Abstract
Mechanical load is an important regulator of cardiac force. Stretching human atrial and ventricular trabeculae elicited a biphasic force increase: an immediate increase (Frank-Starling mechanism) followed by a further slow increase (slow force response, SFR). In ventricle, the SFR was unaffected by AT- and ET-receptor antagonism, by inhibition of protein-kinase-C, PI-3-kinase, and NO-synthase, but attenuated by inhibition of Na+/H+- (NHE) and Na+/Ca2+-exchange (NCX). In atrium, however, neither NHE- nor NCX-inhibition affected the SFR. Stretch elicited a large NHE-dependent [Na+]i increase in ventricle but only a small, NHE-independent [Na+]i increase in atrium. Stretch-activated non-selective cation channels contributed to basal force development in atrium but not ventricle and were not involved in the SFR in either tissue. Interestingly, inhibition of AT-receptors or pre-application of angiotensin II or endothelin-1 reduced the atrial SFR. Furthermore, stretch increased phosphorylation of atrial myosin light chain 2 (MLC2) and inhibition of myosin light chain kinase (MLCK) attenuated the SFR in atrium and ventricle. Thus, in human heart both atrial and ventricular myocardium exhibit a stretch-dependent SFR that might serve to adjust cardiac output to increased workload. In ventricle, there is a robust NHE-dependent (but angiotensin II- and endothelin-1-independent) [Na+]i increase that is translated into a [Ca2+]i and force increase via NCX. In atrium, on the other hand, there is an angiotensin II- and endothelin-dependent (but NHE- and NCX-independent) force increase. Increased myofilament Ca2+ sensitivity through MLCK-induced phosphorylation of MLC2 is a novel mechanism contributing to the SFR in both atrium and ventricle.
Keywords: Stretch, Human myocardium, Slow force response, Atrium, Ventricle, Sodium
1. Introduction
Mechanical load is one of the most important regulators of cardiac function. As early as in the 1860s it was acknowledged by physiologists that diastolic filling of the heart affects stroke volume (for reviews see Katz, 2002; Zimmer, 2002). This mechanism is now known as the “law of the heart” or the Frank-Starling mechanism (FSM), named after its “discoverers” Otto Frank and Ernest Henry Starling. It states that an increase in diastolic filling (preload) causes an increase in stroke volume and thus cardiac output. At the cellular level, increases in diastolic filling cause stretching of the myocytes and this leads to increases in contractile force, which ultimately underlies the increase in cardiac output. The force increase during the FSM is intrinsic to the cardiac myocyte. It is caused by increased Ca2+ sensitivity of the myofilaments (Hibberd and Jewell, 1982; Kentish et al., 1986; Konhilas et al., 2002). The major physiological role of the FSM is to adjust the output between the right and left side of the heart.
The FSM, however, is not the only means by which diastolic filling may regulate cardiac contractility. Following the FSM, there is an additional, slower, load- or stretch-induced increase in contractile force developing over 5-15 minutes termed the slow force response (SFR). Thus, stretch elicits a biphasic increase in force comprising the FSM (1st phase) and the SFR (2nd phase). The SFR was originally characterised in cat papillary muscles (Parmley and Chuck, 1973). Since then, it has been observed in a variety of cardiac preparations ranging from whole hearts to single myocytes in various species including cat (Parmley and Chuck, 1973; Perez et al., 2001), dog (Todaka et al., 1998), ferret (Calaghan and White, 2001), guinea-pig (White et al., 1995), rabbit (von Lewinski et al., 2003), rat (Alvarez et al., 1999; Hongo et al., 1996; Kentish and Wrzosek, 1998), and human (von Lewinski et al., 2004). This implicates that the SFR is a general phenomenon of physiological relevance and that the underlying mechanisms of the SFR, just like the FSM, are intrinsic to the cardiac myocyte.
The SFR has become the subject of intense research in recent years. Despite significant advances in our understanding of this phenomenon, however, it is still not completely understood and some controversies have evolved awaiting resolution. Based on experimental and modelling studies various signalling pathways and mechanisms have been proposed to contribute to the SFR. The list of channels, transporters, and signalling molecules possibly involved in the SFR includes angiotensin II (Alvarez et al., 1999; Perez et al., 2001), endothelins (Alvarez et al., 1999; Calaghan and White, 2001; Ennis et al., 2005; Perez et al., 2001), stretch-activated non-selective cation channels (SACs) (Calaghan and White, 2004; Niederer and Smith, 2007), the Na+/H+ exchanger (NHE) (Alvarez et al., 1999; Calaghan and White, 2004; Luers et al., 2005; Perez et al., 2001; von Lewinski et al., 2003), the Na+/Ca2+ exchanger (NCX) (Luers et al., 2005; Perez et al., 2001; von Lewinski et al., 2003), the Na+/K+ pump (Bluhm et al., 1998), cAMP (Calaghan et al., 1999; Todaka et al., 1998), phosphatidyinositol-3 kinase (PI3K) (Vila Petroff et al., 2001), and nitric oxide (NO) (Vila Petroff et al., 2001), and is likely to be extended further. The significance of each mechanism may vary depending on experimental conditions (e.g. the mode of stretch or increase in load), the preparation (single myocytes versus multicellular trabeculae or whole hearts), the tissue (atrium versus ventricle), and the species.
In this article, we review data on the SFR in human atrium and ventricle. Moreover, we present new data that add to our knowledge of the physiological and pathophysiological roles of the SFR and provide novel insights into the cellular and subcellular mechanisms underlying the SFR in human myocardium. Experiments were conducted on muscle strips isolated from atrial and ventricular myocardium of nonfailing and failing human hearts. The results show that despite similar magnitude and time course of the SFR, there are profound differences in the underlying mechanisms between atrium and ventricle. In human ventricle, the SFR is mediated, in part, by an NHE- and NCX-dependent (but angiotensin II- and endothelin-independent) [Na+]i and [Ca2+]i increase. In human atrium, on the other hand, the SFR is caused by an angiotensin II- and endothelin-dependent (but NHE- and NCX-independent) mechanism. A novel positive inotropic mechanism present in both atrium and ventricle is stretch-induced stimulation of myosin light chain phosphorylation by myosin light chain kinase. Furthermore, a stretch-induced negative inotropic mechanism was identified, which limits the SFR. The nature of this mechanism will be the subject of future research and most certainly reveal additional signalling pathways activated by stretch.
2. Methods
2.1. Human myocardium
Atrial myocardium was obtained from right atrial appendages of patients undergoing bypass or valve replacement surgery. Ventricular myocardium was isolated from end-stage failing explanted hearts. The study was approved by the local ethics committee and all patients gave informed written consent. The investigation conforms with the principles outlined in the Declaration of Helsinki.
The cardiac tissue was stored in cold cardioplegic Tyrode’s solution containing (in mM): Na+ 152, K+ 3.6, Cl- 135, HCO -3 25, Ca2+ 0.2, Mg2+ 0.6, H2PO4- 1.3, SO42- 0.6, glucose 11.2, and 2,3-butanedione-monoxime (BDM) 30, equilibrated with 95% O2 and 5% CO2 to a pH of 7.4 and transported to the laboratory. This cardioplegic solution protects the myocardium during transportation and from cutting injury at the time of dissection with full reversibility of the cardioplegic effects upon washout. Small free-running trabeculae (also referred to as “muscle strips”) with a diameter of <0.8 mm were dissected from the atrial or ventricular tissue with the help of a stereo-microscope. All preparation steps were carried out in the cardioprotective solution, as described before (Meyer et al., 1996; Pieske et al., 2002).
