Abstract
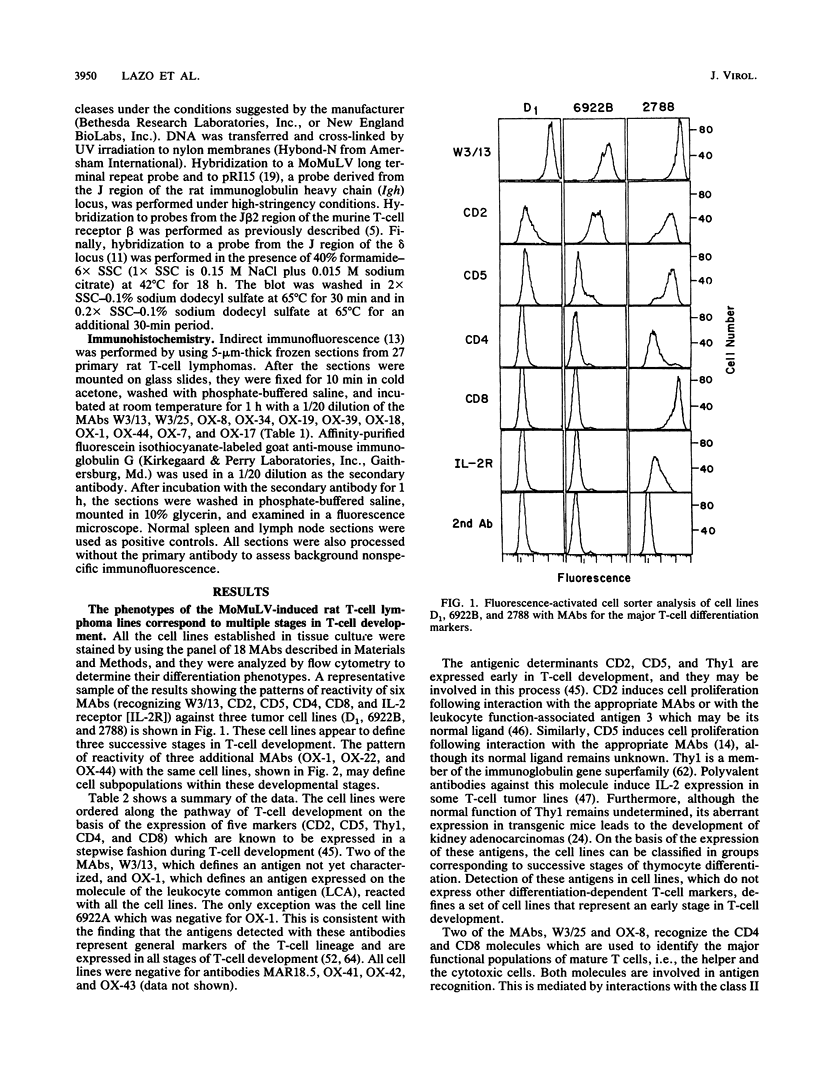
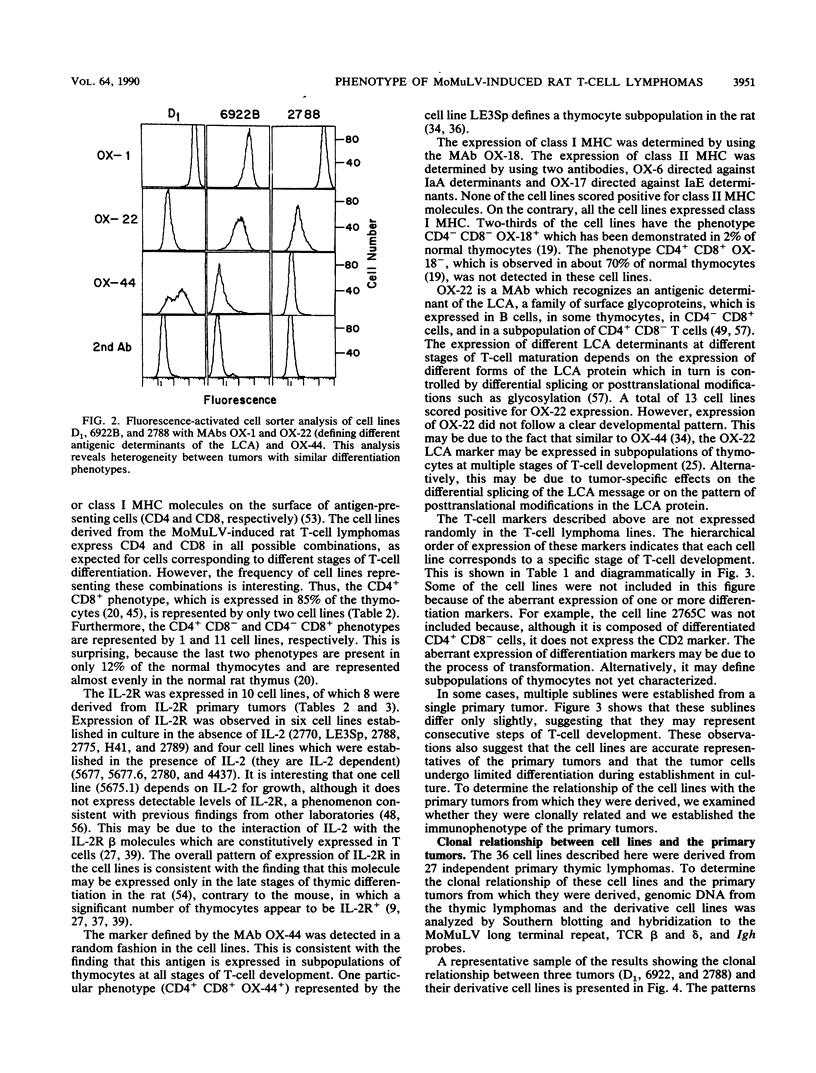
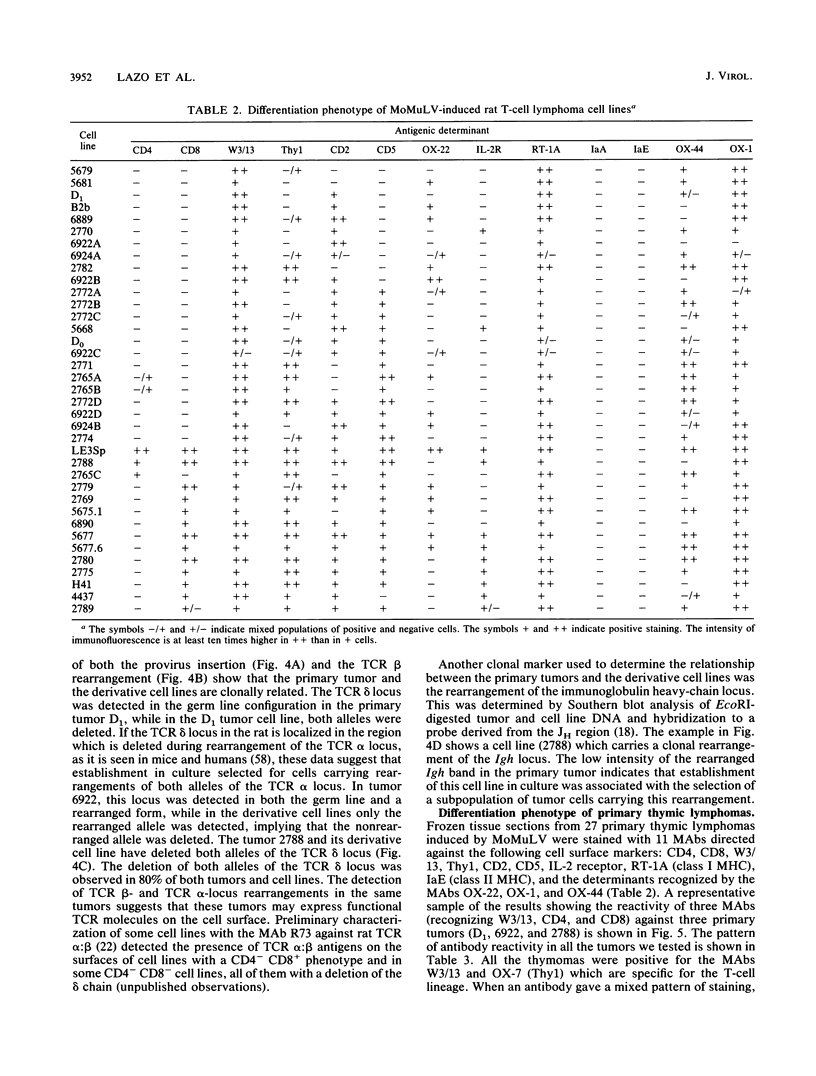
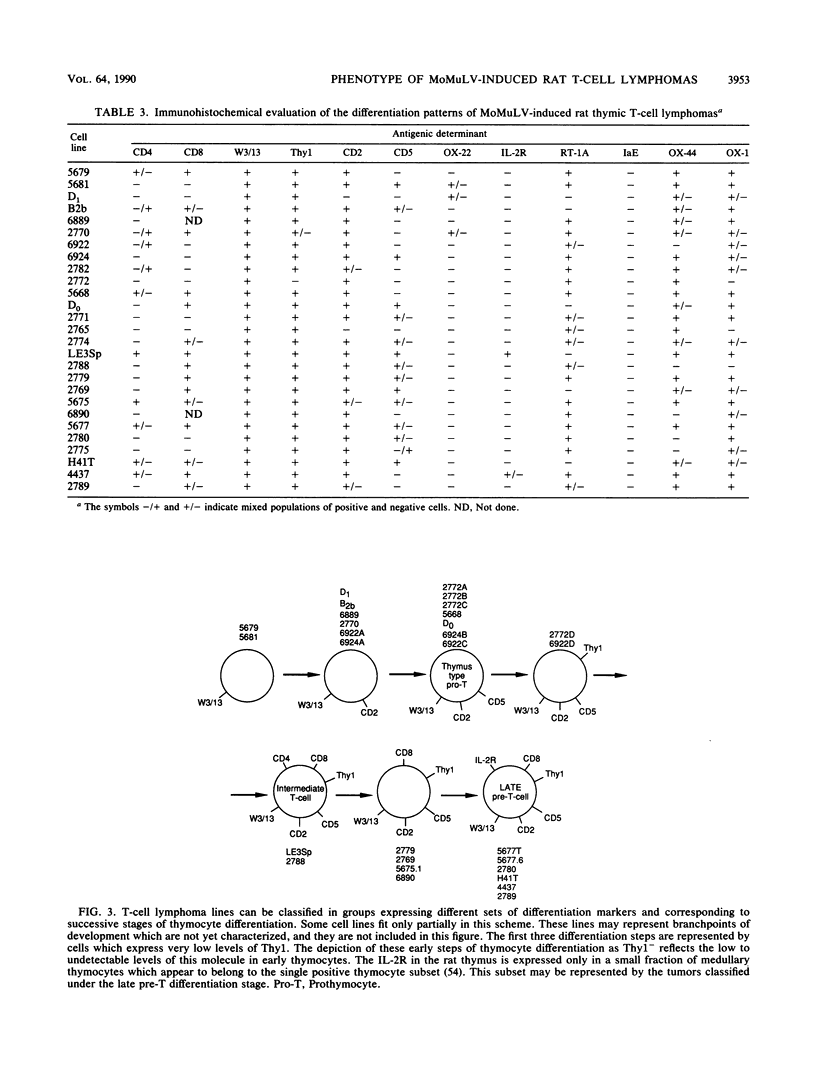
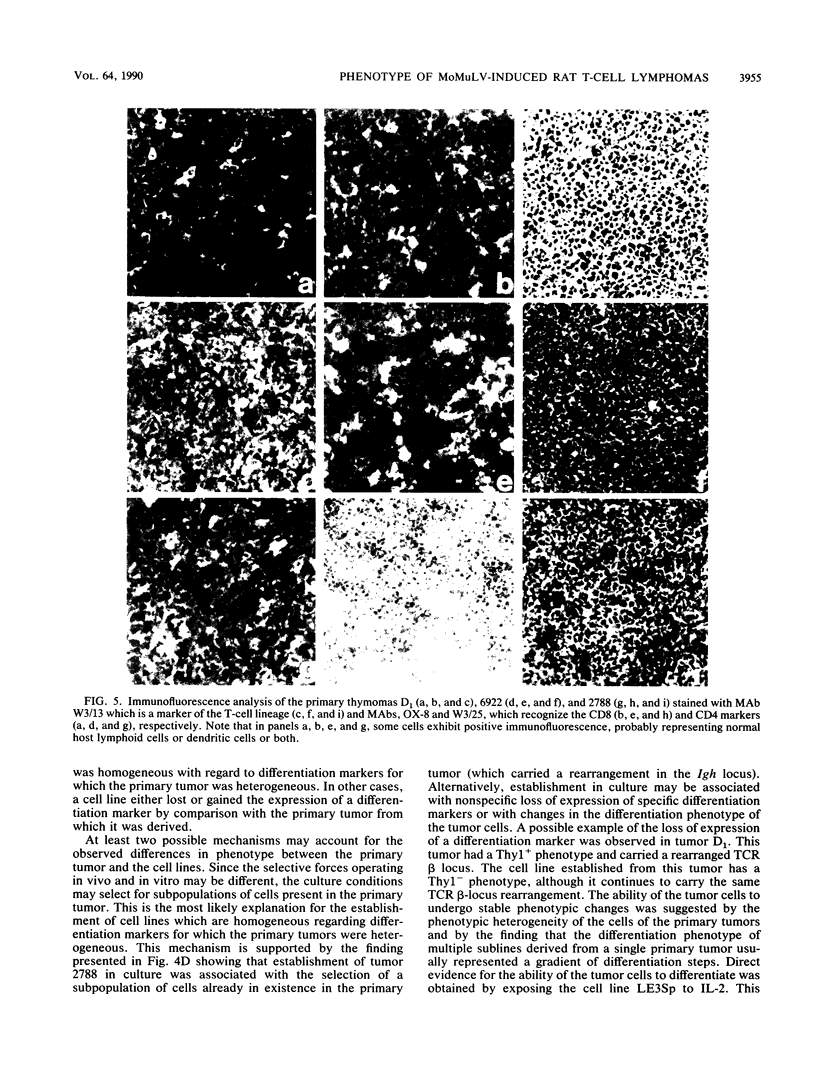
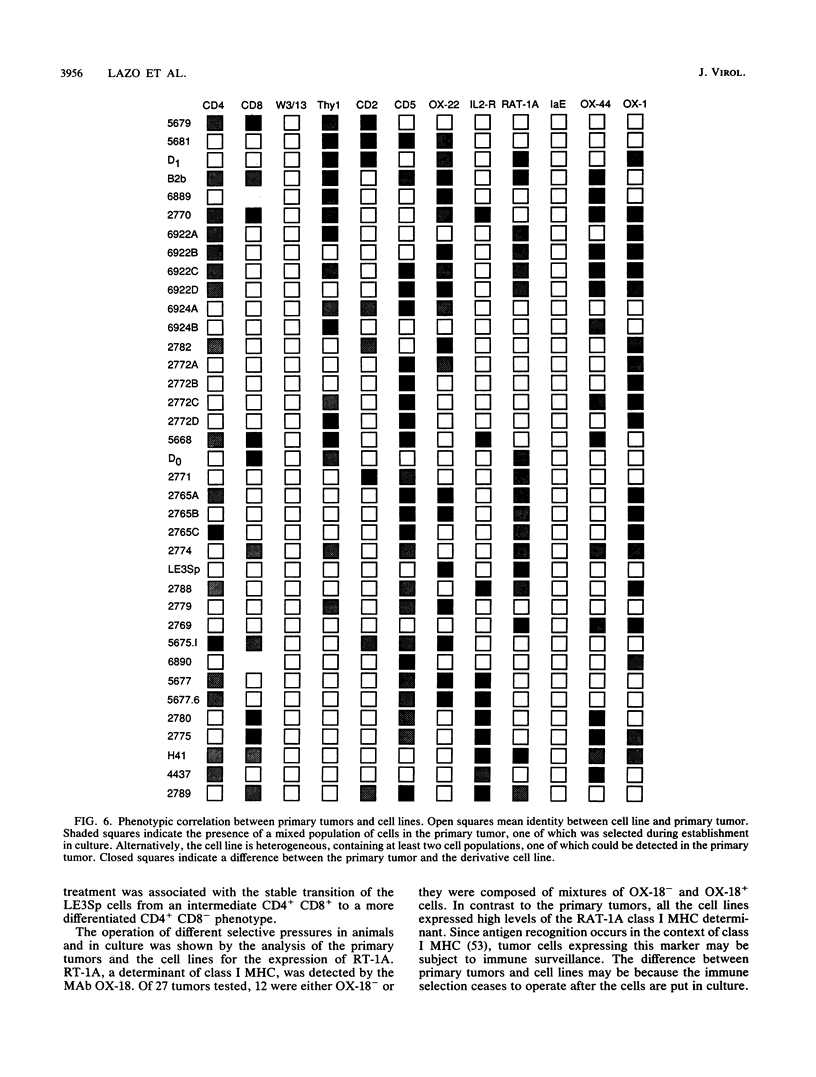
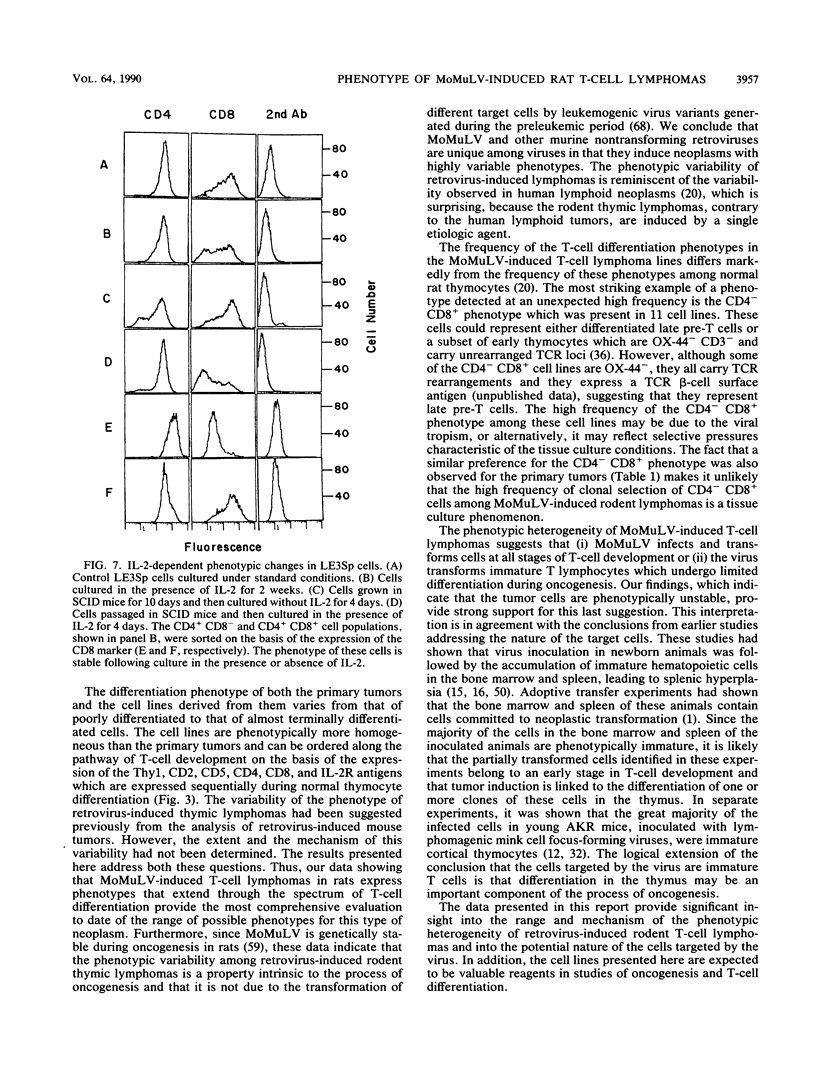
The phenotype of 27 Moloney murine leukemia virus-induced rat thymic lymphomas and 36 cell lines derived from these tumors was determined by using 18 monoclonal antibodies directed against hematopoietic cell surface determinants. The cell lines and the primary tumors from which they were derived were clonally related as determined by the pattern of provirus integration and the pattern of rearrangement of the T-cell receptor beta and delta and Igh loci. The differentiation phenotype of the primary tumors and the cell lines derived from them were related. The differences observed between the primary tumors and the cell lines could be explained either by the selection of subpopulations of tumor cells during establishment in culture or by the phenotypic instability of the tumor cells. One cell line (LE3Sp) underwent the transition from a CD4+ CD8+ to a CD4+ CD8- phenotype following exposure to interleukin-2 in culture. Both the primary tumors and the cell lines derived from them express a wide range of phenotypes which correspond to multiple stages in T-cell development. This observation suggests that the pleiomorphism of retrovirus-induced lymphomas, which had been suggested previously from the analysis of mouse tumors, is an intrinsic property of the process of oncogenesis and is not due to the transformation of different types of cells by spontaneously arising leukemogenic variants of the inoculated virus. The wide spectrum of phenotypes expressed by these tumors suggests that Moloney murine leukemia virus may infect and transform T cells at various stages of development. Alternatively, the target cells may be immature T-cell precursors which, following transformation, continue to differentiate. A host of early findings, suggesting that the repertoire of target cells is restricted to poorly differentiated hematopoietic progenitors, and the ability of the LE3Sp cell line to differentiate in culture indicate that the latter possibility may be more likely. The data in this report address the extent and mechanism of the phenotypic variability