Abstract
An advanced intermediate for the synthesis of amphidinol 3 has been prepared. A cross-metathesis reaction was used to couple the C1-C12 and C13-C26 segments. An unusual β-alkoxy alkyllithium reagent was generated from this segment and added to a Weinreb amide to assemble the C1-C52 section of amphidinol 3. These compounds represent some of the most advanced intermediates reported to date for the synthesis of amphidinol 3.
The amphidinols are a fascinating group of metabolites isolated from the marine dinoflagellates Amphidinium klebsii and Amphidinium carterae.1 Amphidinol 3 (1) exhibits potent hemolytic activity against human erythrocytes as well as antifungal activity against Aspergillus niger.1 A variety of amphidinols have been reported, along with the structurally similar compounds luteophanol A and lingshuiol A and B.2 Common structural features of these natural products are two highly substituted tetrahydropyran (THP) rings, a long, irregular polyol domain, and a skipped polyene chain. Among this family of natural products, only the configuration of amphidinol 3 has been assigned. The absolute stereochemistry of amphidinol 3 was elucidated by Murata and coworkers using a new and powerful J-based NMR spectroscopic technique.3 Amphidinol 3 has emerged as an important synthetic target, and a number of groups, including our own,4 have reported progress towards its synthesis.5 Herein, we describe the synthesis of the fully protected C1–C52 fragment.
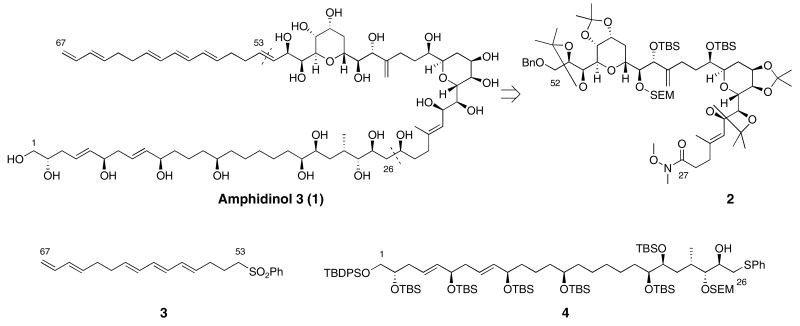
Our retrosynthetic analysis for amphidinol 3 is illustrated in Figure 1. The three components are a bis-tetrahydropyran 2, a polyene sulfone 3, and the protected polyol 4. The bis-THP 2 is similar to and derived from an intermediate we have previously reported.4 Synthesis and coupling reactions with the polyene sulfone have also been described.4a The synthesis of protected polyol phenylthio ether 4 and strategies for its coupling will be the focus of this discussion.
Figure 1.
Retrosynthetic analysis of amphidinol 3
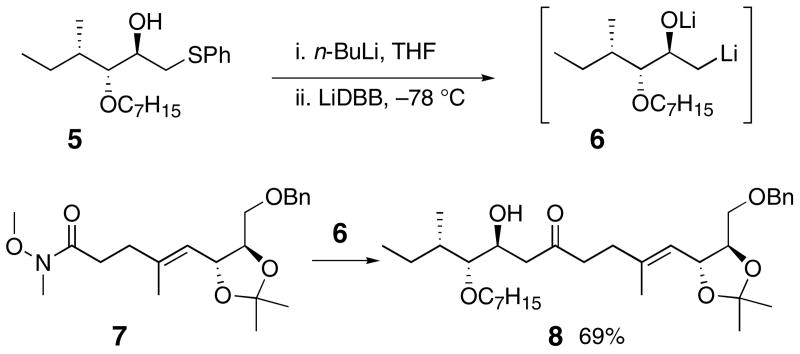
The key coupling reaction between phenylthio ether 4 and Weinreb amide 2 was envisioned as an addition of the alkyllithium reagent derived from 4 to the amide 2. The resulting β-hydroxy ketone would be suitable for stereoselective reduction. Elimination of the C25 oxygen would be avoided by masking it as a lithium alkoxide. Such β-alkoxy alkyllithium reagents are known. They have been prepared by mercury-lithium exchange,6 reductive lithiation of chlorohydrins,7 reductive lithiation of oxiranes,8 and from β-hydroxy phenylthio ethers.9 Several reasonably complex β-alkoxy alkyllithium reagents have been prepared by reduction of oxiranes,10 but these reagents have not been used to couple complex fragments.
