Abstract
Intravenous administration of bacteria leads to their accumulation in tumors and to sporadic tumor regression. We therefore explored the hypothesis that Salmonella typhimurium engineered to express the proapoptotic cytokine Fas ligand (FasL) would exhibit enhanced antitumor activity. Immunocompetent mice carrying tumors derived from syngeneic murine D2F2 breast carcinoma or CT-26 colon carcinoma cells were treated intravenously with FasL-expressing S. typhimurium or with phosphate-buffered saline (PBS; control). Treatment with FasL-expressing S. typhimurium inhibited growth of primary tumors by an average of 59% for D2F2 tumors and 82% for CT-26 tumors (eg, at 25 days after initial treatment, mean volume of PBS-treated CT-26 colon carcinomas = 1385 mm3 and of S. typhimurium FasL-treated CT-26 tumors = 243 mm3, difference = 1142 mm3, 95% confidence interval = 800 mm3 to 1484 mm3, P < .001). Pulmonary D2F2 metastases (as measured by lung weight) were reduced by 34% in S. typhimurium FasL-treated mice compared with PBS-treated mice. FasL-expressing S. typhimurium had similar effects on growth of murine B16 melanoma tumors in wild-type mice but not in lpr/lpr mice, which lack Fas, or in mice with disrupted host inflammatory responses. Antitumor activity was achieved without overt toxicity. These preclinical results raise the possibility that using attenuated S. typhimurium to deliver FasL to tumors may be an effective and well-tolerated therapeutic strategy for some cancers.
CONTEXT AND CAVEATS
Prior knowledge
Salmonella typhimurium, a facultative anaerobe, has been shown to accumulate at tumor sites when injected intravenously into mouse tumor models. Intravenous injection of attenuated strains has been well tolerated.
Study design
Attenuated S. typhimurium were engineered to express Fas ligand (FasL) to deliver this toxic, antitumor cytokine to tumor sites and to thereby enhance therapeutic potential.
Contribution
S. typhimurium expressing FasL dramatically inhibited the growth of D2F2 murine breast carcinoma and CT-26 murine colon carcinoma tumors, as well as the growth of D2F2 pulmonary metastases in mice.
Implications
S. typhimurium expressing FasL may hold promise for the treatment of some human tumors.
Limitations
These experiments involved murine tumor models, and the effects of FasL-expressing S. typhimurium on human tumor models are not yet known.
From the Editors
Attenuated Salmonella typhimurium have an excellent safety profile (1,2), a propensity to home to tumors (3), and a capacity to synthesize large quantities of functional human cytokines (4). Our goal was to capitalize on these properties as a strategy for enhancing the tumoricidal activity of this strain. In this report, we performed a preclinical evaluation of S. typhimurium bacteria engineered to produce the cytokine Fas ligand (FasL), also known as CD95L/APO-1L, using mouse tumor models. Our strategy was to engineer attenuated S. typhimurium to produce cytokines that have known antitumor activity but that are too toxic to use systemically without targeting.
FasL is a membrane protein that belongs to the tumor necrosis factor family of proteins. After binding to its receptor (Fas), it initiates an apoptotic signal in Fas-sensitive cells (5). This mechanism is of particular importance for a variety of physiological and pathological conditions, including the killing of transformed target cells by cytotoxic T lymphocytes and natural killer cells (6). In addition to its proapoptotic activity, however, many other potentially useful functions have been described for FasL [reviewed in (7)], including chemotactic properties toward granulocytes (8,9) that promote tumor rejection (9), induction of IL-23 production by dendritic cells (10), and stimulation of CD8+ T-cell proliferation (11). However, systemic administration of recombinant FasL or of agonistic anti-Fas antibodies has been shown to induce lethal liver injury (12,13), making untargeted systemic delivery an unacceptable strategy. Here, we used FasL as a test case for investigating the feasibility of using attenuated S. typhimurium as a tumor-specific transporter for cytotoxic and immunostimulatory therapeutic proteins.