2.2. Experimental protocol to elicit the SFR
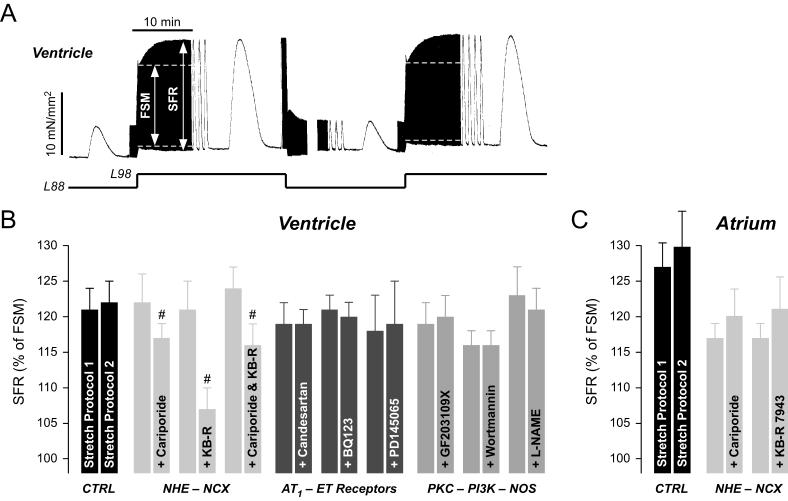
The experimental protocol for eliciting the SFR was identical in atrial and ventricular trabeculae. Trabeculae were transferred to an organ bath containing Tyrode’s solution composed of (mM): Na+ 152, K+ 3.6, Cl- 135, HCO3- 25, Ca2+ 2.5, Mg2+ 0.6, H2PO4- 1.3, SO42- 0.6, glucose 11.2, insulin 5 I.U./l, pH 7.4. The solution was continuously gassed with 95% O2 and 5% CO2. Temperature was 37°C. Muscle strips were attached to a force transducer and electrically stimulated at 1 Hz with rectangular voltage pulses 20-40% above threshold and a pulse duration of 5 ms. Isometric twitch force was measured using a force transducer (Scientific Instruments) and normalised to muscle cross sectional area. The latter was assessed at the beginning of an experiment at slack length. Muscle length was increased stepwise until maximal force development was observed (Lmax). Afterwards, muscle length was reduced to 88% of Lmax (L88) for 30 minutes. Muscles were then stretched acutely to 98% of Lmax (L98). This resulted in a biphasic increase in developed force, an immediate increase (1st phase, Frank-Starling mechanism) followed by a further delayed increase (2nd phase, SFR). The magnitude of the 1st phase was quantified by normalising to developed force measured at L88. The magnitude of the 2nd phase was quantified by normalising to developed force measured during the 1st phase. FSM and SFR were highly reproducible within a given muscle strip (see Fig.1). Thus, all experiments involving drugs included paired stretch protocols, i.e. first a control stretch protocol was conducted in the absence of drug; then the muscle was released to L88 and drug was applied; after 25 minutes a second stretch protocol was conducted in the same muscle strip in the presence of the respective drug.
Fig. 1. The stretch-dependent SFR in human ventricular and atrial myocardium.
(A) Original recording of twitch force in a ventricular muscle strip during a first and second stretch protocol from L88 to L98. The muscle exhibits a reproducible biphasic increase in developed force. The SFR amounts to 140% and to 144% in the first and second stretch protocol, respectively. (B) Average values of the ventricular SFR before and following exposure of the muscle strips to various drugs. CTRL: no drug present during second stretch protocol. Concentrations of drugs were: cariporide, 3 μM (n=11); KB-R 7943, 5 μM (n=7); cariporide, 10 μM & KB-R 7943, 5 μM (n=8); candesartan, 0.1 μM (n=8); BQ123, 0.3 μM (n=12); PD145065, 10 μM (n=6); GF203109X, 1 μM (n=8); wortmannin, 0.1 μM (n=6); L-NAME, 0.5 mM (n=8); # = P<0.05 versus first stretch protocol. (C) Average values of the atrial SFR before and following exposure of the muscle strips to various drugs. CTRL: no drug present during second stretch protocol. Concentrations of drugs were: cariporide, 3 μM (n=6); KB-R 7943, 5 μM (n=8).
Data taken, in part, from (von Lewinski et al., 2004) and (Kockskamper et al., 2008).
2.3. Measuring [Na+]i in isolated trabeculae
Muscle strips were loaded with the fluorescent Na+ indicator SBFI at room temperature by 180 minutes incubation in Tyrode’s solution containing 35 μM of the acetoxymethyl ester of the dye. Trabeculae were mounted in a cylindrical glass cuvette, connected to a force transducer, and superfused with Tyrode’s solution. Because of the long incubation of the muscle strips in SBFI-containing solution, experimental conditions were adjusted to optimise the SFR: [Ca2+] was reduced to 1.25 mM, propranolol (1 μM) was included in the Tyrode’s solution, temperature was reduced to 30°C, and stimulation frequency was set to 0.2 Hz. [Na+]i was measured as described previously (Luers et al., 2005; Pieske et al., 2002). Light from a 100 W mercury lamp was passed through a neutral density filter (1% transmittance) and through bandpass filters (340 nm, 380 nm) in a rotating filter wheel to excite SBFI in the muscle strip. Fluorescence emission was collected by a photomultiplier (Scientific Instruments) after passage through a bandpass filter (505 nm). Fluorescence recording was limited to intervals of ∼20 s every 1-2 minutes following stretch. Fluorescence emission of SBFI-loaded muscles was ∼5 times larger than the autofluorescence. Values of [Na+]i were estimated from the SBFI fluorescence ratio (F340/F380) after subtraction of autofluorescence. SBFI ratios were calibrated at the end of an experiment by exposure of the muscle strip to calibration solutions containing [Na+] of 0, 10, 20, and 30 mM, respectively. For calibration of SBFI fluorescence two calibration solutions were prepared. The first one contained (mM): NaCl 140, HEPES 10, EGTA 1, strophanthidin 0.1, monensin 0.04, gramicidin 0.02 μg/ml, BDM 30, pH 7.4 (NaOH). The second calibration solution contained K+ instead of Na+. The two solutions were mixed to yield [Na+] of 0, 10, 20, and 30 mM, respectively.
In these experiments, force and SBFI fluorescence were measured for 20 minutes following stretch from L88 to L98. Finally, muscles were exposed to the calibration solutions for in vivo calibration of SBFI fluorescence. In some experiments, cariporide (3 μM) was used to inhibit the Na+/H+ exchanger 1 (NHE1). It was applied at L88 20 min prior to stretch and present throughout the experiment.
2.4. Phosphorylation of myosin light chain 2a
Immunoblotting of atrial myosin light chain 2 (MLC2a) was performed with antibodies against phosphorylated and total MLC2a as described before (Grimm et al., 2005; Grimm et al., 2006). Briefly, stretched and non-stretched muscle strips (n=36) were shock frozen in liquid nitrogen. Following homogenisation and centrifugation the pellet was dissolved in Laemmli buffer and subjected to SDS-PAGE and immunodetection using standard Western blot techniques. The antibody directed against total MLC2a (1Ab040; MLC2a) was kindly provided by the CBI Antibody Core at the Center for Biomedical Inventions, University of Texas Southwestern Medical School. The antibody directed against phosphorylated MLC2a (P-MLC2a) was a custom-made antibody from Eurogentec (Seraign, Belgium).