A model coupling reaction was investigated as outlined in Scheme 1. The β-hydroxy phenyl sulfide 5 was prepared and deprotonated with n-BuLi. Addition of lithium di-tert-butylbiphenylide (LiDBB)11 at low temperature generated the β-alkoxy alkyllithium 6. Subsequent addition of the Weinreb amide 7 produced the desired β-hydroxy ketone 8 in 69% yield. The encouraging outcome of this model study emboldened us to move forward with the amphidinol 3 synthesis.
Scheme 1.
Model coupling of β-alkoxy alkyllithium 6 with Weinreb amide 7
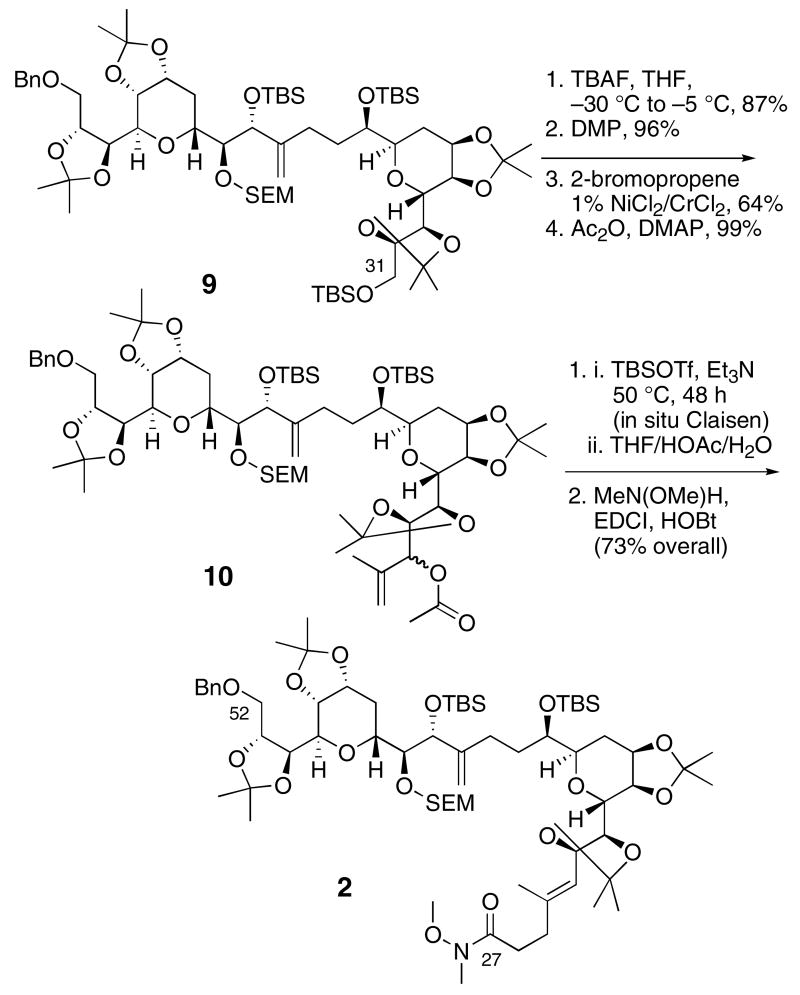
The synthesis of Weinreb amide 2 from the bis-THP intermediate4a 9 is presented in Scheme 2. Low temperature TBAF treatment selectively removed the primary TBS group, and oxidation gave the expected C31 aldehyde. Additions to this aldehyde were very problematic, perhaps because of the steric crowding. A 2-propenyllithium addition failed and the corresponding Grignard addition gave only trace quantities of the expected allylic alcohol products. Finally, a Nozaki-Hiyama-Kishi reaction12 with 2-bromopropene led to the desired alcohol diastereomers in good yield, and acylation gave ester 10. Ireland-ester Claisen rearrangement13 using Nakai's in situ method for generating the silyl ketene acetal14 produced the expected unsaturated silyl ester in good yield. Hydrolysis of the silyl ester and coupling with N-methoxy-N-methylamine produced the Weinreb amide 2 as a single alkene isomer.