of retrovirus-induced rodent T-cell lymphomas. In addition, they demonstrate the potential usefulness of the T-cell lymphoma lines we have established in studies of oncogenesis and T-cell differentiation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asjö B., Skoog L., Palminger I., Wiener F., Isaak D., Cerny J., Fenyö E. M. Influence of genotype and the organ of origin on the subtype of T-cell in Moloney lymphomas induced by transfer of preleukemic cells from athymic and thymus-bearing mice. Cancer Res. 1985 Mar;45(3):1040–1045. [PubMed] [Google Scholar]
- Barbacid M. Oncogenes and human cancer: cause or consequence? Carcinogenesis. 1986 Jul;7(7):1037–1042. doi: 10.1093/carcin/7.7.1037. [DOI] [PubMed] [Google Scholar]
- Barclay A. N. Different reticular elements in rat lymphoid tissue identified by localization of Ia, Thy-1 and MRC OX 2 antigens. Immunology. 1981 Dec;44(4):727–736. [PMC free article] [PubMed] [Google Scholar]
- Barclay A. N. The localization of populations of lymphocytes defined by monoclonal antibodies in rat lymphoid tissues. Immunology. 1981 Apr;42(4):593–600. [PMC free article] [PubMed] [Google Scholar]
- Bear S. E., Bellacosa A., Lazo P. A., Jenkins N. A., Copeland N. G., Hanson C., Levan G., Tsichlis P. N. Provirus insertion in Tpl-1, an Ets-1-related oncogene, is associated with tumor progression in Moloney murine leukemia virus-induced rat thymic lymphomas. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7495–7499. doi: 10.1073/pnas.86.19.7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellacosa A., Lazo P. A., Bear S. E., Shinton S., Tsichlis P. N. Induction of multiple independent T-cell lymphomas in rats inoculated with MOloney murine leukemia virus. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4269–4272. doi: 10.1073/pnas.86.11.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Brideau R. J., Carter P. B., McMaster W. R., Mason D. W., Williams A. F. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980 Aug;10(8):609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- Burny A., Cleuter Y., Kettmann R., Mammerickx M., Marbaix G., Portetelle D., van den Broeke A., Willems L., Thomas R. Bovine leukaemia: facts and hypotheses derived from the study of an infectious cancer. Vet Microbiol. 1988 Jul;17(3):197–218. doi: 10.1016/0378-1135(88)90066-1. [DOI] [PubMed] [Google Scholar]