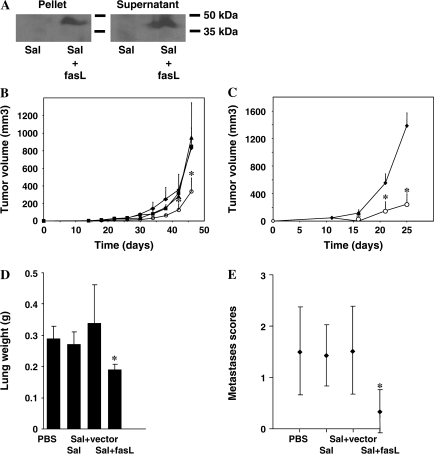
To engineer the attenuated S. typhimurium strain purI0-/msbB- (14) to express FasL, we transformed these bacteria with the plasmid pGEN206, from which a fusion of the extracellular domains of human CD8α and murine FasL [FasL–CD8, a gift from Dr H. Yigaki (15)] with an appended N-terminal leader sequence that directs protein secretion [MISSSSIS (16)] was expressed under the control of an ompC promoter. Plasmid pGEN206 is a modified form of pGEN222 (17), in which we exchanged the parA locus with the parM and parR loci and introduced a repA locus between the multiple cloning sites and the origin of replication. Cell-containing pellets and cleared culture supernatants were tested for CD8–FasL fusion protein by immunoblotting using anti-FasL antibody as described (14). These results demonstrated the presence of CD8–FasL in both the bacterial cells and the culture supernatant (Figure 1, A).
Figure 1.
Inhibition of tumor growth using Fas ligand (FasL)–expressing Salmonella typhimurium. A) FasL expression and secretion. FasL protein expression was verified by immunoblot analysis of cell lysates (pellet) or culture supernatants (sup) from S. typhimurium containing empty vector (Sal) or FasL-expressing plasmid–transformed S. typhimurium (Sal + FasL). Immunoblots were performed as described previously (14). Samples were normalized for total protein content. Blots were probed with rat monoclonal anti-mouse FasL antibody (Alexis, San Diego, CA) at 1:500 dilution. Protein–antibody complexes were visualized by an enhanced chemiluminescence (ECL) method (Amersham). Results are representative of three independent experiments. B) Effect on D2F2 tumor growth. BALB/c mice (n = 8 per group) were subject to subcutaneous injection with 1.5 × 105 D2F2 breast carcinoma cells suspended in 100 μL of phosphate-buffered saline (PBS) followed after 14 days by intravenous treatment with PBS (closed diamonds), S. typhimurium (closed squares), S. typhimurium with empty vector (closed triangles), or S. typhimurium with FasL vector (open circles). All bacterial doses were 5 × 106 colony-forming units per mouse suspended in 100 μL of PBS, a dose selected based on prior preclinical and clinical studies and that is known to be nontoxic or minimally toxic (1,3,16). All graphs indicate average tumor volumes as estimated by external calipers (means with 95% confidence intervals [CIs]) (14). Results are representative of four independent experiments. C) Effect on CT-26 tumor growth. BALB/c mice (n = 5 per group) were injected subcutaneously with 2.5 × 105 CT-26 colon carcinoma cells followed 9, 14, and 19 days later by intravenous treatment with PBS (closed diamonds) or S. typhimurium with FasL vector (open circles). Graphs indicate average tumor volumes from the time that tumor challenge was initiated (means with 95% CIs). Results are representative of four independent experiments. D) and E) Effect on D2F2 metastasis. BALB/c mice (n = 8 per group) were injected intravenously with 7.5 × 104 D2F2 breast carcinoma cells followed 6, 13, and 20 days later by intravenous treatment with PBS, S. typhimurium (Sal), S. typhimurium containing empty vector (Sal + vector), or S. typhimurium containing the FasL plasmid (Sal + FasL). Mice were killed after 41 days with an overdose of intraperitoneal Avertin anesthetic (0.017 mL/g body weight; Aldrich, Milwaukee, WI) followed by cervical dislocation. and lungs were weighed (D) and examined for metastases (E). The fraction of lung surface covered by fused metastases was scored as follows: 0 = 0%, 1 = less than 20%, 2 = 20%–50%, 3 = more than 50%. Values are means with 95% CIs. *P < .05. Results are representative of four independent experiments. D2F2 cells were a gift from Dr Wei-Zen Wei (Karmanos Cancer Institute, Detroit, MI) and CT-26 cells were obtained from ATCC (Manassas, VA). Female BALB/c mice at 6–8 weeks of age were purchased from The Jackson Laboratory (Bar Harbor, ME). Two-tailed Student t tests were used to determine the statistical significance of primary tumor growth differences between control and treatment groups. In the case of metastasis scores, statistical significance was determined by Mann–Whitney U tests. P values of .05 or less were deemed statistically significant.
