition of thermogenic neurons in the DMH and/or the rRPa and, during infection, local or systemic PGE2 binds to their EP3 receptors and reduces their tonic firing which, in turn, leads to disinhibition of thermogenic neurons in caudal brain regions and activation of thermoregulatory effectors to increase heat production and reduce heat loss (Nakamura, 2004; Nakamura et al. 2005).
A role for DMH neurons in driving thermogenesis
The observation that transection of the neuraxis immediately caudal to the POA (approximately 1.3-1.8 mm caudal to bregma) increases BAT SNA and BAT thermogenesis (Chen et al. 1998) suggests that the efferent output of the POA is inhibitory to thermogenesis. In contrast, transections made in the midbrain, just caudal to the hypothalamus (approximately 4.0-4.5 mm caudal to bregma), do not increase basal levels of BAT thermogenesis in normothermic animals (Rothwell et al. 1983) and, in fact, reverse PGE2-evoked increases in BAT SNA and thermogenesis (Morrison et al. 2004; Rathner & Morrison, 2006). Therefore, a simple model consisting of only a long inhibitory pathway from the POA neurons to medullary sympathetic premotor neurons does not provide an adequate explanation of the pathways necessary for mediating BAT thermogenesis in response to removal of the efferent output of the POA or to PGE2 within the POA, though such a pathway may contribute to BAT thermogenesis. Instead, data from experiments involving transection of the neuraxis suggest that a hypothalamic area is necessary for BAT thermogenesis in response to PGE2 acting within the POA.
Several areas of the hypothalamus, including the paraventricular hypothalamus (PVH), the ventromedial hypothalamus (VMH), the posterior hypothalamus (PH) and the dorsomedial hypothalamus/dorsal area of the hypothalamus (DMH/DA), have been implicated in thermogenesis; however, in studies involving the PVH, VMH and PH, the anatomical specificity of the observations has been called into question (Dimicco & Zaretsky, 2007) owing to the large injection volumes used, as well as the lack of appropriate anatomical control injections, or the use of electrical stimulation, electrolytic lesion or other methodological approaches that affect not only cell bodies but also fibres of passage. In contrast, several lines of evidence support a role of neurons in the DMH/DA in thermogenesis. Administration of endotoxin or cold exposure increases the expression of Fos in neurons of the DMH/DA (Elmquist et al. 1996; Cano et al. 2003; Sarkar et al. 2007). Furthermore, disinhibition of neurons within the DMH by blockade of GABAA receptors within the DMH increases BAT SNA (Cao et al. 2004) and thermogenesis (Fig. 5A; Zaretskaia et al. 2002), suggesting a tonic GABAergic inhibitory input to thermogenic neurons within the DMH (Fig. 1). This tonic GABAergic input to neurons within the DMH may originate in the POA, as evidenced by the observation that POA-derived GABAergic axon swellings make close appositions with DMH neurons, including those that project to the rRPa (Fig. 1, DMH inset; Nakamura et al. 2005). In addition, inhibition of neurons in the DMH/DA blocks febrile (Fig. 5B; Zaretskaia et al. 2003; Madden & Morrison, 2004; Morrison et al. 2004; Nakamura et al. 2005) and cold-evoked excitation of BAT SNA and thermogenesis (Nakamura & Morrison, 2007a), as well as shivering thermogenesis (Tanaka et al. 2001). Blockade of ionotropic glutamate receptors within the DMH also blocks the increase in BAT SNA and thermogenesis evoked by PGE2 within the POA (Madden & Morrison, 2004), suggesting that a glutamatergic input to neurons within the DMH is essential for these febrile responses, though the source of this input has yet to be determined. Taken together, these data suggest a model in which GABAergic inhibitory inputs from POA to sympathoexcitatory neurons in DMH are reduced by skin or core cooling or by PGE2 acting on EP3 receptors within the POA.
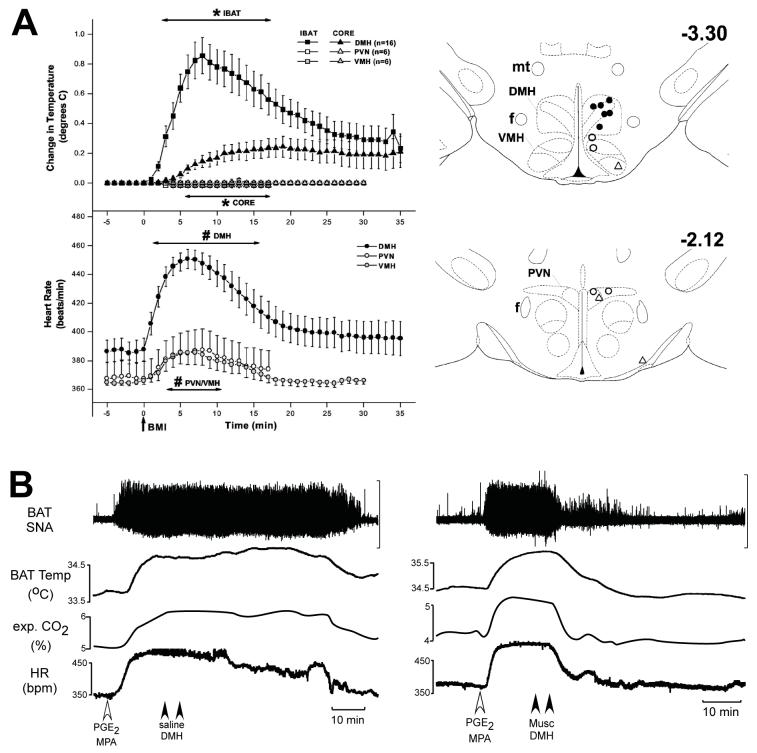
Figure 5. Disinhibition of neurons within the dorsomedial hypothalamus (DMH) increases thermogenesis in brown adipose tissue (BAT), and inhibition of neurons within the DMH reverses febrile-evoked BAT sympathetic nerve activity (SNA) and thermogenesis.
A, left panel, increases in interscapular BAT (IBAT; squares) and core (triangles) temperatures (top panel) and heart rate (HR, bottom panel) following unilateral microinjection of bicuculline (BMI) into the DMH (filled symbols), the paraventricular hypothalamus (PVN; open symbols) or the ventromedial hypothalamus (VMH; shaded symbols). A, right panel, schematic coronal sections at two levels of the hypothalamus depicting representative injection sites in the DMH, the VMH and the PVN that were effective (filled symbols) or ineffective (open symbols) for increasing both IBAT temperature and heart rate. Numbers indicate distance from bregma in millimetres. f, fornix; mt, mammillothalamic tract. Reproduced with permission from Zaretskaia et al. (2002). B, left panel, microinjection of PGE2 into the medial preoptic area (MPA) (open arrowhead) increased BAT SNA, BAT temperature, expired CO2, and HR (bpm, beats min-1). Bilateral nanoinjections of saline vehicle into the DMH (filled arrowheads) had no effect on any of the measured variables. B, right panel, in a different rat, bilateral nanoinjections of muscimol into the DMH (filled arrowheads) completely reversed the PGE2-evoked responses. Vertical scale bars represent 80 μV for BAT SNA. Reproduced with permission from Madden & Morrison (2004).
Since neurons in the DMH do not project directly to sympathetic preganglionic neurons, these neurons probably contribute to thermogenic sympathetic outflow by influencing the activity of the sympathetic premotor neurons responsible for thermogenesis. Neurons in the rostral ventromedial medulla, including the rRPa are the putative sympathetic premotor neurons for BAT thermogenesis (see discussion below) and, indeed, glutamate receptor activation within the rRPa is necessary for the increase in BAT SNA and thermogenesis evoked by disinhibition of neurons within the DMH (Cao & Morrison, 2006). However, the pathway(s) responsible for transmitting the thermogenic drive from the DMH to the rRPa is unclear. A direct monosynaptic pathway from neurons in the DMH to the rRPa has been implicated in transmitting thermogenic drive (Fig. 1), as suggested by the finding that some DMH/DA neurons that are labelled by microinjection of a retrograde tracer into the rRPa express Fos in response to thermogenic stimuli, such as endotoxin administration or stress (Sarkar et al. 2007). Further indirect evidence for the involvement of a monosynaptic pathway from neurons in the DMH to the rRPa in thermogenesis is the observation that some DMH neurons that are labelled by microinjection of a retrograde tracer into the rRPa receive close GABAergic appositions (putative synapses) from neurons in the POA (Fig. 1, DMH inset; Nakamura et al. 2005).
In addition to the evidence for a direct monosynaptic pathway from the DMH/DA to the rRPa, both anatomical and physiological lines of evidence suggest that the caudal periaqueductal grey (cPAG; approximately 8.0-8.7 mm caudal to bregma) may play a role in transmitting a thermogenic signal from the DMH to the rRPa. For example, some of the DMH/DA neurons that are labelled by microinjection of a retrograde tracer into the cPAG express Fos in response to cold exposure (Yoshida et al. 2005). Furthermore, some neurons in the cPAG are labelled by microinjection of pseudorabies virus into BAT (Cano et al. 2003). Many cPAG neurons project directly to the medullary raphe (Hermann et al. 1997), and neurons within the cPAG express Fos in response to cold (Cano et al. 2003); however, a conclusive demonstration of cold-evoked Fos expression in the neurons within cPAG that project to the raphe is lacking. Physiological evidence suggesting a role of the cPAG in non-shivering thermogenesis includes the observation that microinjection of the excitatory amino acid receptor agonist, d,l-homocysteic acid, into the cPAG increases BAT temperature, without a concomitant increase in core temperature (Chen et al. 2002); however, in this study the animals were not paralysed, so there remains the possibility that the thermogenesis was due to shivering in the underlying muscle. In addition, in awake rats the increase in body temperature evoked by microinjection of bicuculline into the DMH is attenuated by ∼50% by prior microinjection of muscimol into the cPAG (de Menezes et al. 2006). In contrast, when BAT SNA and thermogenesis were directly measured in anaesthetized and paralysed rats, during skin cooling the microinjection of muscimol into the cPAG did not reverse the skin cooling-evoked increase in BAT SNA and thermogenesis (Nakamura & Morrison, 2007a). There are many potential explanations for the apparent controversy concerning the role of neurons within the cPAG in thermogenesis, indicating the need for further investigation of the pathways transmitting the thermogenic drive from the hypothalamus to premotor neurons.