- Ceredig R., Lowenthal J. W., Nabholz M., MacDonald H. R. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985 Mar 7;314(6006):98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- Chazan R., Haran-Ghera N. The role of thymus subpopulations in "T" leukemia development. Cell Immunol. 1976 May;23(2):356–375. doi: 10.1016/0008-8749(76)90200-8. [DOI] [PubMed] [Google Scholar]
- Chien Y. H., Iwashima M., Wettstein D. A., Kaplan K. B., Elliott J. F., Born W., Davis M. M. T-cell receptor delta gene rearrangements in early thymocytes. Nature. 1987 Dec 24;330(6150):722–727. doi: 10.1038/330722a0. [DOI] [PubMed] [Google Scholar]
- Cloyd M. W. Characterization of target cells for MCF viruses in AKR mice. Cell. 1983 Jan;32(1):217–225. doi: 10.1016/0092-8674(83)90512-3. [DOI] [PubMed] [Google Scholar]
- Dallman M. J., Thomas M. L., Green J. R. MRC OX-19: a monoclonal antibody that labels rat T lymphocytes and augments in vitro proliferative responses. Eur J Immunol. 1984 Mar;14(3):260–267. doi: 10.1002/eji.1830140311. [DOI] [PubMed] [Google Scholar]
- Davis B. R., Brightman B. K., Chandy K. G., Fan H. Characterization of a preleukemic state induced by Moloney murine leukemia virus: evidence for two infection events during leukemogenesis. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4875–4879. doi: 10.1073/pnas.84.14.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. R., Chandy K. G., Brightman B. K., Gupta S., Fan H. Effects of nonleukemogenic and wild-type Moloney murine leukemia virus on lymphoid cells in vivo: identification of a preleukemic shift in thymocyte subpopulations. J Virol. 1986 Nov;60(2):423–430. doi: 10.1128/jvi.60.2.423-430.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depelchin A., Letesson J. J., Lostrie-Trussart N., Mammerickx M., Portetelle D., Burny A. Bovine leukemia virus (BLV)-infected B-cells express a marker similar to the CD5 T cell marker. Immunol Lett. 1989 Jan 15;20(1):69–76. doi: 10.1016/0165-2478(89)90071-0. [DOI] [PubMed] [Google Scholar]
- Economou-Pachnis A., Lohse M. A., Furano A. V., Tsichlis P. N. Insertion of long interspersed repeated elements at the Igh (immunoglobulin heavy chain) and Mlvi-2 (Moloney leukemia virus integration 2) loci of rats. Proc Natl Acad Sci U S A. 1985 May;82(9):2857–2861. doi: 10.1073/pnas.82.9.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto T., McMaster W. R., Williams A. F. Mouse monoclonal antibodies against rat major histocompatibility antigens. Two Ia antigens and expression of Ia and class I antigens in rat thymus. Eur J Immunol. 1982 Mar;12(3):237–243. doi: 10.1002/eji.1830120313. [DOI] [PubMed] [Google Scholar]