2.5. Drugs
The following drugs were used (source): BQ123 (Calbiochem or Sigma), candesartan or CV11974 (generous gift of AstraZeneca, Mölndal, Sweden), cariporide or HOE642 (generous gift of Aventis Pharma, Frankfurt, Germany), GF203109X (Calbiochem), GsMtx-4 (prepared as described before (Ostrow et al., 2003; Oswald et al., 2002)), KB-R7943 (Tocris), L-NAME (Sigma), ML-7 (Calbiochem), PD145065 (Sigma), streptomycin (Sigma), wortmannin (Calbiochem).
3. Results
3.1. The SFR in human ventricular myocardium — key role for NHE and NCX
Stretching isolated human ventricular muscle from L88 to L98 elicited a biphasic increase in developed force, the 1st phase (due to the FSM) and the delayed 2nd phase or SFR (Fig.1A). Following release of the muscle strip to L88, a second stretch protocol from L88 to L98 revealed a biphasic increase in developed force almost identical to the first stretch protocol. Average data show that there was no difference in the SFR between the first and second stretch protocol (Fig.1B, CTRL). Thus, the SFR was highly reproducible in human ventricular muscle strips. In order to elucidate the role of various membrane channels, transporters, and signalling molecules in the SFR in human ventricle, we used paired stretch protocols: a first control stretch protocol in the absence of a pharmacological inhibitor was followed by a second stretch protocol in the presence of the respective blocker. Average data are presented in Fig.1B. Consistent with results from many animal models, the SFR in human ventricle was reduced by 3 μM cariporide or 5 μM KB-R7943, demonstrating that stretch-induced stimulation/modulation of these Na+-dependent transporters makes an important contribution to the SFR. Dual inhibition of NHE and NCX by 10 μM cariporide and 5 μM KB-R7943 did not further reduce the SFR, indicating that both transporters act via the same pathway. Unlike the situation in animal models, however, antagonism of AT1 (0.1 μM candesartan) and ETA (0.3 μM BQ123) or ETA/B (10 μM PD145065) receptors did not affect the SFR in human ventricle, suggesting that autocrine/paracrine actions of angiotensin II and endothelins are not involved in the SFR in this model. Furthermore, GF203109X (1 μM), wortmannin (0.1 μM), or L-NAME (0.5 mM), inhibitors of protein kinase C (PKC), PI-3 kinase (PI3K), and NO synthase, respectively, also left the SFR unaffected. Taken together, these results suggest that in human ventricle the SFR is mediated by stretch-dependent stimulation of NHE and modulation of NCX activity. The mechanism by which stretch stimulates NHE, however, remains unknown. It does not appear to involve angiotensin II, endothelins, PKC, or the PI3K-NO pathway.
3.2. The SFR in human atrial myocardium — no major role for NHE and NCX
Similar to ventricular myocardium, isolated human atrial muscle strips were also characterised by a highly reproducible, biphasic increase in developed force in response to stretch from L88 to L98 (not shown). On average, the SFR during a first and second stretch protocol amounted to 127±4% and to 130±5%, respectively (Fig.1C, CTRL). This suggested that similar subcellular mechanisms might underlie the SFR in atrium and ventricle. Surprisingly, however, neither blockade of NHE (3 μM cariporide) nor of NCX (5 μM KB-R7943) affected the atrial SFR, indicating that a different mechanism must underlie the stretch-induced SFR in human atrium.
3.3. Stretch differentially affects [Na+]i in atrial versus ventricular myocardium
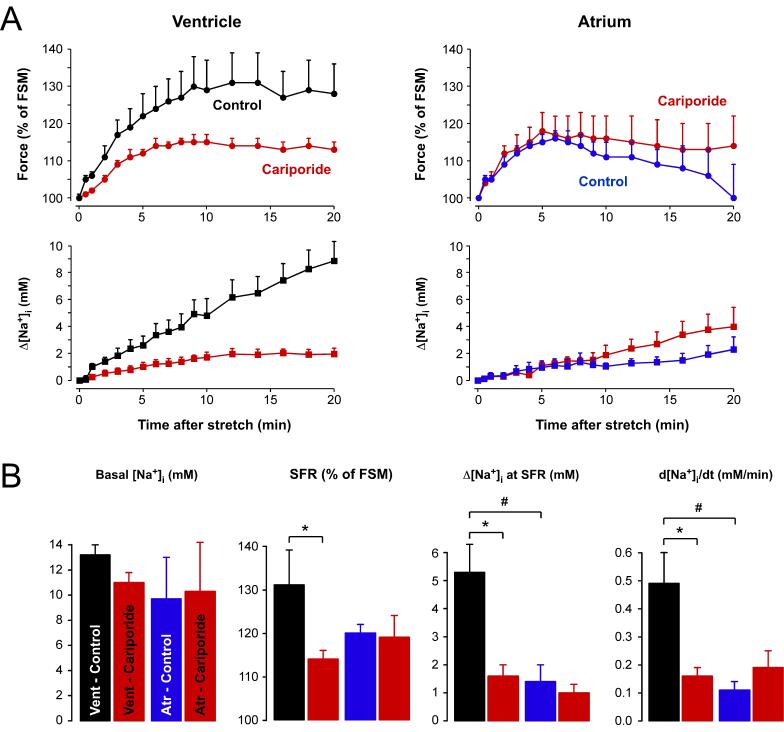
The NHE and NCX dependence of the ventricular SFR suggested that stretch-induced [Na+]i increases might play an important role in the development of the SFR in human ventricle. By contrast, the NHE and NCX independence of the atrial SFR suggested that [Na+]i increases do not occur or that they might be less important for the development of the SFR in human atrium. We therefore measured stretch-induced [Na+]i changes during the SFR in human atrial and ventricular trabeculae by means of SBFI fluorescence. Baseline [Na+]i was 9.9±2.4 mM (n=12) in atrial and 12.2±1.0 mM (n=13) in ventricular muscle strips. There was no difference in basal [Na+]i between control muscle strips and those pre-treated with 3 μM cariporide to inhibit NHE (Fig.2B, left). In ventricular muscle strips, stretch caused a SFR that was associated with an increase in [Na+]i. Fig.2A (left) shows the time course of the stretch-induced increases in twitch force (top) and [Na+]i (bottom) obtained under control conditions (black, n=7) and in the presence of 3 μM cariporide (red, n=6). In control muscle strips, stretch slowly increased developed force until a maximum of ∼130% was reached after 12-14 minutes. Thereafter, force remained almost unchanged. At the same time, [Na+]i rose almost linearly. The rate of rise in [Na+]i, determined by a linear fit to the data, amounted to 0.49±0.11 mM/min. Cariporide significantly reduced the stretch-induced increases in force and [Na+]i by ∼55% and ∼67%, respectively. Fig.2A (right) shows the corresponding data for human atrium (control, blue, n=7; cariporide, red, n=5). The atrial SFR was smaller and peaked earlier than the ventricular SFR with a maximum of ∼117% after 6 minutes. Afterwards, force began to decline. In striking contrast to ventricle, cariporide neither affected the stretch-induced force increase nor the rise in [Na+]i in human atrium. Interestingly, the cariporide-insensitive [Na+]i increase in atrium was comparable to the [Na+]i increase in ventricle in the presence of cariporide. This suggested that the SFR in human atrium was mediated by an NHE-independent mechanism, whereas in human ventricle the SFR was composed of at least two mechanisms, an NHE- and Na+-dependent one as well as an NHE-independent one.
Fig. 2. Stretch-induced changes in [Na+]i during the SFR in atrial and ventricular myocardium.