The rostral ventromedial medulla contains BAT, cardiac and somatic premotor neurons controlling thermogenesis
Within the hierarchical organization of the central network controlling thermogenic thermoregulatory effectors, medullary neurons play key roles as premotor neurons, providing excitatory input to spinal motor neurons, for the circuits regulating sympathetic BAT thermogenesis, heart rate and somatic shivering thermogenesis.
The locations of premotor neurons in sympathetic thermogenic pathways and characterization of their transmitter phenotypes have been determined anatomically through viral retrograde transynaptic transport studies involving tracer injections into the interscapular BAT pad. Neurons in the rostral ventromedial medulla, centred in the rRPa and ventral raphe magnus and extending over the pyramids to the parapyramidal (PPy) area, were consistently infected, including neurons in those studies with short post-inoculation times that are consistent with their having become infected by virtue of their synaptic contact with BAT sympathetic preganglionic neurons (Bamshad et al. 1999; Cano et al. 2003; Yoshida et al. 2003). Other brainstem regions with spinally projecting neurons also became infected following virus injections into BAT and these, as well as the rRPa area, overlapped regions previously shown with conventional retrograde tracing to provide inputs to the intermediolateral cell column (IML) containing sympathetic preganglionic neurons (SPNs). Although the existence of a variety of innervated cell types in all tissues (brown adipocytes and vascular smooth muscle in BAT, for instance) may account for a portion of this anatomical dispersion of potential premotor neurons, there may also be varying degrees of influence from several premotor populations onto the SPNs controlling specific effector cell types. A comparison of the localization of Fos induced by cold exposure, which activates BAT thermogenesis, with the locations of virally-labelled neurons following inoculations of BAT provided function-based evidence that the rRPa and the ventromedial parvicellular subdivision of the paraventricular hypothalamic nucleus were the two potential premotor populations having a principal role in mediating the descending regulation of the spinal sympathetic circuit controlling BAT thermogenesis (Cano et al. 2003). Viral retrograde tracing to identify central sympathetic pathways controlling the heart (Ter Horst et al. 1996) found the heaviest labelling in the rostral ventromedial medulla. Additionally, a potential role for neurons in the rostral ventromedial medulla, including the rRPa, in shivering thermogenesis is suggested by the intense viral retrograde labelling in this region following inoculation into skeletal muscle (Kerman et al. 2003).
Anatomical studies have indicated that rostral ventromedial medullary neurons, including those in rRPa and surrounding the pyramids, that project to the IML to influence the discharge of BAT SPNs contain one or more of the following markers: (a) the vesicular glutamate transporter 3 (VGLUT3), potentially indicative of glutamatergic neurons (Nakamura et al. 2004a; Stornetta et al. 2005); (b) serotonin (5-HT) or tryptophan hydroxylase, a synthetic enzyme for 5-HT (Cano et al. 2003; Nakamura et al. 2004a; Stornetta et al. 2005); and (c) glutamic acid decarboxylase-67 (GAD-67), a marker for GABAergic neurons (Stornetta et al. 2005; Fig. 1, rRPa inset). In addition to serotonin, IML-projecting neurons located in the rRPa and the PPy can contain thyrotrophin-releasing hormone and substance P. The fact that VGLUT3-expressing and serotonin-containing neurons in the rostral ventromedial medulla are functionally related to the control of cold-defense effectors, such as BAT, cardiac or shivering thermogenesis, is suggested by the findings that a significant percentage of VGLUT3-containing neurons in the rRPa express Fos in response to cold exposure or intracerebroventricular PGE2 (Nakamura et al. 2004a) and that physiologically identified, putative serotonergic neurons in the rRPa increase their firing rate in response to PGE2 administration or cold exposure (Martin-Cora et al. 2000).
Physiologically, activation of neurons in the restricted region of the rostral ventromedial medulla containing the rRPa, the PPy and ventral portions of raphe magnus increases BAT SNA, BAT thermogenesis and heart rate. Disinhibition of rRPa neurons following blockade of local GABAA receptors with nanoinjections of bicuculline into rRPa elicits a large and sustained increase in BAT SNA and BAT temperature and a sympathetically mediated tachycardia in anaesthetized rats with external body temperature support (Fig. 6A; Morrison et al. 1999; Cao & Morrison, 2003) and a marked increase in heart rate with no change in body temperature in awake rats (Zaretsky et al. 2003). Increases in muscle EMG activity and shivering are also elicited by disinhibition of rostral ventromedial medullary neurons (Nason & Mason, 2004). These data indicate that when a normothermic body temperature is maintained with an external heat source, the rRPa neurons controlling BAT SNA, the sympathetic outflow to the cardiac sino-atrial node and the discharge of motoneurons for skeletal muscle receive a tonic, GABAergic inhibition and that relief of this inhibition allows a potent increase in rRPa neuronal discharge. The finding that disinhibition of rRPa neurons increases their activity suggests the existence of on-going or bicuculline-activated excitatory inputs to these rRPa neurons or of a complement of membrane ion channels supporting intrinsic activity in these rRPa neurons. The physiological relief of a tonically active inhibitory input to BAT sympathetic premotor neurons in rRPa, potentially from warm-sensitive POA projection neurons (Fig. 1), is postulated to contribute to the stimulation of BAT thermogenesis in cold defense and in fever. The absence of an increase in body temperature following bicuculline administration into rRPa in awake animals in a room temperature environment is consistent with an on-going level of activity in rRPa neurons to drive BAT thermogenesis needed to maintain body temperature. Nanoinjections into rRPa of agonists for either NMDA or non-NMDA glutamate receptors evoke brief, but intense activation of BATSNA and increases in heart rate (Madden & Morrison, 2003). Thus, rRPa neurons capable of increasing the sympathetic drives to BAT and to the heart express NMDA and non-NMDA subtypes of glutamate receptors.
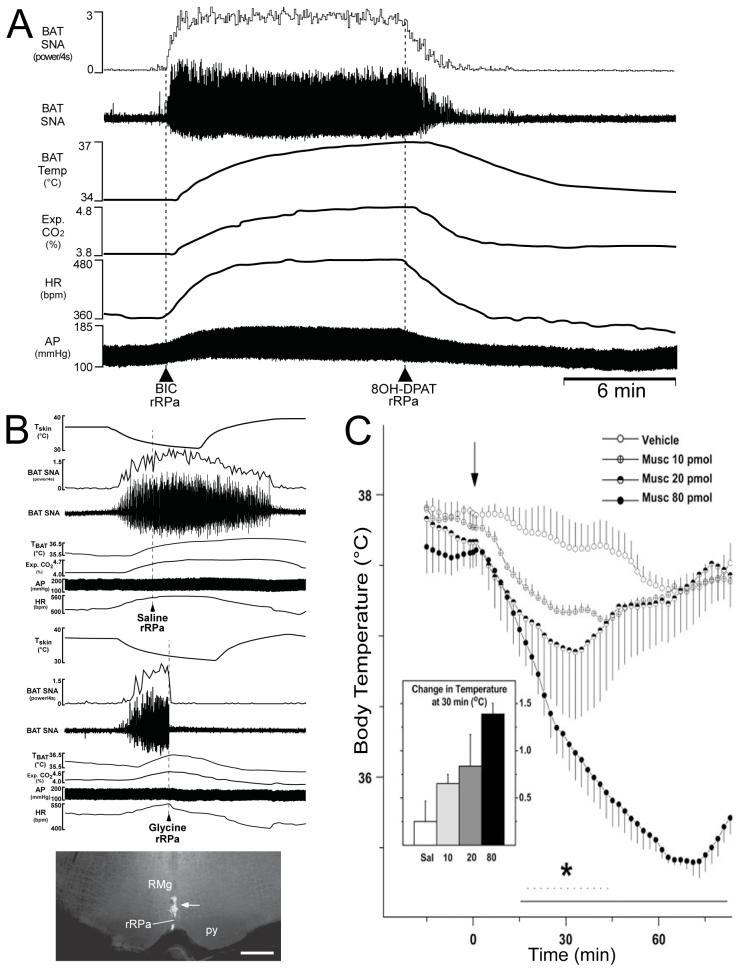
Figure 6. Effects of disinhibition and inhibition of rostral raphe pallidus (rRPa) neurons on BAT thermogenesis and body temperature.
A, blockade of GABAA receptors with bicuculline (BIC) in the rRPa dramatically activates BAT sympathetic nerve activity (SNA) and increases BAT temperature, expired CO2, heart rate (HR) and arterial pressure (AP). All of these responses are reversed by nanoinjection of the 5-HT1A agonist, 8-hydroxy-2-(di-N-propylamino)tetralin hydrobromide (8-OH-DPAT), into rRPa. B, the increases in BAT SNA, BAT temperature, expired CO2 and HR produced by a reduction in skin temperature (Tskin) are blocked by inhibition of neural activity in the rRPa with nanoinjection of glycine. B, bottom panel, deposit of fluorescent beads (arrow) indicates the glycine injection site in rRPa. Abbreviations: RMg, raphe magnus; and py, pyramid. Reproduced with permission from Nakamura & Morrison (2007a)). C, falls in body temperature following nanoinjection of muscimol (Musc, arrow) into the rostral ventromedial medulla to inhibit local neurons in awake rats. Asterisk indicates period of significant falls in core temperature. Reproduced with permission from Zaretsky et al. (2003).