- Greaves M. F. Differentiation-linked leukemogenesis in lymphocytes. Science. 1986 Nov 7;234(4777):697–704. doi: 10.1126/science.3535067. [DOI] [PubMed] [Google Scholar]
- Hogarth P. M., Henning M. M., McKenzie I. F. Alloantigenic phenotype of radiation-induced thymomas in the mouse. J Natl Cancer Inst. 1982 Sep;69(3):619–626. [PubMed] [Google Scholar]
- Hünig T. Cross-linking of the T cell antigen receptor interferes with the generation of CD4+8+ thymocytes from their immediate CD4-8+ precursors. Eur J Immunol. 1988 Dec;18(12):2089–2092. doi: 10.1002/eji.1830181234. [DOI] [PubMed] [Google Scholar]
- Kawashima K., Ikeda H., Stockert E., Takahashi T., Old L. J. Age-related changes in cell surface antigens of preleukemic AKR thymocytes. J Exp Med. 1976 Jul 1;144(1):193–208. doi: 10.1084/jem.144.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollias G., Evans D. J., Ritter M., Beech J., Morris R., Grosveld F. Ectopic expression of Thy-1 in the kidneys of transgenic mice induces functional and proliferative abnormalities. Cell. 1987 Oct 9;51(1):21–31. doi: 10.1016/0092-8674(87)90006-7. [DOI] [PubMed] [Google Scholar]
- Law D. A., Spruyt L. L., Paterson D. J., Williams A. F. Subsets of thymopoietic rat thymocytes defined by expression of the CD2 antigen and the MRC OX-22 determinant of the leukocyte-common antigen CD45. Eur J Immunol. 1989 Dec;19(12):2289–2295. doi: 10.1002/eji.1830191217. [DOI] [PubMed] [Google Scholar]
- Lazo P. A., Lee J. S., Tsichlis P. N. Long-distance activation of the Myc protooncogene by provirus insertion in Mlvi-1 or Mlvi-4 in rat T-cell lymphomas. Proc Natl Acad Sci U S A. 1990 Jan;87(1):170–173. doi: 10.1073/pnas.87.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazo P. A., Tsichlis P. N. Recombination between two integrated proviruses, one of which was inserted near c-myc in a retrovirus-induced rat thymoma: implications for tumor progression. J Virol. 1988 Mar;62(3):788–794. doi: 10.1128/jvi.62.3.788-794.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenthal J. W., Howe R. C., Ceredig R., MacDonald H. R. Functional status of interleukin 2 receptors expressed by immature (Lyt-2-/L3T4-) thymocytes. J Immunol. 1986 Oct 15;137(8):2579–2584. [PubMed] [Google Scholar]