(A) Time course of changes in force (top) and [Na+]i (bottom) following stretch from L88 to L98 in ventricular (left) and atrial (right) muscle strips under control conditions (black, blue) or in the presence of 3 μM cariporide (red). (B) Basal [Na+]i, SFR, [Na+]i increase at SFR, and rate of the stretch-induced [Na+]i increase (from left to right) in atrial and ventricular muscle strips in the absence (black, blue) or presence (red) of 3 μM cariporide. Means±SEM of 7 (control) and 6 (cariporide) trabeculae for ventricle and 7 (control) and 5 (cariporide) trabeculae for atrium. * = P<0.05 ventricle, control versus cariporide; # = P<0.05 control, ventricle versus atrium.
3.4. The role of SACs for basal contractility and the SFR in atrial and ventricular myocardium from human heart
An obvious transducer of stretch are stretch-activated non-selective cation channels (SACs). SACs in atrial and ventricular myocytes preferentially conduct monovalent cations (Isenberg et al., 2003; Kamkin et al., 2003; Zeng et al., 2000) and, to a lesser degree, also Ca2+ (Suchyna et al., 2004). Thus, activation of SACs could increase force via influx of Na+ and/or Ca2+. Evidence from animal models and modelling studies suggested that SACs might be involved in the SFR (Calaghan and White, 2004; Niederer and Smith, 2007). However, disparate results have also been obtained (von Lewinski et al., 2004; von Lewinski et al., 2003) and the role of SACs in the development of the SFR, therefore, is still not understood. One problem in this regard has been the lack of a specific inhibitor of SACs. Many previous studies have relied on either gadolinium or streptomycin to block SACs. Although both can indeed block SACs (Hamill and McBride, 1996; Isenberg et al., 2003), they also have effects on channels other than SACs and thus interpretation of results obtained with these substances alone is difficult. Recently, however, a peptide isolated from the venom of the tarantula Grammostola spatulata, GsMtx-4, was identified as a novel, highly potent and specific inhibitor of SACs (Bowman et al., 2007; Suchyna et al., 2000).
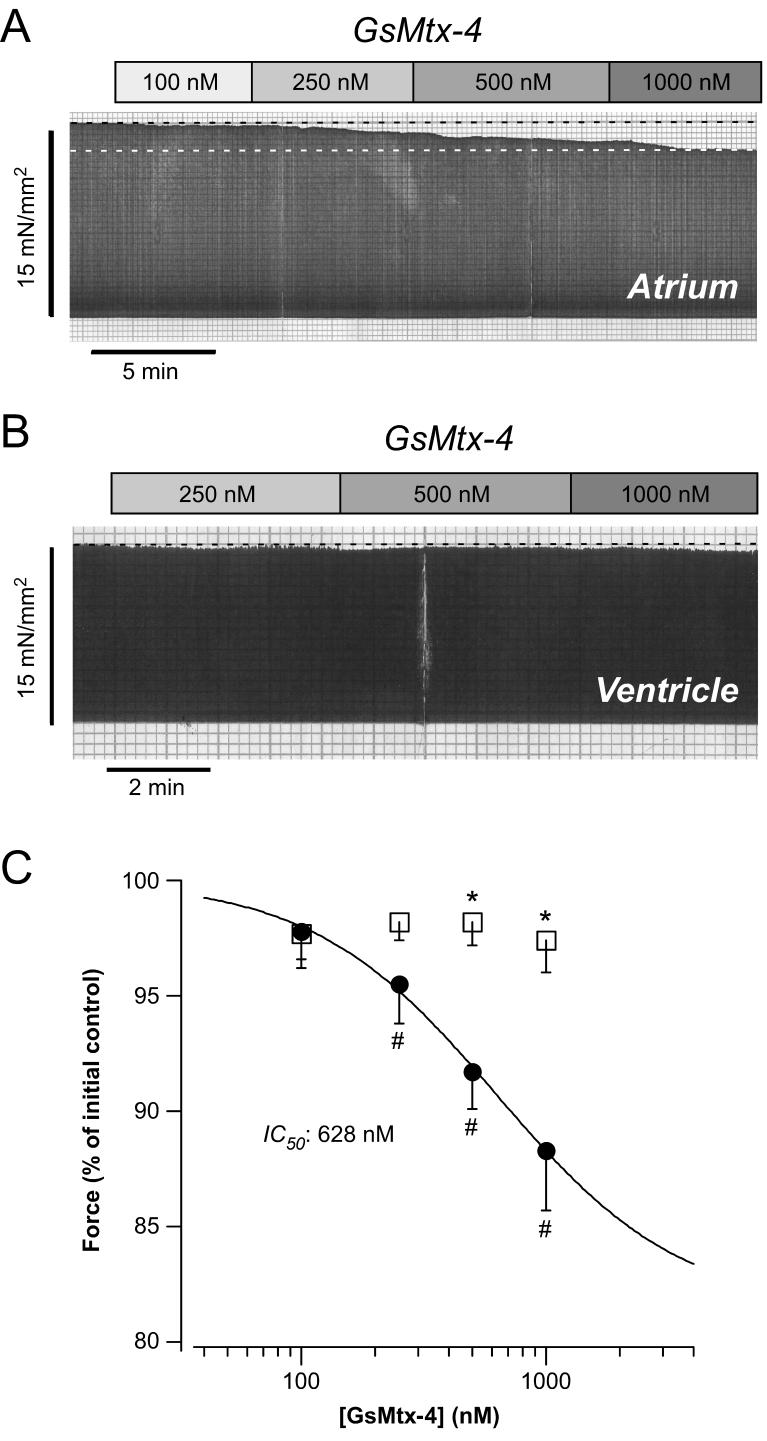
In order to evaluate the functional relevance of SACs in human heart, we tested the effects of GsMtx-4 on basal contractility in atrial and ventricular muscle strips. Fig.3A,B illustrates original recordings from atrium (A) and ventricle (B). In atrium, increasing concentrations of GsMtx-4 reduced basal twitch force at L98. By contrast, the same concentrations of GsMtx-4 did not affect basal twitch force in a ventricular muscle strip under otherwise identical conditions. Average data are presented in Fig.3C. There was a concentration-dependent reduction in twitch force elicited by GsMtx-4 in atrium (circles). At 1000 nM GsMtx-4, the highest concentration tested, basal twitch force decreased by 12±3% (n=4, P<0.05). The IC50 value amounted to 628 nM GsMtx-4 and the Hill coefficient was 1.1. In ventricle, on the other hand, there was no appreciable effect of GsMtx-4 on basal force up to a concentration of 1000 nM (squares). Thus, SACs appear to be activated under baseline conditions and contribute to basal force development in human atrium but not ventricle.
Fig. 3. Effects of GsMtx-4 on basal contractility in atrial and ventricular myocardium.
Original recordings of twitch force at L98 of an atrial (A) and a ventricular (B) muscle strip before and following exposure to increasing concentrations of GsMtx-4. (C) Concentration dependence of GsMtx-4 effects on basal contractility in atrial (circles) and ventricular (squares) muscle strips. Means±SEM of n=4-9 trabeculae. # = P<0.05 versus initial control; * = P<0.05 versus atrium. The line is a fit of the Hill equation to the data points yielding an IC50 of 628 nM GsMtx-4 and a Hill coefficient of 1.1.