Conversely, inhibition of the activity in neurons in the rRPa reverses the increases in BAT SNA, BAT heat production and heart rate elicited by every thermogenic stimulus that has been tested. For example, the elementary cold defense stimulus of skin cooling elicits responses including increases in BAT SNA, BAT temperature (a measure of thermogenesis), expired CO2 (reflecting a stimulation of oxidative metabolism such as in BAT and the heart) and heart rate (Fig. 6B, top panel). Nanoinjection of glycine into the rRPa (Fig. 6B, bottom panel) to inhibit local neurons produces a rapid and complete reversal of the skin cooling-evoked increase in BAT SNA (Fig. 6B, middle panel) and an immediate waning of the accompanying metabolic and cardiac responses, despite the sustained reduction in skin temperature (Nakamura & Morrison, 2007a). Inhibition of rostral ventromedial medullary neurons produces dramatic falls in body temperature in the awake rat (Fig. 6C; Zaretsky et al. 2003), consistent with the possibility that BAT sympathetic premotor neurons in the rRPa and BAT thermogenesis are actively contributing to the maintenance of core temperature in a room temperature environment. Inhibition of rostral ventromedial medullary neurons also attenuates cold-evoked shivering responses (Brown et al. 2007) and eliminates the activation of fusimotor neurons produced by skin cooling (Tanaka et al. 2006). The effects of inhibiting neurons in rRPa have led to the conclusion that the activity of sympathetic premotor neurons in rRPa for BAT and for the heart provides the supraspinal excitatory drive to spinal sympathetic circuits that is both necessary and sufficient for the thermogenic and tachycardic responses to thermoregulatory, febrile and a variety of neurochemical mediators that increase body temperature. Although more detailed investigations are needed, the rostral ventromedial medulla also appears to contain bulbospinal neurons whose activity is required for cold-evoked shivering thermogenesis. In addition to cold defense, other thermogenic stimuli whose stimulated BAT thermogenesis and tachycardia are reversed or prevented by inhibition of neural activity in the rRPa region of the rostral ventromedial medulla include the pyrogenic mediator, PGE2 (Nakamura et al. 2002; Madden & Morrison, 2003; Morrison, 2003; Ootsuka et al. 2008); disinhibition of neurons in the DMH (Cao et al. 2004) or in the lateral hypothalamus (Cerri & Morrison, 2005); activation of central μ-opioid receptors (Cao & Morrison, 2005), central melanocortin receptors (Fan et al. 2007) or preoptic corticotrophin-releasing factor receptors (Cerri & Morrison, 2006); and systemic administration of the adipose tissue hormone, leptin (Morrison, 2004).
Blockade of glutamate receptors in the rRPa is also effective in reversing cold-evoked and pyrogen-mediated stimulations of BAT thermogenesis and of heart rate (Madden & Morrison, 2003; Nakamura & Morrison, 2007a), as well as those elicited by disinhibition of neurons in the DMH (Cao & Morrison, 2006). These findings implicate glutamate receptor activation in the tonic excitation of BAT and cardiac sympathetic premotor neurons that may be revealed by relief of their tonic inhibitory inputs (Fig. 1) or in their excitation from rostral inputs, such as neurons in the DMH (Fig. 1). Together with the anatomical evidence for direct projections from the rRPa region to IML neurons, including the SPNs, that control BAT thermogenesis, these functional data indicate that the rRPa region contains the principal population of BAT sympathetic premotor neurons providing the final common bulbospinal pathway for the sympathoexcitatory drive to the spinal network controlling BAT SNA (Fig. 1).
In addition to BAT sympathetic premotor neurons in the medulla and neurons in the cPAG that may contribute to their excitation, the brainstem also contains sources of inhibition of BAT thermogenesis. The existence of a tonically active inhibition of BAT thermogenesis from neurons in the region of the pontine retrorubral field (Fig. 1) was indicated by the large increases in BAT temperature that followed transections of the neuraxis in the vicinity of the pontomedullary junction (Rothwell et al. 1983), but which were absent if the transection was made rostral to the pons, but caudal to the DMH (Rothwell et al. 1983). Neither the exact location of the neurons mediating this inhibition nor the physiological basis for its control has been determined. The rostral ventromedial PAG in the midbrain contains a population of neurons whose disinhibition can reverse the BAT stimulation in response to PGE2 in the POA and that following disinhibition of neurons in the DMH (Rathner & Morrison, 2006). The BAT sympathoinhibitory effects of these neurons appear to be mediated through a reduced excitability of neurons in rRPa; however, the pathways controlling this inhibition are unknown.
The brainstem also contains the pathways mediating the inhibition of BAT thermogenesis in response to arterial hypoxia, a reflex to restrict oxygen consumption in the face of reduced oxygen availability or compromised oxygen diffusion and transport in the blood. Systemic hypoxia or bolus systemic injections of sodium cyanide produce a prompt and complete reversal of the BAT SNA activations evoked by hypothermia and by PGE2 in the POA, and this response to hypoxia is eliminated by section of the carotid sinus nerves or by inhibition of second-order arterial chemoreceptor sensory neurons in the commissural region of the nucleus of the solitary tract (Madden & Morrison, 2005). Interestingly, hypoxia also eliminates the BAT SNA activation resulting from bicuculline nanoinjection into the rRPa, suggesting that activation of a GABAergic input to BAT sympathetic premotor neurons in rRPa is unlikely to mediate the hypoxic inhibition of BAT thermogenesis. Indirect evidence points to a possible role for a spinal inhibitory mechanism. Similar to arterial hypoxia, disinhibition of neurons in the rostral ventrolateral medulla (RVLM) reduces the BAT SNA activation following bicuculline into the rRPa (Morrison, 1999), and both anatomical (Stornetta et al. 2004) and electrophysiological studies (Deuchars et al. 1997) support the existence of a bulbospinal inhibitory pathway to SPNs from the RVLM. The pathway for the hypoxic inhibition of BAT metabolism between the nucleus of the solitary tract and the BAT SPNs remains to be investigated.
Spinal sympathetic and somatomotor mechanisms in thermogenesis
The discharge of BAT SPNs that determines the level of BAT SNA and BAT thermogenesis, as well as the rhythmic bursting characteristic of BAT SNA, is governed by their supraspinal and segmental inputs as well as those to the network of spinal interneurons that influence BAT SPN excitability. A significant fraction of the BAT sympathetic premotor neurons in rRPa are glutamatergic and/or serotonergic neurons (Fig. 1, rRPa inset). Consistent with these findings from viral retrograde tracing studies, 5-HT-containing (Bacon & Smith, 1988; Vera et al. 1990) and VGLUT3-containing terminals synapse on SPNs (Stornetta et al. 2005) or make close appositions with SPN dendrites (Fig. 1, IML inset; Nakamura et al. 2004a,b). Sympathetic preganglionic neurons contain ionotropic glutamate receptors (Aicher et al. 2000), and several subtypes of 5-HT receptors are located within the IML (Marlier et al. 1991; Thor et al. 1993; Maeshima et al. 1998; Doly et al. 2004), although the specific neuronal subtypes expressing these receptors have not been determined. Viral inoculations of sympathetically innervated tissues (Brooke et al. 2002), including interscapular BAT (Cano et al. 2003), consistently label a population of spinal interneurons in the vicinity of the IML. Spinal GABAergic interneurons would appear to be among this population, since they influence the discharge of SPNs (Deuchars et al. 2005). That such interneurons could receive inputs from the BAT premotor area in the rostral ventromedial medulla is suggested by the demonstration that VGLUT3- and GAD-67-containing terminals synapse on GABAergic neurons in the IML (Stornetta et al. 2005).
In general, SPNs are activated by glutamate receptor agonists (Coote et al. 1981) and by 5-HT (Lewis & Coote, 1990; Pickering et al. 1994). Nanoinjection of glutamate or NMDA into the upper thoracic IML activates BAT SNA and BAT thermogenesis (Nakamura et al. 2004a; Madden & Morrison, 2006). Figure 7A illustrates the brief, but marked increases in BAT SNA consistently evoked by nanoinjections of NMDA into the IML in the fourth thoracic segment, which contains a high concentration of SPNs for interscapular BAT (Cano et al. 2003). Furthermore, blockade of glutamate receptors in the upper thoracic IML suppresses the increase in BAT thermogenesis evoked by disinhibition of rRPa neurons (Fig. 7C; Nakamura et al. 2004a). These findings support the view that the glutamatergic premotor pathway from the rRPa to the IML is an essential mechanism in the control of BAT thermogenesis.
Figure 7. Effects of activation and blockade of spinal glutamate and serotonin receptors on sympathetic activation of BAT and BAT thermogenesis.
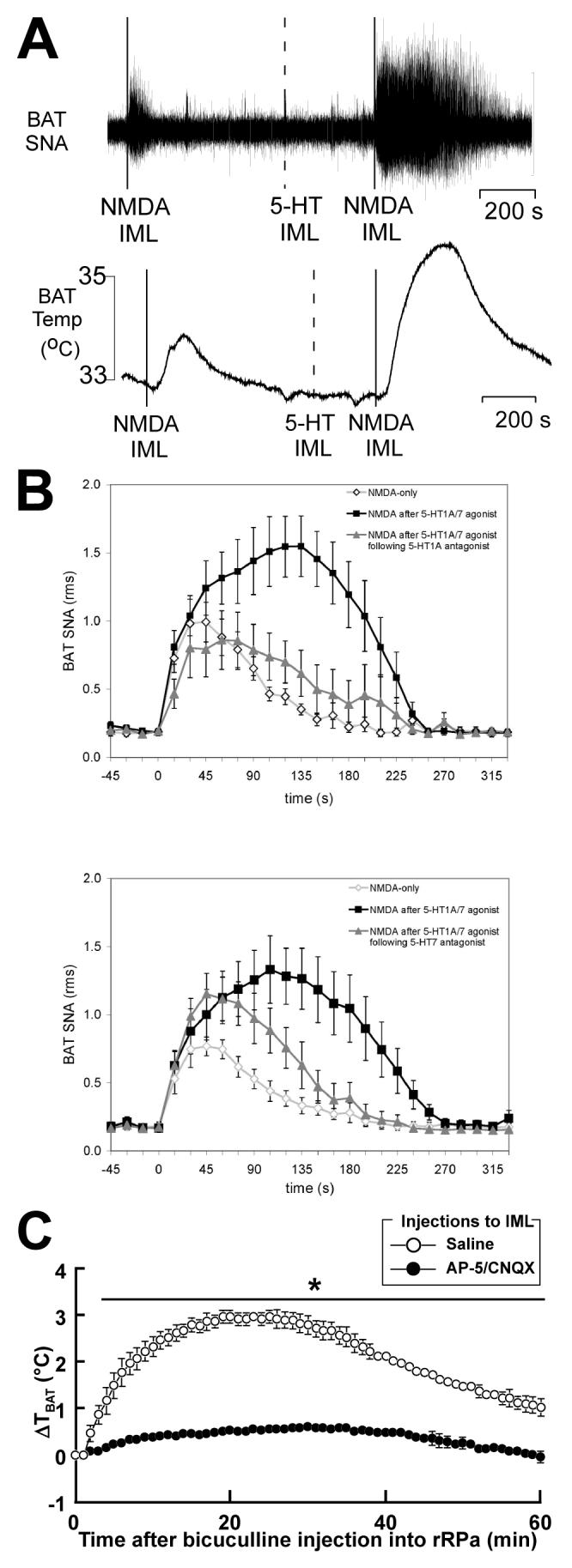
A, microinjection of 5-HT into the T4 intermediolateral nucleus (IML) potentiated (+285%) the NMDA-evoked increase in BAT sympathetic nerve activity (SNA) and in BAT temperature. Vertical scale bar represents 20 μV for BAT SNA. Reproduced with permission from Madden & Morrison (2006). B, the time courses of the inhibition by selective 5-HT receptor antagonists of the 5-HT1A/7 receptor agonist 8-OH-DPAT-mediated potentiation of the increase in BAT SNA evoked by microinjection of NMDA into the T4 IML. The 5-HT1A receptor antagonist, WAY-100635, attenuated both the amplitude and the duration of the 8-OH-DPAT potentiation. The 5-HT7 receptor antagonist, SB269970, attenuated the duration but not the amplitude of the 8-OH-DPAT potentiation. Nanoinjection of NMDA only into the T4 IML (◇), NMDA into the T4 IML after 8-OH-DPAT into the T4 IML (■) and NMDA into the T4 IML following 8-OH-DPAT into the T4 IML after WAY-100635 or SB269970 into the T4 IML (▲). Reproduced with permission from Madden & Morrison (2008). C, nanoinjections of glutamate receptor antagonists into the IML blocked BAT thermogenesis triggered by bicuculline-induced disinhibition of rRPa neurons. Changes in BAT temperature (ΔTBAT) are shown after bicuculline injection into the rRPa in rats microinjected with a mixture of 2-amino-5-phosphonovaleric acid (AP-5, 5 mM) and 6-cyano-7-nitroquinozline-2,3-dione (CNQX, 5mM) or saline into the bilateral T2-T6 IML, using 200 nl/site, every 0.8 - 1.0 mm. The changes in BAT temperature were significantly different between the AP-5/CNQX- and saline-pretreated groups during the time period denoted by a horizontal bar with an asterisk (P < 0.05). Reproduced with permission from Nakamura et al. (2004a).