Scheme 2.
Synthesis of the C27-C52 Weinreb amide 2
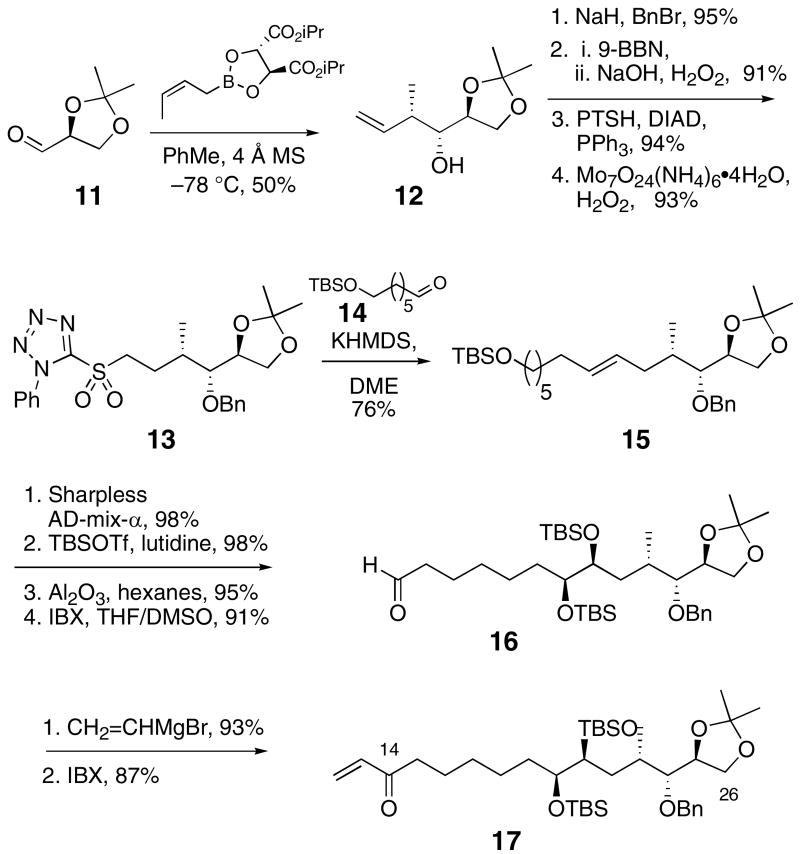
The C14-C26 segment of amphidinol 3 was prepared from (S)-glyceraldehyde acetonide (11) as illustrated in Scheme 3. The syn crotyl adduct 12 was prepared by Roush's procedure.15 A set of standard transformations led to the phenyltetrazole sulfone 13, which was coupled with aldehyde 14 using the Julia-Kocienski method16 to produce the E-alkene 15 as a single isomer. The reaction was optimized (KHMDS, DME, Barbier conditions) to produce high E/Z selectivity to avoid subsequent problematic separations of alkene or diol diastereomers. Sharpless asymmetric dihydroxylation led to a single diastereomer by 1H NMR analysis.17 Protection of the diol, selective deprotection of the primary TBS ether18 and oxidation delivered the aldehyde 16 in excellent overall yield. The enone 17 was prepared by addition of vinylmagnesium bromide to the aldehyde and oxidation. Both 16 and 17 were plausible intermediates for the C14-C26 segment of amphidinol 3.
Scheme 3.