Attenuated S. typhimurium preferentially accumulate in tumors (1–3,19). To test the in vivo antitumor activity of FasL-expressing S. typhimurium, we used mouse cancer models in which mice were either injected subcutaneously with murine D2F2 breast carcinoma cells (n = 8 mice per group) or murine CT-26 colon carcinoma cells (n = 5 mice per group) to form primary tumors or injected intravenously with D2F2 cells to form lung metastases (n = 8 mice per group). All animal experiments were performed according to the National Institutes of Health Guide for Care and Use of Experimental Animals and approved by the Animal Care Committee of the Burnham Institute for Medical Research (#AUF 04-152). Tumor-bearing mice were treated intravenously with phosphate-buffered saline (PBS), with S. typhimurium, or with S. typhimurium containing control (empty) plasmid or FasL-encoding plasmid. In experiments involving subcutaneous tumors, mice were treated after tumors reached a size that was clearly visible, whereas for metastatic tumors, mice were treated at 6, 13, and 20 days after the cancer cells were injected. Tumor volume was assessed with calipers every 4 days from days 14 to 46 for D2F2 breast tumors (Figure 1, B) and on days 11, 16, 21, and 25 for CT-26 colon tumors (Figure 1, C).
In mice treated with S. typhimurium containing the FasL expression vector, tumor growth was statistically significantly reduced compared with that in control groups. For example, after 46 days of treatment, mean D2F2 tumor volumes were 827, 847, 949, and 336 mm3 for the groups treated with PBS, S. typhimurium, S. typhimurium–vector, and S. typhimurium–FasL, respectively (for PBS- vs FasL-treated D2F2 tumors, difference = 491 mm3, 95% confidence interval [CI] = 34 to 984, P = .02) (Figure 1, B). At 25 days after initial treatment, mean CT-26 tumor volumes were 1385 mm3 and 243 mm3 for the groups treated with PBS vs S. typhimurium–FasL, respectively (difference = 1142 mm3, 95% CI = 800 to 1484, P < .001) (Figure 1, C). We also observed that mice treated with FasL-expressing S. typhimurium showed a 34% reduction in the growth of D2F2 experimental metastases (as measured by lung weight) compared with PBS -treated control mice, with lung weights remaining almost normal compared with mice receiving other treatments (measured at 41 days, mean lung weights were 0.29 g, 0.27 g, 0.34 g, and 0.19 g for the PBS, S. typhimurium, S. typhimurium– vector, and S. typhimurium–FasL groups, respectively; for PBS- vs FasL-treated D2F2 metastases, difference = 0.10 g, 95% CI = 0.04 to 0.16, P = .001) (Figure 1, D and E).