Nanoinjection of serotonin into the fourth thoracic IML can also activate BAT SNA and BAT thermogenesis (Madden & Morrison, 2006). Additionally, when nanoinjection of NMDA into the IML is preceded by local injection of a dose of 5-HT that does not activate BAT SNA by itself, the BAT SNA response to subsequent glutamate receptor activations is markedly enhanced (Fig. 7A; Madden & Morrison, 2006). Furthermore, such potentiation by serotonin allows a subthreshold dose of NMDA into the IML to evoke BAT SNA (Madden & Morrison, 2006). This serotonergic potentiation of glutamate receptor-mediated increases in BAT SNA is blocked by antagonists of 5-HT1A/5-HT7 receptors (Fig. 7B; Madden & Morrison, 2008). A functional role for spinal serotonergic neurotransmission in supporting the elaboration of cold-defense responses is suggested by the increase in spinal 5-HT turnover (an index of 5-HT release) during cold exposure (Passerin et al. 1999) and by the effectiveness of 5-HT receptor antagonism in the T3-T6 IML in preventing the cooling-evoked stimulation of interscapular BAT SNA (Madden & Morrison, 2007). These data demonstrate that the serotonergic innervation provided by raphe neurons to IML neurons, including SPNs, plays a critical role in eliciting physiologically relevant levels BAT thermogenesis in cold defense. The significant role of serotonin-containing neurons in normal cold-defense responses is also supported by the recent finding that mice that lack almost all central serotonergic neurons show blunted BAT thermogenesis during cold exposure (Hodges et al. 2008). The mechanisms of the interaction between glutamatergic and serotonergic neurotransmission in the IML remain to be elucidated.
While the parsimony of a model consisting only of supraspinal glutamatergic and serotonergic excitatory inputs to SPNs is attractive, other data suggest that the neurochemical phenotypes of the VGLUT3-containing and tryptophan hydroxylase-containing neurons might not be so straightforward and that more complex spinal circuitry may also influence BAT SNA and thermogenesis. Although exclusively asymmetric (putatively excitatory) synapses for VGLUT3-containing terminals have been described within the IML (Nakamura et al. 2004b), another study indicates that while the majority (∼60%) of the VGLUT3-containing terminals in the IML synapse on SPNs, they form approximately equal numbers of asymmetric and symmetric synapses, suggesting the possibility that these inputs may be either excitatory or inhibitory (Stornetta et al. 2005). Supporting the suggestion that some of the VGLUT3-containing inputs from the ventral medullary raphe to the IML are inhibitory, this study found that the majority of the spinally projecting VGLUT3-containing neurons of the ventral medullary raphe contain GAD-67 and some of the VGLUT3-containing terminals within the IML also contain GAD-67 and make symmetric synapses (Stornetta et al. 2005).
It is also noteworthy that some of the putatively inhibitory inputs within the IML synapse on GABAergic dendrites (Stornetta et al. 2005), providing a potential anatomical substrate for a pathway that increases the activity of SPNs through disinhibition. Similarly, 5-HT terminals form close appositions with GABAergic interneurons in the central autonomic area (Conte et al. 2007). Physiologically, 5-HT in the spinal cord potentiates sympathetic outflow to BAT at least in part via 5-HT1A receptors and, based on the known inhibitory signal transduction systems for 5-HT1A receptors, this effect could be mediated via inhibition of GABA inputs to BAT SPNs (Madden & Morrison, 2008).
Further complexity in the spinal neurocircuitry regulating BAT SNA and thermogenesis may also be introduced by other neurochemical inputs. For example, catecholamines could be postulated to modulate the activity of SPNs regulating BAT, especially considering the excitatory and inhibitory effects of catecholamines on SPNs (Coote et al. 1981; Miyazaki et al. 1989) and the observation of a dense dopamine β-hydroxylase innervation of SPNs that are labelled by pseudorabies virus injection into BAT (Cano et al. 2003). In addition, substance P terminals innervate SPNs (Vera et al. 1990), substance P directly excites the majority of SPNs (Cammack & Logan, 1996) and intrathecally administered substance P affects thermoregulation (Dib, 1987), suggesting a potential role for substance P in modulating sympathetic outflow to BAT. While the present data clearly demonstrate that the spinal cord is much more than a simple relay circuit driving BAT SNA and thermogenesis, the precise details of the spinal neurocircuitry involved in the regulation of thermogenic effector systems await future studies.
The ventral horn of the spinal grey matter contains both α-motoneurons and fusimotor (γ)-neurons projecting to skeletal muscle fibres and to muscle spindles, respectively. During shivering, two patterns of EMG activity occur to varying degrees: (a) a continuous, low-amplitude activity representing an increase in tone in type I muscle fibres specialized for lipid fuel consumption; and (b) bursts of large-amplitude activity, indicating synchronous activation of type II muscle fibres preferentially consuming carbohydrate fuel (Haman, 2006). These two patterns of EMG activity presumably are the result of similar patterns of activation of the respective α-motoneurons innervating these muscle fibre types. During shivering, the dynamic fusimotor system activates muscle spindles (Schafer & Schafer, 1973a), and this cooling-evoked activation of fusimotor neurons is significantly dependent on the activity of neurons in the rostral medullary raphe (Sato et al. 1990; Tanaka et al. 2006). The increase in Ia afferent activity arising from muscle spindle activation could elicit an increase in α-motoneuron discharge and the muscle tone corresponding to the first pattern of EMG activity in shivering. The mechanisms underlying synchronous discharge of the populations of α-motoneurons producing the large-amplitude bursts in EMG activity are unknown, although an instability in the stretch reflex loop and a ‘pacemaker’-like function of spinal motor control circuits have been proposed (Schafer & Schafer, 1973b).
Summary and future directions
Thermogenesis is regulated in BAT, the heart and skeletal muscle by what appear to be parallel networks in the central nervous system which respond to the feedforward afferent signals from cutaneous and core body thermoreceptors and to feedback signals from brain thermosensitive neurons to activate specific populations of sympathetic and somatic efferents. We have summarized the research leading to a model (Fig. 1) of the feedforward reflex pathway through which environmental cold stimulates thermogenesis, including the influence on this thermoregulatory network of the pyrogenic mediator, PGE2, to increase body temperature. The cold thermal afferent circuit from cutaneous thermal receptors ascends via second-order thermosensory neurons in the medullary and spinal dorsal horn to activate neurons in the LPBel which drive GABAergic interneurons in the MnPO to inhibit warm-sensitive, inhibitory output neurons of the MPO. The resulting disinhibition of thermogenesis-promoting neurons in the DMH and possibly of sympathetic and somatic premotor neurons in the rostral ventromedial medulla, including the rRPa, activates excitatory inputs to spinal sympathetic and somatic motor circuits to drive thermogenesis.
Although significant recent research progress has enhanced our understanding of the central control of thermogenesis and body temperature, several areas remain poorly understood. As with the circuits controlling other autonomic functions, the sources and mechanisms responsible for the tonic discharge and the synchronous bursting that characterizes the sympathetic outflow to thermogenic effectors remain to be delineated. The neural basis for the tremor-like discharge in somatic motor neurons during shivering is not known. The sites and mechanisms underlying the critical integration of thermogenesis with other homeostatic systems regulating oxygen and fuel substrate availability and energy balance remain to be investigated. The projection neurons connecting important regions in the central thermogenic pathways remain to be characterized, including the inhibitory output neurons of the POA, the excitatory output neurons in the DMH and the sympathetic premotor neurons in the rRPa and PPy regions of the medulla. The marked differences in temperature response thresholds among thermoregulatory effectors suggests that significant differences should be expected among the populations of warm-sensitive POA projection neurons that control these effectors. The microcircuitry of the POA and the role and regulation of the pontine inhibitory area for thermogenesis also represent significant lacunae in our understanding of the functional organization of the model circuit for central control of thermogenesis that we have proposed. We hope that this review will inspire research into some of these questions.