Synthesis of the C14-C26 aldehyde 16 and enone 17
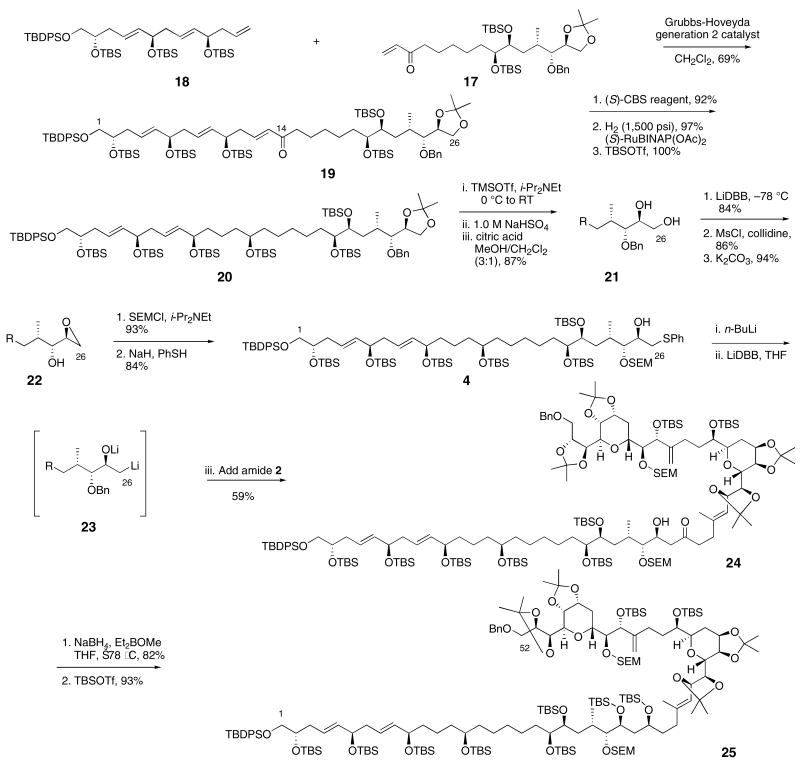
Initially we planned to add a C1-C13 organozinc reagent to aldehyde 16 to assemble the C1-C26 fragment.19 We prepared the protected polyol 18 using a modification of Cossy's elegant metathesis route (Scheme 4).5a,20 Unfortunately, numerous attempts to prepare a dialkylzinc reagent from 18 failed; the hydroboration was successful but the transmetalation was not. Ultimately the successful coupling strategy used a cross-metathesis reaction, Scheme 4.21 Enone 17 was combined with triene 18 in a 1:1 molar ratio and exposed to the Grubbs-Hoveyda catalyst (10 mol%).22 The reaction produced enone 19 in 69% yield. Stereoselective reduction of the C14 ketone was accomplished using the CBS reagent23 to deliver the required C14-R diastereomer with 17:1 selectivity in 92% yield. Following Roush's precedent,5b hydroxyl-directed reduction using Noyori's catalyst24, saturated the C12-C13 alkene and produced the C1-C26 segment 20 after protection. The proposed dialkylzinc strategy would have required fewer steps, but the metathesis route was very effective.
Scheme 4.
Coupling fragments to assemble the C1-C52 segment of amphidinol 3
To set up the coupling between the bis-THP fragment and the C1-C26 polyol segment, the phenyl sulfide needed to be introduced and the protecting groups adjusted. Selective hydrolysis of the acetonide was accomplished by enol ether formation and hydrolysis.25 Removal of the benzyl ether, epoxide formation, SEM protection and treatment with thiophenol and base delivered the desired C1-C26 synthon 4 in good overall yield.
The final segment coupling between protected polyol 4 and bis-THP 2 is illustrated in Scheme 4. Deprotonation of the C25 alcohol with n-BuLi, followed by reductive lithiation with LiDBB generated the β-alkoxy alkyllithium 23. The bis-THP Weinreb amide 2 was added to the alkyllithium solution in THF at −78 °C, and the reaction was allowed to proceed for 21 h at that temperature. The β-hydroxy ketone 24 was isolated in 59% yield. A four-fold excess of the C1-C26 segment was used in the coupling, but this ratio was largely dictated by the relative abundance of the two coupling partners. Model studies to prepare ketone 8 used a three-fold excess, but neither of these ratios were optimized. Synthesis of the C1-C52 segment of amphidinol 3 was completed by hydroxyl-directed reduction of the C27 ketone using Prasad's conditions,26 followed by alcohol protection to produce the amphidinol 3 intermediate 25.
We have developed an efficient construction of an advanced intermediate for the synthesis of amphidinol 3. The route features segment coupling reactions using a cross-metathesis and a β-alkoxy alkyllithium addition to a Weinreb amide. These validated coupling strategies will undoubtedly be useful in the synthesis of other complex natural products.
Supporting Information Available
Preparation and characterization of compounds 2-4 and 10-25 are described. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
This work was supported by the National Institute of General Medical Sciences (GM-65338) and by a generous donation from the Schering Plough Research Institute. J.d.V. acknowledges the Spanish Ministry of Education and Science for a postdoctoral fellowship.