- Mathieson B. J., Campbell P. S., Potter M., Asofsky R. Expression of Ly 1, Ly 2, Thy 1, and TL differentiation antigens on mouse T-cell tumors. J Exp Med. 1978 Apr 1;147(4):1267–1279. doi: 10.1084/jem.147.4.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster W. R., Williams A. F. Identification of Ia glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol. 1979 Jun;9(6):426–433. doi: 10.1002/eji.1830090603. [DOI] [PubMed] [Google Scholar]
- Newcomb E. W., Binari R., Fleissner E. A comparative analysis of radiation- and virus-induced leukemias in BALB/c mice. Virology. 1985 Jan 15;140(1):102–112. doi: 10.1016/0042-6822(85)90449-0. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Tumor cell instability, diversification, and progression to the metastatic phenotype: from oncogene to oncofetal expression. Cancer Res. 1987 Mar 15;47(6):1473–1487. [PubMed] [Google Scholar]
- O'Donnell P. V., Woller R., Chu A. Stages in development of mink cell focus-inducing (MCF) virus-accelerated leukemia in AKR mice. J Exp Med. 1984 Sep 1;160(3):914–934. doi: 10.1084/jem.160.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen F. L., Strauss W. M., Murre C., Duby A. D., Hiai H., Seidman J. G. AKR murine thymic leukemias are from a distinct thymic cell lineage and do not express the beta chain of the T-cell antigen receptor. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7434–7437. doi: 10.1073/pnas.83.19.7434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson D. J., Green J. R., Jefferies W. A., Puklavec M., Williams A. F. The MRC OX-44 antigen marks a functionally relevant subset among rat thymocytes. J Exp Med. 1987 Jan 1;165(1):1–13. doi: 10.1084/jem.165.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson D. J., Jefferies W. A., Green J. R., Brandon M. R., Corthesy P., Puklavec M., Williams A. F. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol Immunol. 1987 Dec;24(12):1281–1290. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- Paterson D. J., Williams A. F. An intermediate cell in thymocyte differentiation that expresses CD8 but not CD4 antigen. J Exp Med. 1987 Nov 1;166(5):1603–1608. doi: 10.1084/jem.166.