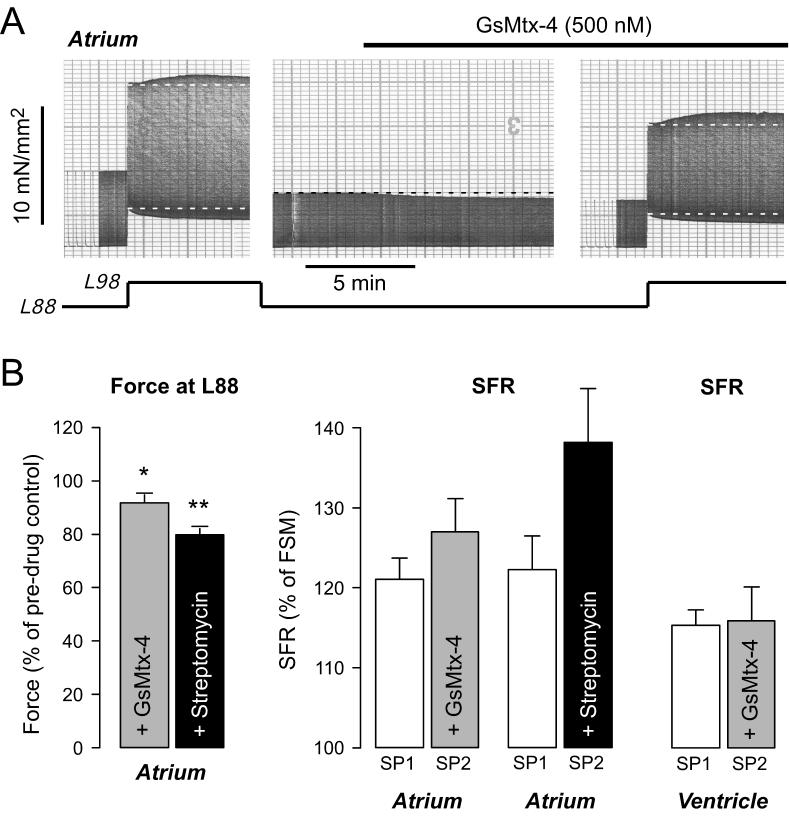
We next assessed whether SACs might be involved in the SFR in human atrium or ventricle. Fig.4 illustrates an original recording (A) and average results (B) from human atrial muscle strips. Following a control stretch response with a SFR of 117%, the muscle strip was released to L88 and exposed to 500 nM GsMtx-4. The peptide reduced basal twitch force at L88 by 12%. When the stretch protocol was repeated in the presence of GsMtx-4, the SFR was not reduced and amounted to 127%. On average, GsMtx-4 (500 nM, n=8) reduced basal twitch force at L88 in human atrium by 9±3% (Fig.4B, left; P<0.05). There was, however, no difference between the SFR elicited in the absence and presence of the peptide (Fig.4B, middle). Similar results were obtained using 1000 nM GsMtx-4 (n=3, data not shown). For comparison, we also conducted experiments with the unspecific SAC inhibitor streptomycin (Fig.4B). Streptomycin (80 μM, n=6) reduced basal force development at L88 by 20±2% (Fig.4B, left; P<0.01). It tended to increase the SFR, but this effect did not reach statistical significance (P=0.08, Fig.4B, middle). Finally, we tested for the involvement of SACs in the SFR in human ventricular muscle strips using 1000 nM GsMtx-4 (n=3). GsMtx-4 did not affect the ventricular SFR (Fig.4B, right), thus confirming previous experiments using 70 μM streptomycin or 10 μM gadolinium to block SACs (von Lewinski et al., 2004). From these results we conclude that SACs are unlikely to contribute to the SFR in either human atrium or ventricle.
Fig. 4. GsMtx-4 does not affect the SFR in atrial or ventricular myocardium.
(A) Original recording of SFR of an atrial muscle strip in the absence (left) and presence (right) of 500 nM GsMtx-4. Muscle length is indicated below the force recording. GsMtx-4 was applied at L88 before the second stretch protocol and reduced twitch force by 12%. SFR amounted to 117% and 127% in the first and second stretch protocol, respectively. (B) Effects of 500 nM GsMtx-4 (n=8) and 80 μM streptomycin (n=6) on basal force development in atrial muscle strips (left); * and ** indicate P<0.05 and P<0.01 versus initial control. SFR in atrium (middle) or ventricle (right) in the absence (SP1) and presence (SP2) of either GsMtx-4 or streptomycin, as indicated. Means±SEM of 8 (atrium, GsMtx-4), 6 (atrium, streptomycin), and 3 (ventricle, GsMtx-4) trabeculae. SP1 and SP2 indicate first and second stretch protocol, respectively.
Data taken, in part, from (Kockskamper et al., 2008).
3.5. In search of new mechanisms underlying the SFR in human atrium: the peptide case
Despite the fact that antagonism of AT and ET receptors did not affect the SFR in human ventricle, we considered the possibility that these peptides might be involved in the SFR in human atrium, because many cardioactive peptides act preferentially on the atrium rather than the ventricle. Intriguingly, angiotensin II exerts a positive inotropic effect in human atrium, but not in ventricle (Holubarsch et al., 1993). Moreover, atrial myocytes produce the peptide hormone atrial natriuretic peptide (ANP), which is stored in granules and released upon stretch. Therefore, we investigated whether angiotensin II, endothelin-1, or ANP might be involved in the SFR in human atrial myocardium. We used two approaches: application of the peptide itself and, if the SFR was altered by the peptide, application of the respective receptor antagonist. Fig.5A shows an original recording of an atrial muscle strip treated with 500 nM angiotensin II. In the absence of the octapeptide, the SFR amounted to 124%. Exposure to angiotensin II elicited a positive inotropic effect (compare force at L88 before stretch between first and second stretch protocol). When the muscle was stretched in the presence of angiotensin II, the SFR was blunted and amounted to 108%. Subsequent elevation of extracellular [Ca2+] to 5 mM induced an additional positive inotropic effect of ∼50% on top of the SFR, indicating that reduction of the SFR by angiotensin II was not caused by exhaustion of inotropic reserve. On average, angiotensin II increased basal force by 247±46% (n=8, P<0.05 versus initial control) and reduced the SFR from 120±4% to 113±3% (n=8, P<0.05, Fig.5B). Similar to angiotensin II, endothelin-1 (25 nM) also elicited a positive inotropic effect (427±75%, n=8, P<0.01 versus initial control) and largely reduced the SFR from 127±6% to 105±2% (n=8, P<0.01, Fig.5B). By contrast, ANP (20 nM) induced a negative inotropic effect (87±5%, n=6, P<0.05 versus initial control) and left the SFR unaffected (n=6, P=N.S., Fig.5B). These results suggested that angiotensin II and endothelin-1, but not ANP, were involved in the SFR in human atrium. We therefore tested whether receptor antagonism might also reduce the SFR. Saralasin (5 μM), an unselective AT receptor antagonist, significantly reduced the SFR from 125±6% to 111±2% (n=9, P<0.05, Fig.5B). ET receptor antagonists were not tested, however, because preliminary experiments indicated that neither BQ123 (0.25-1.0 μM) nor PD145065 (10 μM) were able to reduce the positive inotropic effect of bath-applied endothelin-1, presumably due to the unique pharmacology of ET receptors in human atrium (Burrell et al., 2000). Nevertheless, taken together these results suggested that autocrine/paracrine actions of angiotensin II and endothelin contributed to the atrial SFR. The intracellular signalling cascade, however, did not involve NHE and NCX.
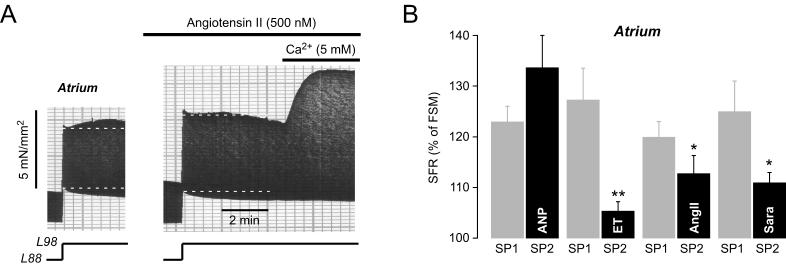
Fig. 5. Effects of cardioactive peptides on the SFR in atrium.