Acknowledgements
The authors are grateful for the institutional support of the research that contributed to this review: National Institutes of Health grants NS40987 (S.F.M.), DK57838 (S.F.M.), DK65401 (C.J.M.) and the Japan Society for the Promotion of Science fellowship (K.N.).
References
- Aicher SA, Hahn B, Milner TA. N-Methyl-d-aspartate-type glutamate receptors are found in post-synaptic targets of adrenergic terminals in the thoracic spinal cord. Brain Research. 2000;856:1–11. doi: 10.1016/s0006-8993(99)02145-9. [DOI] [PubMed] [Google Scholar]
- Andrew D, Craig AD. Spinothalamic lamina I neurones selectively sensitive to cutaneous warming in cats. J Physiol. 2001;537:489–495. doi: 10.1111/j.1469-7793.2001.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon SJ, Smith AD. Preganglionic sympathetic neurones innervating the rat adrenal medulla: immunocytochemical evidence of synaptic input from nerve terminals containing substance P, GABA or 5-hydroxytryptamine. J Auton Nerv Syst. 1988;24:97–122. doi: 10.1016/0165-1838(88)90140-3. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 1999;276:R1569–R1578. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Dallel R, Raboisson P, Villanueva L, Le Bars D. Organization of the efferent projections from the spinal cervical enlargement to the parabrachial area and periaqueductal gray: a PHA-L study in the rat. J Comp Neurol. 1995;353:480–505. doi: 10.1002/cne.903530403. [DOI] [PubMed] [Google Scholar]
- Boulant JA. Counterpoint: Heat-induced membrane depolarization of hypothalamic neurons: an unlikely mechanism of central thermosensitivity. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1481–R1484. doi: 10.1152/ajpregu.00655.2005. discussion R1484. [DOI] [PubMed] [Google Scholar]
- Boulant JA, Dean JB. Temperature receptors in the central nervous system. Annu Rev Physiol. 1986;48:639–654. doi: 10.1146/annurev.ph.48.030186.003231. [DOI] [PubMed] [Google Scholar]
- Boulant JA, Hardy JD. The effect of spinal and skin temperatures on the firing rate and thermosensitivity of preoptic neurones. J Physiol. 1974;240:639–660. doi: 10.1113/jphysiol.1974.sp010627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratincsak A, Palkovits M. Activation of brain areas in rat following warm and cold ambient exposure. Neuroscience. 2004;127:385–397. doi: 10.1016/j.neuroscience.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Bratincsak A, Palkovits M. Evidence that peripheral rather than intracranial thermal signals induce thermoregulation. Neuroscience. 2005;135:525–532. doi: 10.1016/j.neuroscience.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Brooke RE, Pyner S, McLeish P, Buchan S, Deuchars J, Deuchars SA. Spinal cord interneurones labelled transneuronally from the adrenal gland by a GFP-herpes virus construct contain the potassium channel subunit Kv3.1b. Auton Neurosci. 2002;98:45–50. doi: 10.1016/s1566-0702(02)00030-9. [DOI] [PubMed] [Google Scholar]
- Brown JW, Sirlin EA, Benoit AM, Hoffman JM, Darnall RA. Activation of 5-HT1A receptors in medullary raphe disrupts sleep and decreases shivering during cooling in the conscious piglet. Am J Physiol Regul Integr Comp Physiol. 2007;294:R884–R894. doi: 10.1152/ajpregu.00655.2007. [DOI] [PubMed] [Google Scholar]
- Cammack C, Logan SD. Excitation of rat sympathetic preganglionic neurones by selective activation of the NK1 receptor. J Auton Nerv Syst. 1996;57:87–92. doi: 10.1016/0165-1838(95)00103-4. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol. 2003;460:303–326. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- Cao WH, Fan W, Morrison SF. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience. 2004;126:229–240. doi: 10.1016/j.neuroscience.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Cao WH, Morrison SF. Disinhibition of rostral raphe pallidus neurons increases cardiac sympathetic nerve activity and heart rate. Brain Res. 2003;980:1–10. doi: 10.1016/s0006-8993(03)02981-0. [DOI] [PubMed] [Google Scholar]
- Cao WH, Morrison SF. Brown adipose tissue thermogenesis contributes to fentanyl-evoked hyperthermia. Am J Physiol Regul Integr Comp Physiol. 2005;288:R723–R732. doi: 10.1152/ajpregu.00669.2004. [DOI] [PubMed] [Google Scholar]
- Cao WH, Morrison SF. Glutamate receptors in the raphe pallidus mediate brown adipose tissue thermogenesis evoked by activation of dorsomedial hypothalamic neurons. Neuropharmacology. 2006;51:426–437. doi: 10.1016/j.neuropharm.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Standaert DG, Saper CB. Spinal and trigeminal dorsal horn projections to the parabrachial nucleus in the rat. J Comp Neurol. 1985;240:153–160. doi: 10.1002/cne.902400205. [DOI] [PubMed] [Google Scholar]
- Cerri M, Morrison SF. Activation of lateral hypothalamic neurons stimulates brown adipose tissue thermogenesis. Neuroscience. 2005;135:627–638. doi: 10.1016/j.neuroscience.2005.06.039. [DOI] [PubMed] [Google Scholar]
- Cerri M, Morrison SF. Corticotropin releasing factor increases in brown adipose tissue thermogenesis and heart rate through dorsomedial hypothalamus and medullary raphe pallidus. Neuroscience. 2006;140:711–721. doi: 10.1016/j.neuroscience.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Chen XM, Hosono T, Yoda T, Fukuda Y, Kanosue K. Efferent projection from the preoptic area for the control of non-shivering thermogenesis in rats. J Physiol. 1998;512:883–892. doi: 10.1111/j.1469-7793.1998.883bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XM, Nishi M, Taniguchi A, Nagashima K, Shibata M, Kanosue K. The caudal periaqueductal gray participates in the activation of brown adipose tissue in rats. Neurosci Lett. 2002;331:17–20. doi: 10.1016/s0304-3940(02)00757-7. [DOI] [PubMed] [Google Scholar]
- Christensen CR, Clark PB, Morton KA. Reversal of hypermetabolic brown adipose tissue in F-18 FDG PET imaging. Clin Nucl Med. 2006;31:193–196. doi: 10.1097/01.rlu.0000204199.33136.05. [DOI] [PubMed] [Google Scholar]
- Colburn RW, Lubin ML, Stone DJ, Jr, Wang Y, Lawrence D, D’Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Conte D, Deuchars SA, Deuchars J. Do serotonin immunoreactive terminals innervate GABAergic neurons in the central autonomic area of the mouse spinal cord. FASEB J. 2007;21:A885. [Google Scholar]
- Coote JH, Macleod VH, Fleetwood-Walker S, Gilbey MP. The response of individual sympathetic preganglionic neurones to microelectrophoretically applied endogenous monoamines. Brain Res. 1981;215:135–145. doi: 10.1016/0006-8993(81)90497-2. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD, Bushnell MC, Zhang ET, Blomqvist A. A thalamic nucleus specific for pain and temperature sensation. Nature. 1994;372:770–773. doi: 10.1038/372770a0. [DOI] [PubMed] [Google Scholar]
- Craig AD, Krout K, Andrew D. Quantitative response characteristics of thermoreceptive and nociceptive lamina I spinothalamic neurons in the cat. J Neurophysiol. 2001;86:1459–1480. doi: 10.1152/jn.2001.86.3.1459. [DOI] [PubMed] [Google Scholar]
- De Castro e Silva E, Fregoneze JB, Johnson AK. Corticotropin-releasing hormone in the lateral parabrachial nucleus inhibits sodium appetite in rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1136–R1141. doi: 10.1152/ajpregu.00075.2003. [DOI] [PubMed] [Google Scholar]
- De Luca LA, Jr, Vendramini RC, Pereira DT, Colombari DA, David RB, de Paula PM, Menani JV. Water deprivation and the double-depletion hypothesis: common neural mechanisms underlie thirst and salt appetite. Braz J Med Biol Res. 2007;40:707–712. doi: 10.1590/s0100-879x2007000500015. [DOI] [PubMed] [Google Scholar]
- de Menezes RC, Zaretsky DV, Fontes MA, DiMicco JA. Microinjection of muscimol into caudal periaqueductal gray lowers body temperature and attenuates increases in temperature and activity evoked from the dorsomedial hypothalamus. Brain Res. 2006;1092:129–137. doi: 10.1016/j.brainres.2006.03.080. [DOI] [PubMed] [Google Scholar]
- Deuchars SA, Milligan CJ, Stornetta RL, Deuchars J. GABAergic neurons in the central region of the spinal cord: a novel substrate for sympathetic inhibition. J Neurosci. 2005;25:1063–1070. doi: 10.1523/JNEUROSCI.3740-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuchars SA, Spyer KM, Gilbey MP. Stimulation within the rostral ventrolateral medulla can evoke monosynaptic GABAergic IPSPs in sympathetic preganglionic neurons in vitro. J Neurophysiol. 1997;77:229–235. doi: 10.1152/jn.1997.77.1.229. [DOI] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Dib B. Thermoregulatory behaviour induced by intrathecal injection of substance P in the rat. Eur J Pharmacol. 1987;133:147–153. doi: 10.1016/0014-2999(87)90145-2. [DOI] [PubMed] [Google Scholar]
- Dimicco JA, Zaretsky DV. The dorsomedial hypothalamus: a new player in thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R47–R63. doi: 10.1152/ajpregu.00498.2006. [DOI] [PubMed] [Google Scholar]
- Doly S, Madeira A, Fischer J, Brisorgueil MJ, Daval G, Bernard R, Verge D, Conrath M. The 5-HT2A receptor is widely distributed in the rat spinal cord and mainly localized at the plasma membrane of postsynaptic neurons. J Comp Neurol. 2004;472:496–511. doi: 10.1002/cne.20082. [DOI] [PubMed] [Google Scholar]
- Driver J, Noesselt T. Multisensory interplay reveals crossmodal influences on ‘sensory-specific’ brain regions, neural responses, and judgments. Neuron. 2008;57:11–23. doi: 10.1016/j.neuron.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist JK, Scammell TE, Jacobson CD, Saper CB. Distribution of Fos-like immunoreactivity in the rat brain following intravenous lipopolysaccharide administration. J Comp Neurol. 1996;371:85–103. doi: 10.1002/(SICI)1096-9861(19960715)371:1<85::AID-CNE5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Scammell TE, Saper CB. Mechanisms of CNS response to systemic immune challenge: the febrile response. Trends Neurosci. 1997;20:565–570. doi: 10.1016/s0166-2236(97)01138-7. [DOI] [PubMed] [Google Scholar]