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse M., Gallagher P., Wilson A., Wu L., Fisicaro N., Miller J. F., Scollay R., Shortman K. Molecular characterization of T-cell antigen receptor expression by subsets of CD4- CD8- murine thymocytes. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6082–6086. doi: 10.1073/pnas.85.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar E., Potter M., Singer P. A., Sklar M. D. Synthesis, surface deposition, and secretion of immunoglobulins by Abelson virus-transformed lymphosarcoma cell lines. Cell. 1975 Oct;6(2):149–159. doi: 10.1016/0092-8674(75)90005-7. [DOI] [PubMed] [Google Scholar]
- Raulet D. H. Expression and function of interleukin-2 receptors on immature thymocytes. Nature. 1985 Mar 7;314(6006):101–103. doi: 10.1038/314101a0. [DOI] [PubMed] [Google Scholar]
- Richie E. R., Nairn R. S., Becker F. F. Proviral structure and differentiation antigen phenotype of spontaneous and chemically induced AKR lymphomas. Cancer Res. 1985 Jun;45(6):2802–2806. [PubMed] [Google Scholar]
- Risser R. The pathogenesis of Abelson virus lymphomas of the mouse. Biochim Biophys Acta. 1982 Jun 28;651(4):213–244. doi: 10.1016/0304-419x(82)90013-0. [DOI] [PubMed] [Google Scholar]
- Robinson A. P., White T. M., Mason D. W. MRC OX-43: a monoclonal antibody which reacts with all vascular endothelium in the rat except that of brain capillaries. Immunology. 1986 Feb;57(2):231–237. [PMC free article] [PubMed] [Google Scholar]
- Robinson A. P., White T. M., Mason D. W. Macrophage heterogeneity in the rat as delineated by two monoclonal antibodies MRC OX-41 and MRC OX-42, the latter recognizing complement receptor type 3. Immunology. 1986 Feb;57(2):239–247. [PMC free article] [PubMed] [Google Scholar]
- Schüpbach J. Human retrovirology. Facts and concepts. Curr Top Microbiol Immunol. 1989;142:1–115. [PubMed] [Google Scholar]
- Scollay R., Wilson A., D'Amico A., Kelly K., Egerton M., Pearse M., Wu L., Shortman K. Developmental status and reconstitution potential of subpopulations of murine thymocytes. Immunol Rev. 1988 Aug;104:81–120. doi: 10.1111/j.1600-065x.1988.tb00760.x. [DOI] [PubMed] [Google Scholar]