(A) Original recording of the SFR of an atrial muscle strip before and following exposure to 500 nM angiotensin II. After the maximum of the second SFR had occurred, extracellular [Ca2+] was increased to 5 mM. (B) Average values for the atrial SFR obtained in the absence and presence, respectively, of 20 nM ANP (n=6), 25 nM endothelin-1 (ET, n=8), 500 nM angiotensin II (AngII, n=8), and 5 μM saralasin (Sara, n=9). ** = P<0.05 and * = P<0.05 versus first control stretch protocol. SP1 and SP2 indicate first and second stretch protocol, respectively.
Data taken, in part, from (Kockskamper et al., 2008).
3.6. In search of new mechanisms underlying the SFR in human atrium: myosin light chain phosphorylation by MLCK
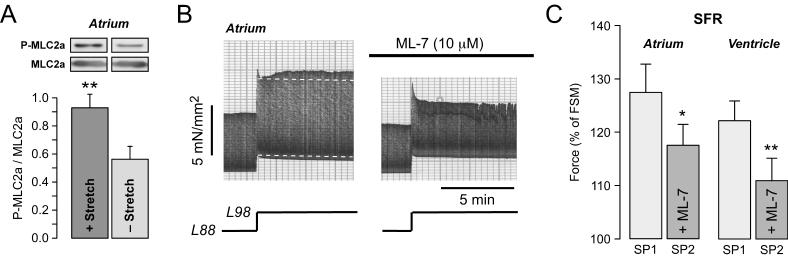
Contractile force can be modulated by changes in the amplitude of the [Ca2+]i transient or by alterations in myofilament Ca2+ responsiveness. Since the atrial SFR appeared to be independent of the NHE-[Na+]i-NCX pathway, we studied whether it might be mediated by a mechanism targeting the myofilaments. In atrium, phosphorylation of atrial myosin light chain 2 (MLC2a) regulates force development. Increased phosphorylation of MLC2a by a specific kinase, myosin light chain kinase (MLCK), causes an increase in the Ca2+ sensitivity of the myofilaments and thereby an increase in force development (Morano, 1999). Fig.6A shows that stretch of atrial muscle strips increased phosphorylation of MLC2a, as determined by immunoblotting of phosphorylated and total MLC2a in stretched versus non-stretched muscle strips. In stretched muscle strips the average ratio of phosphorylated to total MLC2a amounted to 0.93±0.10 (n=18) versus 0.56±0.09 in non-stretched muscle strips (n=18; P<0.01). Inhibition of MLCK by 10 μM ML-7 reduced basal force development by 18±3% in atrium (n=8; P<0.01). Fig.6B illustrates the effect of ML-7 on the SFR in atrial myocardium. Under control conditions, the SFR amounted to 114%. In the presence of the MLCK inhibitor, however, the SFR was completely absent. On average (Fig.6C), ML-7 reduced the SFR from 127±5% to 117±4% (n=8; P<0.05). We also tested the effects of ML-7 on the SFR in ventricular myocardium. In ventricle, ML-7 reduced the SFR from 122±4% to 111±4% (n=5; P<0.01, Fig.6C, right). Thus, MLCK inhibition caused a ∼40-50% reduction of the SFR in atrial and ventricular myocardium, suggesting that MLCK-mediated MLC phosphorylation is involved in the regulation of the SFR in both human atrium and ventricle.
Fig. 6. Involvement of MLCK in the SFR in atrial and ventricular myocardium.
(A) Original immunoblots (top) of phosphorylated (P-MLC2a) and total (MLC2a) atrial MLC2 in a stretched versus non-stretched atrial muscle strips. Means±SEM of the ratio of P-MLC2a to MLC2a are shown below. ** = P<0.01 versus non-stretched. n=18 muscle strips for each group. (B) Original recording of the SFR in an atrial muscle strip in the absence and presence of 10 μM ML-7. (C) Average data of the SFR in the absence and presence of 10 μM ML-7 in atrial (left, n=8) or ventricular (right, n=5) trabeculae. * = P<0.05 and ** = P<0.01 versus first control stretch protocol. SP1 and SP2 indicate first and second stretch protocol, respectively.
Data taken, in part, from (Kockskamper et al., 2008).
3.7. Evidence for a negative inotropic response mediated by stretch: the slow force decline, SFD
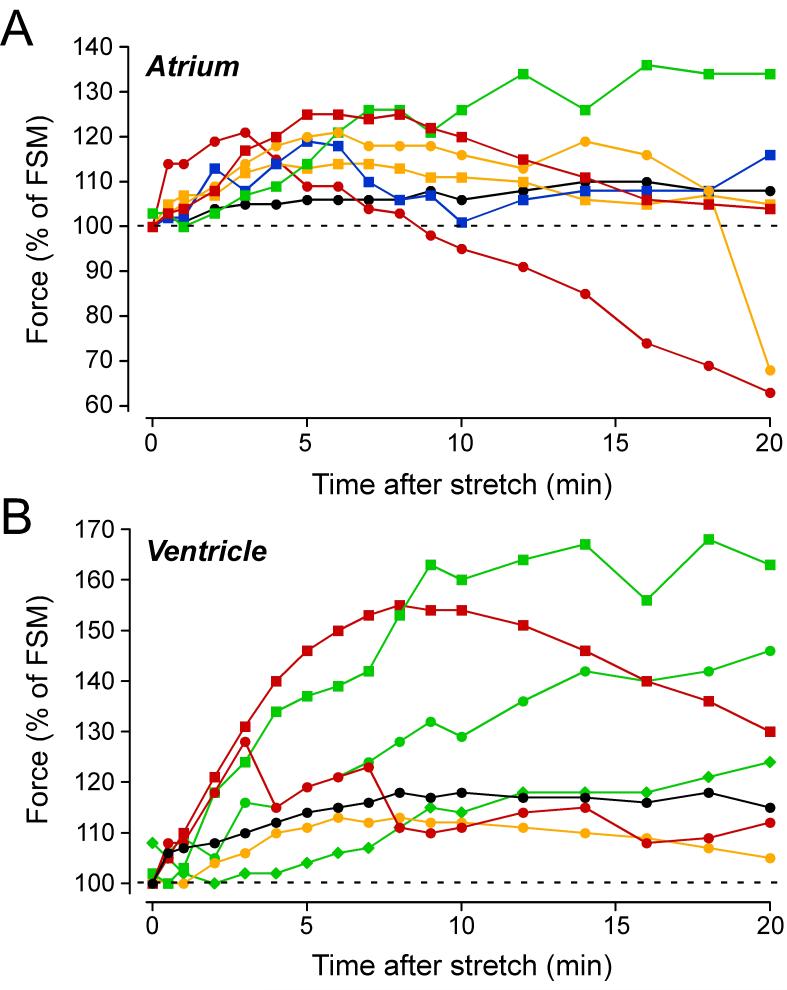
As evident from Fig.2A, atrial muscle strips were characterised by a triphasic change in force induced by stretch: the FSM and SFR were followed by a slow force decline (SFD) or 3rd phase. Fig.7A shows the time courses of the stretch-induced changes in force of the 7 individual atrial muscle strips from Fig.2. Each muscle strip is encoded by a different symbol and colour. Of the seven atrial muscle strips, only two (green squares, black circles) exhibited a continuous rise in force with a plateau after ∼15-20 minutes. The other five muscle strips displayed a maximal force increase after 4-8 minutes followed by a force decline, which was particularly pronounced in two trabeculae (red symbols).