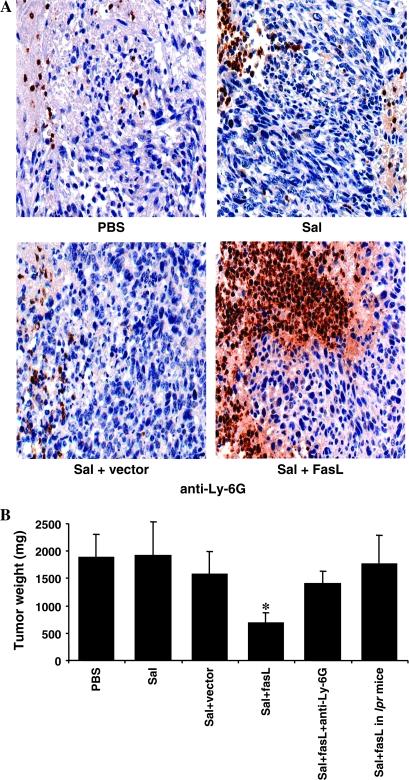
To investigate the dependence of the antitumor effect on the expression of endogenous Fas in the host, we implanted B16 mouse melanoma cells into C57/B16 mice with wild-type or lpr/lpr backgrounds. FasL-expressing S. typhimurium failed to inhibit B16 tumor growth in lpr/lpr-mice, which lack Fas, in contrast to wild-type control mice. Mean tumor volumes were 1882 mm3 for PBS (control)-treated wild-type mice vs 1761 mm3 for S. typhimurium–FasL-treated lpr/lpr mice (difference = 121 mm3, 95% CI = −811 mm3 to 1053 mm3, P = .73) at 22 days after treatment (Figure 2, B). Thus, the antitumor activity of FasL-expressing S. typhimurium depends at least in part on expression of intact Fas on host cells rather than tumor cells.
Figure 2.
Roles of neutrophils and Fas in observed antitumor effects. A) Accumulation of neutrophils in Fas ligand (FasL)–treated D2F2 breast tumors. Treated and untreated mice were killed with an overdose of intraperitoneal Avertin anesthetic (0.017 mL/g body weight; Aldrich, Milwaukee, WI) followed by cervical dislocation. Tumor tissues from BALB/c mice (n = 3 per group) were fixed in paraformaldehyde, embedded in paraffin, and subjected to histopathologic analyses using staining with hematoxylin and eosin and with an antibody specific for the Ly-6G antigen (BD, Franklin Lakes, NJ). Shown are high-power field examples (×400 magnification) of D2F2 breast tumors from mice treated intravenously with phosphate-buffered saline (PBS), S. typhimurium (Sal), S. typhimurium plus empty vector (Sal + vector), or S. typhimurium plus FasL-bearing vector (Sal + FasL). B) Dependence of treatment on host neutrophils and Fas. C57/Bl6 mice (n = 5 per group) were injected subcutaneously with 105 B16 murine melanoma cells. After 7 days, mice were injected intravenously with PBS, S. typhimurium (Sal), S. typhimurium plus empty vector (Sal + vector), or S. typhimurium plus FasL-bearing vector (Sal + FasL). Test groups also included mice treated with Sal + FasL 5 and 15 days after intraperitoneal treatment with 500 μg per mouse of Ly-6G antibody, an antibody that depletes neutrophils (Sal + FasL + anti–Ly-6G), and C57/Bl6 mice lacking Fas (Sal + FasL in lpr mice). Mice were killed after 22 days, and tumor weights were determined (means with 95% confidence intervals). *P < .05. Results are representative of three independent experiments. B16 cells were obtained from ATCC (Manassas, VA). Female C57/Bl6 and lpr/lpr mice (6–8 weeks of age) were supplied by The Jackson Laboratory (Bar Harbor, ME).
It has been previously demonstrated that FasL is chemotactic for neutrophils, and this activity can be indispensable for efficient inhibition of tumor growth under some circumstances (9). In this regard, we observed an increase in inflammatory cells, particularly neutrophils, within subcutaneously grown D2F2 breast tumors in mice treated with FasL-expressing S. typhimurium, as determined by hematoxylin and eosin staining and by immunohistochemistry using anti–Ly-6G staining (Figure 2, A and Supplementary Figure, available online). To experimentally investigate the role of neutrophils in the antitumor activity of FasL-expressing S. typhimurium, we depleted neutrophils in vivo using an anti–Ly-6G antibody (20) before beginning treatment of B16 melanoma–bearing mice with FasL-expressing S. typhimurium (Figure 2, B). Tumor weights from killed mice showed that the antitumor effects of targeted FasL were abolished when neutrophils were depleted (mean tumor weight for mice treated with S. typhimurium–FasL + anti–Ly-6G = 1405 mg, tumor weight for mice treated with S. typhimurium–FasL in which neutrophils were not depleted = 679 mg, difference = 726 mg, 95% CI = 355 to 1097 mg, P = .001).