- Fan W, Morrison SF, Cao WH, Yu P. Thermogenesis activated by central melanocortin signaling is dependent on neurons in the rostral raphe pallidus (rRPa) area. Brain Res. 2007;1179:61–69. doi: 10.1016/j.brainres.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Feil K, Herbert H. Topographic organization of spinal and trigeminal somatosensory pathways to the rat parabrachial and Kölliker-Fuse nuclei. J Comp Neurol. 1995;353:506–528. doi: 10.1002/cne.903530404. [DOI] [PubMed] [Google Scholar]
- Forster RE, Ferguson TB. Relationship between hypothalamic temperature and thermo-regulatory effectors in unanesthetized cat. Am J Physiol. 1952;169:255–269. doi: 10.1152/ajplegacy.1952.169.2.255. [DOI] [PubMed] [Google Scholar]
- Gavva NR, Bannon AW, Surapaneni S, Hovland DN, Jr, Lehto SG, Gore A, Juan T, Deng H, Han B, Klionsky L, Kuang R, Le A, Tamir R, Wang J, Youngblood B, Zhu D, Norman MH, Magal E, Treanor JJ, Louis JC. The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci. 2007;27:3366–3374. doi: 10.1523/JNEUROSCI.4833-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerling JC, Loewy AD. Aldosterone-sensitive neurons in the nucleus of the solitary tract: bidirectional connections with the central nucleus of the amygdala. J Comp Neurol. 2006;497:646–657. doi: 10.1002/cne.21019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerling JC, Loewy AD. Central regulation of sodium appetite. Exp Physiol. 2008;93:178–209. doi: 10.1113/expphysiol.2007.039891. [DOI] [PubMed] [Google Scholar]
- Golozoubova V, Cannon B, Nedergaard J. UCP1 is essential for adaptive adrenergic nonshivering thermogenesis. Am J Physiol Endocrinol Metab. 2006;291:E350–E357. doi: 10.1152/ajpendo.00387.2005. [DOI] [PubMed] [Google Scholar]
- Gong H, McGinty D, Guzman-Marin R, Chew KT, Stewart D, Szymusiak R. Activation of c-fos in GABAergic neurones in the preoptic area during sleep and in response to sleep deprivation. J Physiol. 2004;556:935–946. doi: 10.1113/jphysiol.2003.056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guieu JD, Hardy JD. Effects of heating and cooling of the spinal cord on preoptic unit activity. J Appl Physiol. 1970;29:675–683. doi: 10.1152/jappl.1970.29.5.675. [DOI] [PubMed] [Google Scholar]
- Guler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22:6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta BN, Nier K, Hensel H. Cold-sensitive afferents from the abdomen. Pflugers Arch. 1979;380:203–204. doi: 10.1007/BF00582158. [DOI] [PubMed] [Google Scholar]
- Haman F. Shivering in the cold: from mechanisms of fuel selection to survival. J Appl Physiol. 2006;100:1702–1708. doi: 10.1152/japplphysiol.01088.2005. [DOI] [PubMed] [Google Scholar]
- Hammel HT, Hardy JD, Fusco MM. Thermoregulatory responses to hypothalamic cooling in unanesthetized dogs. Am J Physiol. 1960;198:481–486. doi: 10.1152/ajplegacy.1960.198.3.481. [DOI] [PubMed] [Google Scholar]
- Hellon RF, Hensel H, Schafer K. Thermal receptors in the scrotum of the rat. J Physiol. 1975;248:349–357. doi: 10.1113/jphysiol.1975.sp010978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom B, Hammel HT. Some characteristics of temperature regulation in the unanesthetized dog. Am J Physiol. 1967;213:547–556. doi: 10.1152/ajplegacy.1967.213.2.547. [DOI] [PubMed] [Google Scholar]
- Hensel H, Iggo A, Witt I. A quantitative study of sensitive cutaneous thermoreceptors with C afferent fibres. J Physiol. 1960;153:113–126. doi: 10.1113/jphysiol.1960.sp006522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Hermann DM, Luppi PH, Peyron C, Hinckel P, Jouvet M. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars α demonstrated by iontophoretic application of choleratoxin (subunit b) J Chem Neuroanat. 1997;13:1–21. doi: 10.1016/s0891-0618(97)00019-7. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori T. Capsaicin and central control of thermoregulation. Pharmacol Ther. 1984;26:389–416. doi: 10.1016/0163-7258(84)90041-x. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Anton F, Nahin RL. Spinal lamina I projection neurons in the rat: collateral innervation of parabrachial area and thalamus. Neuroscience. 1989;28:27–37. doi: 10.1016/0306-4522(89)90229-7. [DOI] [PubMed] [Google Scholar]
- Iida T, Shimizu I, Nealen ML, Campbell A, Caterina M. Attenuated fever response in mice lacking TRPV1. Neurosci Lett. 2005;378:28–33. doi: 10.1016/j.neulet.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Imai-Matsumura K, Matsumura K, Nakayama T. Involvement of ventromedial hypothalamus in brown adipose tissue thermogenesis induced by preoptic cooling in rats. Jpn J Physiol. 1984;34:939–943. doi: 10.2170/jjphysiol.34.939. [DOI] [PubMed] [Google Scholar]
- Iriuchijima J, Zotterman Y. The specificity of afferent cutaneous C fibres in mammals. Acta Physiol Scand. 1960;49:267–278. doi: 10.1111/j.1748-1716.1960.tb01952.x. [DOI] [PubMed] [Google Scholar]
- Ishiwata T, Saito T, Hasegawa H, Yazawa T, Kotani Y, Otokawa M, Aihara Y. Changes of body temperature and thermoregulatory responses of freely moving rats during GABAergic pharmacological stimulation to the preoptic area and anterior hypothalamus in several ambient temperatures. Brain Res. 2005;1048:32–40. doi: 10.1016/j.brainres.2005.04.027. [DOI] [PubMed] [Google Scholar]
- Jancso-Gabor A, Szolcsanyi J, Jancso N. Irreversible impairment of thermoregulation induced by capsaicin and similar pungent substances in rats and guinea-pigs. J Physiol. 1970;206:495–507. doi: 10.1113/jphysiol.1970.sp009027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Jubrias SA, Vollestad NK, Gronka RK, Kushmerick MJ. Contraction coupling efficiency of human first dorsal interosseous muscle. J Physiol. 2008;586:1993–2002. doi: 10.1113/jphysiol.2007.146829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerman IA, Enquist LW, Watson SJ, Yates BJ. Brainstem substrates of sympatho-motor circuitry identified using trans-synaptic tracing with pseudorabies virus recombinants. J Neurosci. 2003;23:4657–4666. doi: 10.1523/JNEUROSCI.23-11-04657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Osaka T. Involvement of the parabrachial nucleus in thermogenesis induced by environmental cooling in the rat. Pflugers Arch. 2003;446:760–765. doi: 10.1007/s00424-003-1119-7. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Hori A, Matsumura K, Hosokawa H. Point: Heat-induced membrane depolarization of hypothalamic neurons: a putative mechanism of central thermosensitivity. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1479–R1480. doi: 10.1152/ajpregu.00655.2005. discussion R1484. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Lazarus M, Yoshida K, Coppari R, Bass CE, Mochizuki T, Lowell BB, Saper CB. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci. 2007;10:1131–1133. doi: 10.1038/nn1949. [DOI] [PubMed] [Google Scholar]
- Lee H, Iida T, Mizuno A, Suzuki M, Caterina MJ. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J Neurosci. 2005;25:1304–1310. doi: 10.1523/JNEUROSCI.4745.04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DI, Coote JH. The influence of 5-hydroxytryptamine agonists and antagonists on identified sympathetic preganglionic neurones in the rat, in vivo. Br J Pharmacol. 1990;99:667–672. doi: 10.1111/j.1476-5381.1990.tb12987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Xiong K, Pang Y, Dong Y, Kaneko T, Mizuno N. Medullary dorsal horn neurons providing axons to both the parabrachial nucleus and thalamus. J Comp Neurol. 2006;498:539–551. doi: 10.1002/cne.21068. [DOI] [PubMed] [Google Scholar]
- Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4-/- mice. Proc Natl Acad Sci USA. 2003;100:13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax P, Malveaux E, Smith RE. Brain temperatures in the rat during exposure to low environmental temperatures. Am J Physiol. 1964;207:736–739. doi: 10.1152/ajplegacy.1964.207.3.736. [DOI] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Excitatory amino acid receptor activation in the raphe pallidus area mediates prostaglandin-evoked thermogenesis. Neuroscience. 2003;122:5–15. doi: 10.1016/s0306-4522(03)00527-x. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Excitatory amino acid receptors in the dorsomedial hypothalamus mediate prostaglandin-evoked thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2004;286:R320–R325. doi: 10.1152/ajpregu.00515.2003. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Hypoxic activation of arterial chemoreceptors inhibits sympathetic outflow to brown adipose tissue in rats. J Physiol. 2005;566:559–573. doi: 10.1113/jphysiol.2005.086322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Serotonin potentiates sympathetic responses evoked by spinal NMDA. J Physiol. 2006;577:525–537. doi: 10.1113/jphysiol.2006.116574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Role of spinal serotonin in cold-evoked sympathetic activation of brown adipose tissue. FASEB J. 2007;21:A885. [Google Scholar]
- Madden CJ, Morrison SF. Brown adipose tissue sympathetic nerve activity is potentiated by activation of 5-hydroxytryptamine (5-HT)1A/5-HT7 receptors in the rat spinal cord. Neuropharmacology. 2008;54:487–496. doi: 10.1016/j.neuropharm.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeshima T, Ito R, Hamada S, Senzaki K, Hamaguchi-Hamada K, Shutoh F, Okado N. The cellular localization of 5-HT2A receptors in the spinal cord and spinal ganglia of the adult rat. Brain Res. 1998;797:118–124. doi: 10.1016/s0006-8993(98)00360-6. [DOI] [PubMed] [Google Scholar]