Fig. 7. A stretch-induced slow force decline (SFD) in atrial and ventricular muscle strips.
Time course of stretch-induced changes of twitch force following stretch from L88 to L98 in individual muscle strips from atrium (A) or ventricle (B). Same muscle strips as in Fig.2. Each muscle strip is encoded by a different symbol and colour. Trabeculae with a sustained SFR and no SFD are shown in green, whereas those with a clear SFD are shown in red or yellow.
Fig.2A also suggests that stretch might activate a negative inotropic mechanism in ventricular myocardium: despite a continuous rise in [Na+]i, force exhibited a maximum and did not further increase, as if the Na+-dependent, positive inotropic mechanism was limited by a negative inotropic mechanism. Analysis of the time courses of stretch-induced changes in force of the 7 individual ventricular muscle strips revealed that some of them were indeed characterised by a triphasic change in force (Fig.7B). Of the seven ventricular muscle strips, three exhibited a continuous rise in force (green symbols), one displayed a plateau (black symbol), and three a clear force decline following a maximum after 3-8 minutes (red and yellow symbols).
Taken together, these data show that atrial and ventricular trabeculae can exhibit a stretch-induced SFD that limits the SFR. The SFD was more frequent and more pronounced in atrium than in ventricle.
3.8. The SFR in the diseased heart
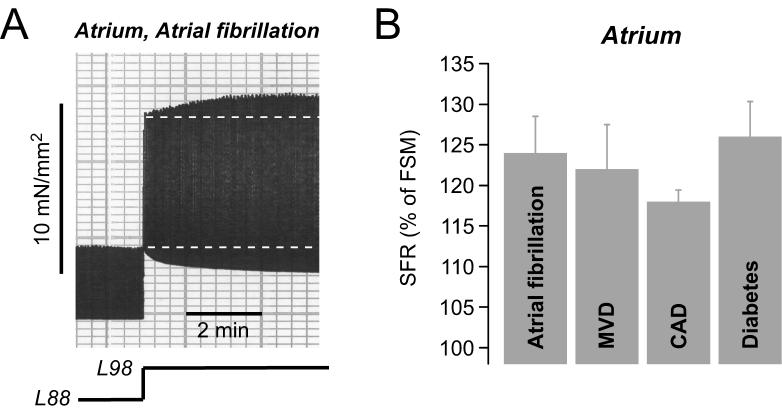
Finally, we aimed to evaluate the functional relevance of the SFR in cardiac disease by comparing the magnitude of the SFR in atrial and ventricular muscle strips isolated from patients afflicted by various cardiovascular diseases. Fig.8A illustrates an original recording of twitch force of an atrial muscle strip isolated from a patient with atrial fibrillation, a cardiac disease associated with electrical, contractile and structural remodelling of the atria (Allessie et al., 2002). Interestingly, stretching this muscle strip from L88 to L98 elicited a normal biphasic stretch response with a SFR of 133%. The average SFR in atrial muscle strips from 6 patients with atrial fibrillation amounted to 124±4% (Fig.8B). Similarly, muscle strips from patients with mitral valve disease (MVD, N=5), which causes dilatation of the atria, exhibited a normal SFR of 122±5% (Fig.8B). Furthermore, patients with coronary artery disease (CAD, N=57) or diabetes mellitus (N=15) had a normal SFR of ∼120-125% (Fig.8B). There was no statistically significant difference in the magnitude of the SFR between these groups.
Fig. 8. The SFR in atrial and ventricular myocardium from patients with various cardiac diseases.
(A) Original recording of stretch-induced changes in twitch force of an atrial muscle strip from a patient with atrial fibrillation. (B) Average values for the SFR in atrial trabeculae from patients with atrial fibrillation (N=6), mitral valve disease (MVD, N=5), coronary artery disease (CAD, N=57), or diabetes mellitus (N=15).
Data taken, in part, from (Kockskamper et al., 2008).
In human ventricle from end-stage failing hearts, a SFR of ∼120-125% was observed (see Fig.1). The magnitude of the SFR was comparable in muscle strips from patients with either dilatative or ischaemic cardiomyopathy (not shown). Further analysis revealed that neither age, nor gender, nor ejection fraction affected the SFR in either atrium or ventricle (not shown).
4. Discussion
4.1. The SFR in human heart - a universal phenomenon
Cumulative data from more than 250 atrial trabeculae of more than 100 patients and from more than 150 ventricular trabeculae of more than 70 patients indicate that defined stretch elicits a SFR of ∼120-125%, i.e. exceeding the stretch-induced force increase caused by the Frank-Starling mechanism by 20-25%. Analysis of these data further revealed the existence of the SFR in failing and nonfailing muscle strips of atria and ventricles from patients with various cardiac diseases. In addition, the SFR was independent of age, gender, and ejection fraction in both atrium and ventricle. Further analysis in atrium suggests that the pre-medication of the patients does not affect the SFR (Kockskamper et al., 2008). In fact, so far we have not identified conditions under which the SFR was completely absent. Together with data obtained from various mammalian species (including cat, dog, ferret, guinea-pig, rabbit, rat), these observations demonstrate that the SFR is a universal phenomenon occurring in atrial and ventricular preparations on the level of single myocytes, multicellular muscle strips and whole hearts. One possible reason for the universality of the SFR may be that it is mediated by activation of several stretch-dependent pathways rather than a single mechanism (see below). This redundancy ensures that, even if one of the stretch-activated pathways was compromised (e.g. in cardiac disease), the remaining pathways could kick in and still elicit a substantial SFR. It also implies that the SFR serves important physiological functions.
4.2. Various pathways and mechanisms contribute to the stretch-induced changes in force
An important insight from recent studies on the SFR in human atrium and ventricle, but also from animal models and modelling studies, is that stretch activates various signalling pathways and mechanisms in parallel to regulate contractile force.
Early evidence indicated that the SFR in cat ventricular muscle is caused by an increase in the underlying [Ca2+]i transient (Allen and Kurihara, 1982). Since then, this finding has been confirmed in other ventricular preparations (Alvarez et al., 1999; Calaghan and White, 2004; Hongo et al., 1996; Kentish and Wrzosek, 1998; Luers et al., 2005; Todaka et al., 1998). In human ventricle, a stretch-induced slow increase in the [Ca2+]i transient has not been demonstrated directly so far. However, blocking SR function greatly reduced the SFR in human ventricle, suggesting an important role for alterations in SR Ca2+ load and thus the electrically stimulated [Ca2+]i transient (von Lewinski et al., 2004). In line with this finding, caffeine (which releases Ca2+ from the SR through ryanodine receptors) was found to reduce the SFR in cat ventricle by an SR-dependent mechanism (Chuck and Parmley, 1980). On the other hand, inhibition of SR function did not diminish the SFR in rat (Calaghan and White, 2004; Kentish and Wrzosek, 1998) and rabbit ventricle (Bluhm and Lew, 1995). Taken together, these results suggest that in mammalian ventricle the SFR can be mediated by a mechanism dependent on changes in SR Ca2+ load and the electrically stimulated [Ca2+]i transient and an additional mechanism.