Although demonstrating antitumor activity, FasL-expressing S. typhimurium did not cause greater systemic toxicity than the unmodified attenuated S. typhimurium strain. Histological analysis of multiple organs (lungs, kidneys, spleens, and heart) showed minimal changes (data not shown) that were comparable to the results obtained previously with control S. typhimurium that did not produce FasL (7–10). Thus, addition of FasL did not worsen the toxicity observed with S. typhimurium, which has been shown previously to be well tolerated in humans (1,4).
Here, we have demonstrated that systemic delivery of attenuated S. typhimurium expressing a soluble version of FasL reduces growth of primary tumors and pulmonary metastases in mouse cancer models (21) using multidrug-resistant murine tumors in immunocompetent animals. In mice depleted in vivo of neutrophils, the antitumor effect of treatment with FasL-expressing bacteria was severely impaired, corroborating findings from previous studies in which FasL was applied intratumorally (9). Moreover, Fas expression on host rather than tumor cells was found to be critical for antitumor activity, based on experiments using lpr/lpr mice, which lack the receptor for FasL. Thus, the observed antitumor effects of FasL-expressing S. typhimurium appear to rely largely on a host inflammatory reaction to the tumor, rather than on a direct proapoptotic effect on tumor cells.
Unlike systemically delivered agonistic anti-Fas antibodies or FasL, which have toxic effects, mice appeared to tolerate FasL-expressing bacterial therapy well; however, formal preclinical toxicology studies should be performed. Histopathologic analyses of various organs showed only modest changes that were similar in extent for control and FasL-expressing S. typhimurium, thus confirming previous reports (2,19). Together with other preclinical (1,19) and clinical (22) studies that have documented minimal side effects of intravenous administration of attenuated S. typhimurium, these results from murine cancer models suggest that FasL-expressing S. typhimurium could offer an acceptable strategy for employing FasL and possibly other toxic cytokines for cancer therapy.
Funding
NIH-CA69381; Austrian Program for Advanced Research and Technology (Austrian Academy of Sciences).
Supplementary Material
Footnotes
M. Loeffler and J. C. Reed should be considered senior authors.
The organizations funding this work had no role in design or execution of the studies.
References
- 1.Thamm DH, Kurzman ID, King I, et al. Systemic administration of an attenuated, tumor-targeting Salmonella typhimurium to dogs with spontaneous neoplasia: phase I evaluation. Clin Cancer Res. 2005;11(13):4827–4834. doi: 10.1158/1078-0432.CCR-04-2510. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Spiess PJ, Kleiner DE. Antitumor effects in mice of the intravenous injection of attenuated Salmonella typhimurium. J Immunother. 2002;25(3):218–225. doi: 10.1097/01.CJI.0000014623.45316.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clairmont C, Lee KC, Pike J, et al. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. J Infect Dis. 2000;181(6):1996–2002. doi: 10.1086/315497. [DOI] [PubMed] [Google Scholar]
- 4.Carrier MJ, Chatfield SN, Dougan G, et al. Expression of human IL-1 beta in Salmonella typhimurium. A model system for the delivery of recombinant therapeutic proteins in vivo. J Immunol. 1992;148(4):1176–1181. [PubMed] [Google Scholar]
- 5.Thorburn A. Death receptor-induced cell killing. Cell Signal. 2004;16(2):139–144. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Linkermann A, Qian J, Janssen O. Slowly getting a clue on CD95 ligand biology. Biochem Pharmacol. 2003;66(8):1417–1426. doi: 10.1016/s0006-2952(03)00492-1. [DOI] [PubMed] [Google Scholar]