- Marlier L, Teilhac JR, Cerruti C, Privat A. Autoradiographic mapping of 5-HT1, 5-HT1A, 5-HT1B and 5-HT2 receptors in the rat spinal cord. Brain Research. 1991;550:15–23. doi: 10.1016/0006-8993(91)90400-p. [DOI] [PubMed] [Google Scholar]
- Martin-Cora FJ, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic medullary and pontine raphe neurons to environmental cooling in freely moving cats. Neuroscience. 2000;98:301–309. doi: 10.1016/s0306-4522(00)00133-0. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Hori T, Nakashima T. Thermal and PGE2 sensitivity of the organum vasculosum lamina terminalis region and preoptic area in rat brain slices. J Physiol. 1992;454:197–212. doi: 10.1113/jphysiol.1992.sp019260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura K, Cao C, Ozaki M, Morii H, Nakadate K, Watanabe Y. Brain endothelial cells express cyclooxygenase-2 during lipopolysaccharide-induced fever: light and electron microscopic immunocytochemical studies. J Neurosci. 1998;18:6279–6289. doi: 10.1523/JNEUROSCI.18-16-06279.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Coote JH, Dun NJ. Excitatory and inhibitory effects of epinephrine on neonatal rat sympathetic preganglionic neurons in vitro. Brain Research. 1989;497:108–116. doi: 10.1016/0006-8993(89)90976-1. [DOI] [PubMed] [Google Scholar]
- Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, Patapoutian A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- Morrison SF. RVLM and raphe differentially regulate sympathetic outflows to splanchnic and brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 1999;276:R962–R973. doi: 10.1152/ajpregu.1999.276.4.R962. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Raphe pallidus neurons mediate prostaglandin E2-evoked increases in brown adipose tissue thermogenesis. Neuroscience. 2003;121:17–24. doi: 10.1016/s0306-4522(03)00363-4. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Activation of 5-HT1A receptors in raphe pallidus inhibits leptin-evoked increases in brown adipose tissue thermogenesis. Am J Physiol Regul Integr Comp Physiol. 2004;286:R832–R837. doi: 10.1152/ajpregu.00678.2003. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Cao W-H, Madden CJ. Dorsomedial hypothalamic and brainstem pathways controlling thermogenesis in brown adipose tissue. J Therm Biol. 2004;29:333–337. [Google Scholar]
- Morrison SF, Sved AF, Passerin AM. GABA-mediated inhibition of raphe pallidus neurons regulates sympathetic outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 1999;276:R290–R297. doi: 10.1152/ajpregu.1999.276.2.R290. [DOI] [PubMed] [Google Scholar]
- Nakamura K. Fever-inducing sympathetic neural pathways. J Therm Biol. 2004;29:339–344. [Google Scholar]
- Nakamura K, Kaneko T, Yamashita Y, Hasegawa H, Katoh H, Ichikawa A, Negishi M. Immunocytochemical localization of prostaglandin EP3 receptor in the rat hypothalamus. Neurosci Lett. 1999;260:117–120. doi: 10.1016/s0304-3940(98)00962-8. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kaneko T, Yamashita Y, Hasegawa H, Katoh H, Negishi M. Immunohistochemical localization of prostaglandin EP3 receptor in the rat nervous system. J Comp Neurol. 2000;421:543–569. doi: 10.1002/(sici)1096-9861(20000612)421:4<543::aid-cne6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Hubschle T, Nakamura Y, Hioki H, Fujiyama F, Boldogkoi Z, Konig M, Thiel HJ, Gerstberger R, Kobayashi S, Kaneko T. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J Neurosci. 2004a;24:5370–5380. doi: 10.1523/JNEUROSCI.1219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Kaneko T, Kobayashi S, Katoh H, Negishi M. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J Neurosci. 2002;22:4600–4610. doi: 10.1523/JNEUROSCI.22-11-04600.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2007a;292:R127–R136. doi: 10.1152/ajpregu.00427.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. Identification of lateral parabrachial neurons mediating cutaneous thermoregulatory afferent signals to the preoptic area. Soc Neurosci Abstr. 2007b;33:731–713. [Google Scholar]
- Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci. 2008a;11:62–71. doi: 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. Preoptic mechanism for cold-defensive responses to skin cooling. J Physiol. 2008b;586:2611–2620. doi: 10.1113/jphysiol.2008.152686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Wu SX, Fujiyama F, Okamoto K, Hioki H, Kaneko T. Independent inputs by VGLUT2- and VGLUT3-positive glutamatergic terminals onto rat sympathetic preganglionic neurons. Neuroreport. 2004b;15:431–436. doi: 10.1097/00001756-200403010-00010. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Nakamura K, Matsumura K, Kobayashi S, Kaneko T, Morrison SF. Direct pyrogenic input from prostaglandin EP3 receptor-expressing preoptic neurons to the dorsomedial hypothalamus. Eur J Neurosci. 2005;22:3137–3146. doi: 10.1111/j.1460-9568.2005.04515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T, Eisenman JS, Hardy JD. Single unit activity of anterior hypothalamus during local heating. Science. 1961;134:560–561. doi: 10.1126/science.134.3478.560. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Hammel H, Hardy J, Eisenman J. Thermal stimulation of electrical activity of single units of the preoptic region. Am J Physiol. 1963;204:1122–1126. [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Nason MW, Jr, Mason P. Modulation of sympathetic and somatomotor function by the ventromedial medulla. J Neurophysiol. 2004;92:510–522. doi: 10.1152/jn.00089.2004. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- Negishi M, Irie A, Sugimoto Y, Namba T, Ichikawa A. Selective coupling of prostaglandin E receptor EP3D to Gi and Gs through interaction of α-carboxylic acid of agonist and arginine residue of seventh transmembrane domain. J Biol Chem. 1995;270:16122–16127. doi: 10.1074/jbc.270.27.16122. [DOI] [PubMed] [Google Scholar]
- Oka T, Oka K, Kobayashi T, Sugimoto Y, Ichikawa A, Ushikubi F, Narumiya S, Saper CB. Characteristics of thermoregulatory and febrile responses in mice deficient in prostaglandin EP1 and EP3 receptors. J Physiol. 2003;551:945–954. doi: 10.1113/jphysiol.2003.048140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T, Oka K, Scammell TE, Lee C, Kelly JF, Nantel F, Elmquist JK, Saper CB. Relationship of EP(1-4) prostaglandin receptors with rat hypothalamic cell groups involved in lipopolysaccharide fever responses. J Comp Neurol. 2000;428:20–32. doi: 10.1002/1096-9861(20001204)428:1<20::aid-cne3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, Blessing WW, Steiner AA, Romanovsky AA. Fever response to intravenous prostaglandin E2 is mediated by the brain but does not require afferent vagal signaling. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1294–R1303. doi: 10.1152/ajpregu.00709.2007. [DOI] [PubMed] [Google Scholar]
- Osaka T. Cold-induced thermogenesis mediated by GABA in the preoptic area of anesthetized rats. Am J Physiol Regul Integr Comp Physiol. 2004a;287:R306–R313. doi: 10.1152/ajpregu.00003.2004. [DOI] [PubMed] [Google Scholar]
- Osaka T. Thermogenesis elicited by skin cooling in anaesthetized rats: lack of contribution of the cerebral cortex. J Physiol. 2004b;555:503–513. doi: 10.1113/jphysiol.2003.053215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passerin AM, Bellush LL, Henley WN. Activation of bulbospinal serotonergic neurons during cold exposure. Can J Physiol Pharmacol. 1999;77:250–258. [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002a;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, Bevan S, Patapoutian A. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002b;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- Rall JA, Woledge RC. Influence of temperature on mechanics and energetics of muscle contraction. Am J Physiol Regul Integr Comp Physiol. 1990;259:R197–R203. doi: 10.1152/ajpregu.1990.259.2.R197. [DOI] [PubMed] [Google Scholar]
- Ranels HJ, Griffin JD. The effects of prostaglandin E2 on the firing rate activity of thermosensitive and temperature insensitive neurons in the ventromedial preoptic area of the rat hypothalamus. Brain Res. 2003;964:42–50. doi: 10.1016/s0006-8993(02)04063-5. [DOI] [PubMed] [Google Scholar]
- Rathner JA, Morrison SF. Rostral ventromedial periaqueductal gray: a source of inhibition of the sympathetic outflow to brown adipose tissue. Brain Res. 2006;1077:99–107. doi: 10.1016/j.brainres.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Riedel W. Warm receptors in the dorsal abdominal wall of the rabbit. Pflugers Arch. 1976;361:205–206. doi: 10.1007/BF00583468. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Stock MJ, Thexton AJ. Decerebration activates thermogenesis in the rat. J Physiol. 1983;342:15–22. doi: 10.1113/jphysiol.1983.sp014836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci. 2002;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Zaretskaia MV, Zaretsky DV, Moreno M, DiMicco JA. Stress- and lipopolysaccharide-induced c-fos expression and nNOS in hypothalamic neurons projecting to medullary raphe in rats: a triple immunofluorescent labeling study. Eur J Neurosci. 2007;26:2228–2238. doi: 10.1111/j.1460-9568.2007.05843.x. [DOI] [PubMed] [Google Scholar]
- Sato H, Hashitani T, Isobe Y, Furuyama F, Nishino H. Descending influences from nucleus rahe magnus on fusimotor neurone activity in rats. J Therm Biol. 1990;15:259–265. [Google Scholar]
- Scammell TE, Elmquist JK, Griffin JD, Saper CB. Ventromedial preoptic prostaglandin E2 activates fever-producing autonomic pathways. J Neurosci. 1996;16:6246–6254. doi: 10.1523/JNEUROSCI.16-19-06246.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer SS, Schafer S. The behavior of the proprioceptors of the muscle and the innervation of the fusimotor system during cold shivering. Exp Brain Res. 1973a;17:364–380. doi: 10.1007/BF00234100. [DOI] [PubMed] [Google Scholar]