References
- 1.Murata M, Matsuoka S, Matsumori N, Paul GK, Tachibana K. J Am Chem Soc. 1999;121:870–871. [Google Scholar]
- 2.a Paul GK, Matsumori N, Murata M, Tachibana K. Tetrahedron Lett. 1995;36:6279–6282. [Google Scholar]; b Satake M, Murata M, Yasumoto T, Fujita T, Naoki H. J Am Chem Soc. 1991;113:9859–9861. [Google Scholar]; c Houdai T, Matsuoka S, Murata M, Satake M, Ota S, Oshima Y, Rhodes LL. Tetrahedron. 2001;57:5551–5555. [Google Scholar]; d Paul GK, Matsumori N, Konoki K, Murata M, Tachibana K. J Mar Biotechnol. 1997;5:124–128. [Google Scholar]; e Echigoya R, Rhodes L, Oshima Y, Satake M. Harmful Algae. 2005;4:383–389. [Google Scholar]; f Doi Y, Ishibshi M, Nakamichi H, Kosaka T, Ishikawa T, Kobayashi Ji. J Org Chem. 1997;62:3820–3823. [Google Scholar]; g Huang XC, Zhao D, Guo YW, Wu HM, Trivellone E, Cimino G. Tetrahedron Lett. 2004;45:5501–5504. [Google Scholar]
- 3.Matsumori N, Kaneno D, Murata M, Nakamura H, Tachibana K. J Org Chem. 1999;64:866–876. doi: 10.1021/jo981810k. [DOI] [PubMed] [Google Scholar]
- 4.a de Vicente J, Huckins JR, Rychnovsky SD. Angew Chem, Int Ed. 2006;45:7258–7262. doi: 10.1002/anie.200602742. [DOI] [PubMed] [Google Scholar]; b De Vicente J, Betzemeier B, Rychnovsky SD. Org Lett. 2005;7:1853–1856. doi: 10.1021/ol050477l. [DOI] [PubMed] [Google Scholar]
- 5.a BouzBouz S, Cossy J. Org Lett. 2001;3:1451–1454. doi: 10.1021/ol0157095. [DOI] [PubMed] [Google Scholar]; b Flamme EM, Roush WR. Org Lett. 2005;7:1411–1414. doi: 10.1021/ol050250q. [DOI] [PubMed] [Google Scholar]; c Hicks JD, Flamme EM, Roush WR. Org Lett. 2005;7:5509–5512. doi: 10.1021/ol052322j. [DOI] [PubMed] [Google Scholar]; c Chang SK, Paquette LA. Synlett. 2005:2915–2918. [Google Scholar]; d Paquette LA, Chang SK. Org Lett. 2005;7:3111–3114. doi: 10.1021/ol0511833. [DOI] [PubMed] [Google Scholar]; e Dubost C, Marko IE, Bryans J. Tetrahedron Lett. 2005;46:4005–4009. [Google Scholar]; f Bedore MW, Chang SK, Paquette LA. Org Lett. 2007;9:513–516. doi: 10.1021/ol062875+. [DOI] [PubMed] [Google Scholar]
- 6.a Barluenga J, Fananas FJ, Yus M. J Org Chem. 1979;44:4798–4801. [Google Scholar]; b Barluenga J, Fananas FJ, Yus M. J Org Chem. 1981;46:1281–1283. [Google Scholar]
- 7.a Barluenga J, Florez J, Yus M. J Chem Soc, Chem Commun. 1982:1153–1154. [Google Scholar]; b Barluenga J, Florez J, Yus M. J Chem Soc, Perkin 1. 1983:3019–3026. [Google Scholar]; c Najera C, Yus M, Seebach D. Helv Chim Acta. 1984;67:289–300. [Google Scholar]
- 8.a Bartmann E. Angew Chem, Int Ed. 1986;25:653–655. [Google Scholar]; b Cohen T, Jeong IH, Mudryk B, Bhupathy M, Awad MMA. J Org Chem. 1990;55:1528–1536. [Google Scholar]; c Bachki A, Foubelo F, Yus M. Tetrahedron: Asymmetry. 1996;7:2997–3008. [Google Scholar]