- Selvaraj P., Plunkett M. L., Dustin M., Sanders M. E., Shaw S., Springer T. A. The T lymphocyte glycoprotein CD2 binds the cell surface ligand LFA-3. 1987 Mar 26-Apr 1Nature. 326(6111):400–403. doi: 10.1038/326400a0. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Smith R. T., Norcross M. A., Maino V. C. Mechanisms controlling TCGF production by cloned sublines of EL-4 azgr in response to stimulation by anti-Thy-1 antibody. J Immunol. 1982 Jan;128(1):296–301. [PubMed] [Google Scholar]
- Smith K. A. Interleukin 2. Annu Rev Immunol. 1984;2:319–333. doi: 10.1146/annurev.iy.02.040184.001535. [DOI] [PubMed] [Google Scholar]
- Spickett G. P., Brandon M. R., Mason D. W., Williams A. F., Woollett G. R. MRC OX-22, a monoclonal antibody that labels a new subset of T lymphocytes and reacts with the high molecular weight form of the leukocyte-common antigen. J Exp Med. 1983 Sep 1;158(3):795–810. doi: 10.1084/jem.158.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch T. G., Arnstein P., Manohar V., Leiserson W. M., Chused T. M. Proliferation of infected lymphoid precursors before Moloney murine leukemia virus-induced T-cell lymphoma. J Natl Cancer Inst. 1985 Jan;74(1):137–143. [PubMed] [Google Scholar]
- Sunderland C. A., McMaster W. R., Williams A. F. Purification with monoclonal antibody of a predominant leukocyte-common antigen and glycoprotein from rat thymocytes. Eur J Immunol. 1979 Feb;9(2):155–159. doi: 10.1002/eji.1830090212. [DOI] [PubMed] [Google Scholar]
- Swain S. L. T cell subsets and the recognition of MHC class. Immunol Rev. 1983;74:129–142. doi: 10.1111/j.1600-065x.1983.tb01087.x. [DOI] [PubMed] [Google Scholar]
- Takács L., Ruscetti F. W., Kovacs E. J., Rocha B., Brocke S., Diamantstein T., Mathieson B. J. Immature, double negative (CD4-,CD8-) rat thymocytes do not express IL-2 receptors. J Immunol. 1988 Dec 1;141(11):3810–3818. [PubMed] [Google Scholar]
- Teshigawara K., Wang H. M., Kato K., Smith K. A. Interleukin 2 high-affinity receptor expression requires two distinct binding proteins. J Exp Med. 1987 Jan 1;165(1):223–238. doi: 10.1084/jem.165.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. L., Lefrançois L. Differential expression of the leucocyte-common antigen family. Immunol Today. 1988 Oct;9(10):320–326. doi: 10.1016/0167-5699(88)91326-6. [DOI] [PubMed] [Google Scholar]
- Toyonaga B., Mak T. W. Genes of the T-cell antigen receptor in normal and malignant T cells. Annu Rev Immunol. 1987;5:585–620. doi: 10.1146/annurev.iy.05.040187.003101. [DOI] [PubMed] [Google Scholar]