- Schafer SS, Schafer S. The role of the primary afference in the generation of a cold shivering tremor. Exp Brain Res. 1973b;17:381–393. doi: 10.1007/BF00234101. [DOI] [PubMed] [Google Scholar]
- Schoener EP, Wang SC. Effects of locally administered prostaglandin E1 on anterior hypothalamic neurons. Brain Res. 1976;117:157–162. doi: 10.1016/0006-8993(76)90567-9. [DOI] [PubMed] [Google Scholar]
- Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- Soderlund V, Larsson SA, Jacobsson H. Reduction of FDG uptake in brown adipose tissue in clinical patients by a single dose of propranolol. Eur J Nucl Med Mol Imaging. 2007;34:1018–1022. doi: 10.1007/s00259-006-0318-9. [DOI] [PubMed] [Google Scholar]
- Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, Bannon AW, Norman MH, Louis JC, Treanor JJ, Gavva NR, Romanovsky AA. Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci. 2007;27:7459–7468. doi: 10.1523/JNEUROSCI.1483-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt JT. Prosaglandin E1 fever induced in rabbits. J Physiol. 1973;232:163–179. doi: 10.1113/jphysiol.1973.sp010262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, McQuiston TJ, Guyenet PG. GABAergic and glycinergic presympathetic neurons of rat medulla oblongata identified by retrograde transport of pseudorabies virus and in situ hybridization. J Comp Neurol. 2004;479:257–270. doi: 10.1002/cne.20332. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Rosin DL, Simmons JR, McQuiston TJ, Vujovic N, Weston MC, Guyenet PG. Coexpression of vesicular glutamate transporter-3 and γ-aminobutyric acidergic markers in rat rostral medullary raphe and intermediolateral cell column. J Comp Neurol. 2005;492:477–494. doi: 10.1002/cne.20742. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Swanson DM, Dubin AE, Shah C, Nasser N, Chang L, Dax SL, Jetter M, Breitenbucher JG, Liu C, Mazur C, Lord B, Gonzales L, Hoey K, Rizzolio M, Bogenstaetter M, Codd EE, Lee DH, Zhang SP, Chaplan SR, Carruthers NI. Identification and biological evaluation of 4-(3-trifluoro-methylpyridin-2-yl)piperazine-1-carboxylic acid (5-trifluo-romethylpyridin-2-yl)amide, a high affinity TRPV1 (VR1) vanilloid receptor antagonist. J Med Chem. 2005;48:1857–1872. doi: 10.1021/jm0495071. [DOI] [PubMed] [Google Scholar]
- Szelenyi Z, Hummel Z, Szolcsanyi J, Davis JB. Daily body temperature rhythm and heat tolerance in TRPV1 knockout and capsaicin pretreated mice. Eur J Neurosci. 2004;19:1421–1424. doi: 10.1111/j.1460-9568.2004.03221.x. [DOI] [PubMed] [Google Scholar]
- Szymusiak R, Satinoff E. Acute thermoregulatory effects of unilateral electrolytic lesions of the medial and lateral preoptic area in rats. Physiol Behav. 1982;28:161–170. doi: 10.1016/0031-9384(82)90118-4. [DOI] [PubMed] [Google Scholar]
- Tajino K, Matsumura K, Kosada K, Shibakusa T, Inoue K, Fushiki T, Hosokawa H, Kobayashi S. Application of menthol to the skin of whole trunk in mice induces autonomic and behavioral heat-gain responses. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2128–R2135. doi: 10.1152/ajpregu.00377.2007. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Owens NC, Nagashima K, Kanosue K, McAllen RM. Reflex activation of rat fusimotor neurons by body surface cooling, and its dependence on the medullary raphe. J Physiol. 2006;572:569–583. doi: 10.1113/jphysiol.2005.102400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Tonouchi M, Hosono T, Nagashima K, Yanase-Fujiwara M, Kanosue K. Hypothalamic region facilitating shivering in rats. Jpn J Physiol. 2001;51:625–629. doi: 10.2170/jjphysiol.51.625. [DOI] [PubMed] [Google Scholar]
- Ter Horst GJ, Hautvast RW, De Jongste MJ, Korf J. Neuroanatomy of cardiac activity-regulating circuitry: a transneuronal retrograde viral labelling study in the rat. Eur J Neurosci. 1996;8:2029–2041. doi: 10.1111/j.1460-9568.1996.tb00723.x. [DOI] [PubMed] [Google Scholar]
- Thor KB, Nickolaus S, Helke CJ. Autoradiographic localization of 5-hydroxytryptamine1A, 5-hydroxytryptamine1B and 5-hydroxytryptamine1C/2 binding sites in the rat spinal cord. Neuroscience. 1993;55:235–252. doi: 10.1016/0306-4522(93)90469-v. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Uschakov A, Gong H, McGinty D, Szymusiak R. Efferent projections from the median preoptic nucleus to sleep- and arousal-regulatory nuclei in the rat brain. Neuroscience. 2007;150:104–120. doi: 10.1016/j.neuroscience.2007.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushikubi F, Segi E, Sugimoto Y, Murata T, Matsuoka T, Kobayashi T, Hizaki H, Tuboi K, Katsuyama M, Ichikawa A, Tanaka T, Yoshida N, Narumiya S. Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature. 1998;395:281–284. doi: 10.1038/26233. [DOI] [PubMed] [Google Scholar]
- Vera PL, Holets VR, Miller KE. Ultrastructural evidence of synaptic contacts between substance P-, enkephalin-, and serotonin-immunoreactive terminals and retrogradely labeled sympathetic preganglionic neurons in the rat: a study using a double-peroxidase procedure. Synapse. 1990;6:221–229. doi: 10.1002/syn.890060303. [DOI] [PubMed] [Google Scholar]
- Viana F, de la Pena E, Belmonte C. Specificity of cold thermotransduction is determined by differential ionic channel expression. Nat Neurosci. 2002;5:254–260. doi: 10.1038/nn809. [DOI] [PubMed] [Google Scholar]
- Wang L, Cardin S, Martínez V, Taché Y, Lloyd KC. Duodenal loading with glucose induces Fos expression in rat brain: selective blockade by devazepide. Am J Physiol Regul Integr Comp Physiol. 1999;277:R667–R674. doi: 10.1152/ajpregu.1999.277.3.R667. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002;277:47044–47051. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- Wechselberger M, Wright CL, Bishop GA, Boulant JA. Ionic channels and conductance-based models for hypothalamic neuronal thermosensitivity. Am J Physiol Regul Integr Comp Physiol. 2006;291:R518–R529. doi: 10.1152/ajpregu.00039.2006. [DOI] [PubMed] [Google Scholar]
- Williams JW, Rudy TA, Yaksh TL, Viswanathan CT. An extensive exploration of the rat brain for sites mediating prostaglandin-induced hyperthermia. Brain Res. 1977;120:251–262. doi: 10.1016/0006-8993(77)90904-0. [DOI] [PubMed] [Google Scholar]
- Wilson JD, Nicklous DM, Aloyo VJ, Simansky KJ. An orexigenic role for μ-opioid receptors in the lateral parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1055–R1065. doi: 10.1152/ajpregu.00108.2003. [DOI] [PubMed] [Google Scholar]
- Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci. 2006;9:628–635. doi: 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
- Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Matsumura K, Inoue W, Shiraki T, Suzuki K, Yasuda S, Sugiura H, Cao C, Watanabe Y, Kobayashi S. Coexpression of microsomal-type prostaglandin E synthase with cyclooxygenase-2 in brain endothelial cells of rats during endotoxin-induced fever. J Neurosci. 2001;21:2669–2677. doi: 10.1523/JNEUROSCI.21-08-02669.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Sawa K. c-Fos-like immunoreactivity in the brainstem following gastric loads of various chemical solutions in rats. Brain Res. 2000;866:135–143. doi: 10.1016/s0006-8993(00)02241-1. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Konishi M, Nagashima K, Saper CB, Kanosue K. Fos activation in hypothalamic neurons during cold or warm exposure: projections to periaqueductal gray matter. Neuroscience. 2005;133:1039–1046. doi: 10.1016/j.neuroscience.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Maruyama M, Hosono T, Nagashima K, Fukuda Y, Gerstberger R, Kanosue K. Fos expression induced by warming the preoptic area in rats. Brain Research. 2002;933:109–117. doi: 10.1016/s0006-8993(02)02287-4. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Nakamura K, Matsumura K, Kanosue K, Konig M, Thiel HJ, Boldogkoi Z, Toth I, Roth J, Gerstberger R, Hubschle T. Neurons of the rat preoptic area and the raphe pallidus nucleus innervating the brown adipose tissue express the prostaglandin E receptor subtype EP3. Eur J Neurosci. 2003;18:1848–1860. doi: 10.1046/j.1460-9568.2003.02919.x. [DOI] [PubMed] [Google Scholar]
- Zaretskaia MV, Zaretsky DV, DiMicco JA. Role of the dorsomedial hypothalamus in thermogenesis and tachycardia caused by microinjection of prostaglandin E2 into the preoptic area in anesthetized rats. Neurosci Lett. 2003;340:1–4. doi: 10.1016/s0304-3940(03)00047-8. [DOI] [PubMed] [Google Scholar]
- Zaretskaia MV, Zaretsky DV, Shekhar A, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Res. 2002;928:113–125. doi: 10.1016/s0006-8993(01)03369-8. [DOI] [PubMed] [Google Scholar]
- Zaretsky DV, Hunt JL, Zaretskaia MV, DiMicco JA. Microinjection of prostaglandin E2 and muscimol into the preoptic area in conscious rats: comparison of effects on plasma adrenocorticotrophic hormone (ACTH), body temperature, locomotor activity, and cardiovascular function. Neurosci Lett. 2006;397:291–296. doi: 10.1016/j.neulet.2005.12.032. [DOI] [PubMed] [Google Scholar]
- Zaretsky DV, Zaretskaia MV, DiMicco JA. Stimulation and blockade of GABAA receptors in the raphe pallidus: effects on body temperature, heart rate, and blood pressure in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R110–R116. doi: 10.1152/ajpregu.00016.2003. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Yanase-Fujiwara M, Hosono T, Kanosue K. Warm and cold signals from the preoptic area: which contribute more to the control of shivering in rats? J Physiol. 1995;485:195–202. doi: 10.1113/jphysiol.1995.sp020723. [DOI] [PMC free article] [PubMed] [Google Scholar]