- 7.Rescigno M, Piguet V, Valzasina B, et al. Fas engagement induces the maturation of dendritic cells (DCs), the release of interleukin (IL)-1beta, and the production of interferon gamma in the absence of IL-12 during DC-T cell cognate interaction: a new role for Fas ligand in inflammatory responses. J Exp Med. 2000;192(11):1661–1668. doi: 10.1084/jem.192.11.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ottonello L, Tortolina G, Amelotti M, Dallegri F. Soluble Fas ligand is chemotactic for human neutrophilic polymorphonuclear leukocytes. J Immunol. 1999;162(6):3601–3606. [PubMed] [Google Scholar]
- 9.Arai H, Gordon D, Nabel EG, Nabel GJ. Gene transfer of Fas ligand induces tumor regression in vivo. Proc Natl Acad Sci U S A. 1997;94(25):13862–13867. doi: 10.1073/pnas.94.25.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kidoya H, Umemura M, Kawabe T, et al. Fas ligand induces cell-autonomous IL-23 production in dendritic cells, a mechanism for Fas ligand-induced IL-17 production. J Immunol. 2005;175(12):8024–8031. doi: 10.4049/jimmunol.175.12.8024. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki I, Martin S, Boursalian TE, Beers C, Fink PJ. Fas ligand costimulates the in vivo proliferation of CD8+ T cells. J Immunol. 2000;165(10):5537–5543. doi: 10.4049/jimmunol.165.10.5537. [DOI] [PubMed] [Google Scholar]
- 12.Ogasawara J, Watanabe-Fukunaga R, Adachi M, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 13.Rensing-Ehl A, Frei K, Flury R, et al. Local Fas/APO-1 (CD95) ligand-mediated tumor cell killing in vivo. Eur J Immunol. 1995;25(8):2253–2258. doi: 10.1002/eji.1830250821. [DOI] [PubMed] [Google Scholar]
- 14.Clairmont C, Lee KC, Pike J, et al. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. J Infect Dis. 2000;181(6):1996–2002. doi: 10.1086/315497. [DOI] [PubMed] [Google Scholar]
- 15.Kayagaki N, Yamaguchi N, Nagao F, et al. Polymorphism of murine Fas ligand that affects the biological activity. Proc Natl Acad Sci U S A. 1997;94(8):3914–3919. doi: 10.1073/pnas.94.8.3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lloyd SA, Sjostrom M, Andersson S, Wolf-Watz H. Molecular characterization of type III secretion signals via analysis of synthetic N-terminal amino acid sequences. Mol Microbiol. 2002;43(1):51–59. doi: 10.1046/j.1365-2958.2002.02738.x. [DOI] [PubMed] [Google Scholar]
- 17.Galen JE, Nair J, Wang JY, et al. Optimization of plasmid maintenance in the attenuated live vector vaccine strain Salmonella typhi CVD 908-htrA. Infect Immun. 1999;67(12):6424–6433. doi: 10.1128/iai.67.12.6424-6433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Euhus DM, Hudd C, LaRegina MC, Johnson FE. Tumor measurement in the nude mouse. J Surg Oncol. 1986;31(4):229–234. doi: 10.1002/jso.2930310402. [DOI] [PubMed] [Google Scholar]
- 19.Loeffler M, Le’Negrate G, Krajewska M, Reed JC. Attenuated Salmonella engineered to produce human cytokine LIGHT inhibit tumor growth. Proc Natl Acad Sci U S A. 2007;104(31):12879–12883. doi: 10.1073/pnas.0701959104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol. 1993;151(5):2399–2408. [PubMed] [Google Scholar]
- 21.Loeffler M, Kruger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest. 2006;116(7):1955–1962. doi: 10.1172/JCI26532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toso JF, Gill VJ, Hwu P, et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol. 2002;20(1):142–152. doi: 10.1200/JCO.2002.20.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.