- Tsichlis P. N., Shepherd B. M., Bear S. E. Activation of the Mlvi-1/mis1/pvt-1 locus in Moloney murine leukemia virus-induced T-cell lymphomas. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5487–5491. doi: 10.1073/pnas.86.14.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsichlis P. N., Strauss P. G., Hu L. F. A common region for proviral DNA integration in MoMuLV-induced rat thymic lymphomas. 1983 Mar 31-Apr 6Nature. 302(5907):445–449. doi: 10.1038/302445a0. [DOI] [PubMed] [Google Scholar]
- Tsichlis P. N., Strauss P. G., Lohse M. A. Concerted DNA rearrangements in Moloney murine leukemia virus-induced thymomas: a potential synergistic relationship in oncogenesis. J Virol. 1985 Oct;56(1):258–267. doi: 10.1128/jvi.56.1.258-267.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N., Clark S. J., Paterson D. J., Willis A. C. Similarities in sequences and cellular expression between rat CD2 and CD4 antigens. J Exp Med. 1987 Feb 1;165(2):368–380. doi: 10.1084/jem.165.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Galfrè G., Milstein C. Analysis of cell surfaces by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977 Nov;12(3):663–673. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]
- Woollett G. R., Barclay A. N., Puklavec M., Williams A. F. Molecular and antigenic heterogeneity of the rat leukocyte-common antigen from thymocytes and T and B lymphocytes. Eur J Immunol. 1985 Feb;15(2):168–173. doi: 10.1002/eji.1830150211. [DOI] [PubMed] [Google Scholar]
- Yetter R. A., Morse H. C., 3rd Cell surface antigen phenotypes of MCF-induced thymic lymphomas in AKR mice. J Immunol. 1984 May;132(5):2644–2648. [PubMed] [Google Scholar]
- Zielinski C. C., Datta S. K., Waksal S. D. Department of Medicine, Tufts University School of Medicine, Boston, Massachusetts. Immunogenetics. 1981;14(1-2):169–176. doi: 10.1007/BF00344310. [DOI] [PubMed] [Google Scholar]
- Zielinski C. C., Waksal S. D., Tempelis L. D., Khiroya R. H., Schwartz R. S. Surface phenotypes in T-cell leukaemia are determined by oncogenic retroviruses. Nature. 1980 Dec 4;288(5790):489–491. doi: 10.1038/288489a0. [DOI] [PubMed] [Google Scholar]
- Zielinski C. C., Waters D. L., Datta S. K., Waksal S. D. Analysis of intrathymic differentiation patterns during the course of AKR leukemogenesis. Cell Immunol. 1981 Apr;59(2):355–366. doi: 10.1016/0008-8749(81)90415-9. [DOI] [PubMed] [Google Scholar]