- 9.a Foubelo F, Gutierrez A, Yus M. Tetrahedron Lett. 1997;38:4837–4840. [Google Scholar]; b Foubelo F, Gutierrez A, Yus M. Synthesis. 1999:503–514. [Google Scholar]
- 10.a Soler T, Bachki A, Falvello LR, Foubelo F, Yus M. Tetrahedron: Asymmetry. 2000;11:493–517. [Google Scholar]; b Falvello LR, Foubelo F, Soler T, Yus M. Tetrahedron: Asymmetry. 2000;11:2063–2066. [Google Scholar]; c Yus M, Soler T, Foubelo F. Tetrahedron: Asymmetry. 2001;12:801–810. [Google Scholar]
- 11.Freeman PK, Hutchinson LL. J Org Chem. 1980;45:1924–1930. [Google Scholar]
- 12.Wessjohann LA, Scheid G. Synthesis. 1999:1–36. [Google Scholar]
- 13.Ireland RE, Mueller RH, Willard AK. J Am Chem Soc. 1976;98:2868–2877. [Google Scholar]
- 14.Kobayashi M, Masumoto K, Nakai Ei, Nakai T. Tetrahedron Lett. 1996;37:3005–3008. [Google Scholar]
- 15.Roush WR, Adam MA, Walts AE, Harris DJ. J Am Chem Soc. 1986;108:3422–3434. [Google Scholar]
- 16.a Baudin JB, Hareau G, Julia SA, Ruel O. Tetrahedron Lett. 1991;32:1175–1178. [Google Scholar]; b Blakemore PR, Cole WJ, Kocienski PJ, Morley A. Synlett. 1998:26–28. [Google Scholar]; c Blakemore PR. J Chem Soc, Perkin Trans 1. 2002:2563–2585. [Google Scholar]
- 17.Kolb HC, Van Nieuwenhze MS, Sharpless KB. Chem Rev. 1994;94:2483–2547. [Google Scholar]
- 18.Feixas J, Capdevila A, Guerrero A. Tetrahedron. 1994;50:8539–8550. [Google Scholar]
- 19.a Pu L, Yu HB. Chem Rev. 2001;101:757–824. doi: 10.1021/cr000411y. [DOI] [PubMed] [Google Scholar]; b Knochel P, Singer RD. Chem Rev. 1993;93:2117–2188. [Google Scholar]; c Lutz C, Knochel P. J Org Chem. 1997;62:7895–7898. [Google Scholar]
- 20.Preparation of triene 18 is presented in the Supporting Information section.
- 21.Chatterjee AK, Choi TL, Sanders DP, Grubbs RH. J Am Chem Soc. 2003;125:11360–11370. doi: 10.1021/ja0214882. [DOI] [PubMed] [Google Scholar]
- 22.Kingsbury JS, Harrity JPA, Bonitatebus PJ, Hoveyda AM. J Am Chem Soc. 1999;121:791–799. [Google Scholar]
- 23.a Corey EJ, Bakshi K, Shibata S. J Am Chem Soc. 1987;109:5551–5553. [Google Scholar]; b Corey EJ, Helal CJ. Angew Chem, Int Ed. 1998;37:1986–2012. doi: 10.1002/(SICI)1521-3773(19980817)37:15<1986::AID-ANIE1986>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 24.Takaya H, Ohta T, Sayo N, Kumobayashi H, Akutagawa S, Inoue SI, Kasahara I, Noyori R. J Am Chem Soc. 1987;109:1596–1597. [Google Scholar]
- 25.a Rychnovsky SD, Kim J. Tetrahedron Lett. 1991;32:7219–7222. [Google Scholar]; b Rychnovsky SD, Kim J. Tetrahedron Lett. 1991;32:7223–7224. [Google Scholar]; c Gassman PG, Burns SJ. J Org Chem. 1988;53:5574–5576. [Google Scholar]
- 26.Chen KM, Hardtman GE, Prasad K, Repic O, Shapiro MJ. Tetrahedron Lett. 1987;28:155–158. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preparation and characterization of compounds 2-4 and 10-25 are described. This material is available free of charge via the Internet at http://pubs.acs.org.