Three major mechanisms have recently been proposed to account for the increase in [Ca2+]i transients during the SFR: (1) activation of SACs (which may act via increased Na+ and/or Ca2+ influx); (2) stimulation of the PI3K-NO pathway (which may act via modulation of SR function); and (3) stimulation of NHE activity through autocrine/paracrine actions of stretch-released angiotensin II and endothelin. The first two are particularly controversial. Using a specific SAC blocker, GsMtx-4, we have shown here that SACs do not contribute significantly to the SFR in human ventricular myocardium. Furthermore, our previous study using unspecific SAC blockers came to the same conclusion (von Lewinski et al., 2004). Inhibition of PI3K or NO synthase also did not affect the SFR in human ventricle, consistent with a recent study in rat ventricle (Calaghan and White, 2004) and modelling studies (Niederer and Smith, 2007). We thus conclude that in human ventricular trabeculae from end-stage failing hearts neither SACs nor the PI3K-NO pathway play an important role for the development of the SFR. In contrast, there is convincing evidence for a key role of NHE and NCX in the SFR in human ventricle (von Lewinski et al., 2004). We have further substantiated this evidence by demonstrating, for the first time in human ventricle, a stretch-induced increase in [Na+]i associated with the SFR. This finding is in line with previous studies revealing stretch-induced increases in [Na+]i in rat, rabbit, and mouse ventricle (Alvarez et al., 1999; Isenberg et al., 2003; Kondratev and Gallitelli, 2003; Luers et al., 2005; Perez et al., 2001; Wilhelm et al., 2006), although one study failed to detect a stretch-induced [Na+]i increase (Hongo et al., 1996). How NHE is stimulated by stretch, however, is unknown at present. Angiotensin II and endothelin, although of importance in some animal species (Alvarez et al., 1999; Calaghan and White, 2001; Perez et al., 2001), are not involved in human ventricle. Future studies will have to unravel the mechanism underlying the stretch-induced NHE stimulation in human ventricle.
In addition to the NHE-[Na+]i-NCX-SR pathway, we have identified a novel mechanism contributing to the SFR in human ventricle: MLCK-dependent MLC phosphorylation and increased myofilament Ca2+ responsiveness (Andersen et al., 2002). This mechanism may explain why blockade of the NHE-NCX pathway is unable to completely suppress the SFR (Fig.1B) and why in some studies suppression of SR function did not inhibit the SFR (Bluhm and Lew, 1995; Calaghan and White, 2004; Kentish and Wrzosek, 1998). How MLCK is stimulated by stretch remains to be determined. Since MLCK is dependent on Ca2+/calmodulin, one possibility is that the stretch-induced increase in the [Ca2+]i transient also stimulates MLCK via Ca2+/calmodulin. In this case, however, MLCK would be downstream of the NHE-[Na+]i-NCX-SR axis and inhibition of this pathway should cause complete suppression of the SFR (which it does not). Thus, alternative pathways might exist by which stretch can stimulate MLCK.
A further important finding of the current study is the existence of a stretch-induced negative inotropic mechanism. This 3rd phase or slow force decline (SFD) limits the SFR in both human atrium and ventricle. The SFD is not unprecedented. A study in dog heart has noted this phenomenon before (Todaka et al., 1998). So far, the cellular mechanisms underlying this stretch-induced negative inotropic response are not known. Most recent experiments from our laboratory suggest the involvement of p38 mitogen-activated protein kinase (unpublished data, manuscript in preparation), which mediates negative inotropic effects in rat cardiomyocytes (Palomeque et al., 2006). Nevertheless, whatever the precise mechanism of the SFD, it reveals the existence of yet another inotropic signalling pathway activated by stretch and adds to the complexity of stretch-induced mechanisms in human myocardium. It suggests that the individual magnitude and time course of each inotropic mechanism activated by stretch decides about the overall response of the preparation to stretch, i.e. SFR and SFD, and may explain part of the variability in stretch-induced inotropic responses between individual trabeculae (see Fig.7) as well as between preparations and species.
In summary, current evidence indicates that in human ventricle stretch activates at least three signalling pathways that regulate contractility: two exerting a positive inotropic effect and one that exerts a negative inotropic effect. The first positive inotropic mechanism is Na+- and Ca2+-dependent and mediated by increased [Ca2+]i transients via the NHE-[Na+]i-NCX-SR axis (but not autocrine/paracrine actions of angiotensin II and endothelin), whereas the second positive inotropic mechanism appears to be mediated by an MLCK-dependent increase in myofilament Ca2+ responsiveness. The negative inotropic mechanism limits the stretch-induced force increase during the SFR and can even induce a decline of contractile force.
In human atrium, stretch elicited a similar SFR as in ventricle, in line with previous studies on rat atrium (Tavi et al., 1998; Tavi et al., 1999; Tavi et al., 2000). Analysis of the underlying mechanisms revealed that, despite similar magnitude and time course, the atrial SFR was independent of NHE and NCX function. Since either pre-application of angiotensin II and endothelin-1 or antagonism of AT receptors largely reduced the atrial SFR, we propose that it is mediated by autocrine/paracrine release and action of these cardioactive peptides, similar to what has been found in ventricle of some animal species (Alvarez et al., 1999; Calaghan and White, 2001; Perez et al., 2001). In human atrium, Gq-coupled receptors increase force via MLCK-dependent increases in the phosphorylation of MLC2a and increased myofilament Ca2+ responsiveness (Grimm et al., 2005). Angiotensin II and endothelin-1 both act via Gq-coupled receptors and we found clear evidence that the atrial SFR is mediated by MLCK-dependent increases in MLC2a phosphorylation. As noted above, MLCK activity depends on Ca2+/calmodulin. Elevation of [Ca2+]i, therefore, could link AT and ET receptor activation with stimulation of MLCK. Indeed, in rat atrium direct evidence for stretch-induced increases in the [Ca2+]i transient during the SFR has been obtained (Tavi et al., 1998; Tavi et al., 1999). How angiotensin II and endothelin-1 might elevate [Ca2+]i remains speculative, but the recent observation that endothelin-1 acts via inositol 1,4,5-trisphosphate (IP3) to increase the atrial [Ca2+]i transient (Mackenzie et al., 2002; Zima and Blatter, 2004) makes IP3-induced SR Ca2+ release a likely candidate. Furthermore, an earlier study has shown that stretch increases IP3 in cardiac myocytes (Dassouli et al., 1993). Thus, based on our experimental results in human atrium and data from the literature, we propose that the following chain of events underlies the SFR in human atrium: Stretch causes the release of angiotensin II and endothelin, which act in an autocrine/paracrine way on AT and ET receptors on atrial myocytes. These receptors activate the phospholipase C - IP3 pathway to increase the atrial [Ca2+]i transient. This leads to a Ca2+-dependent increase in contractile force and to Ca2+/calmodulin-dependent stimulation of MLCK, which in turn phosphorylates MLC2a to elicit an additional increase in force via increased myofilament Ca2+ responsiveness.
An unexpected finding was that SACs are apparently not involved in the SFR in human myocardium, neither in atrium nor ventricle, despite convincing evidence for the expression and functional relevance of SACs in atrium and ventricle of many mammalian species (Isenberg et al., 2003; Suchyna et al., 2000; Zeng et al., 2000) including humans (Kamkin et al., 2003), and evidence for the involvement of SACs in the SFR in rat ventricle (Calaghan and White, 2004; Niederer and Smith, 2007). We have used a specific blocker of SACs in this study, GsMtx-4, to evaluate the role of SACs in human atrium and ventricle for baseline force development and the SFR. GsMtx-4 was found to attenuate baseline force in human atrium but not ventricle. The IC50 of GsMtx-4 was 628 nM, in perfect agreement (∼630 nM) with studies on isolated astrocytes and cardiac myocytes (Suchyna et al., 2000). This finding is important for two reasons: (1) it demonstrates that the peptide was functional and effective in a multicellular muscle preparation and (2) it provides the first evidence for the contribution of SACs to force development in isolated human atrial myocardium. GsMtx-4, however, did not attenuate the SFR, neither in atrium nor ventricle. This indicates, in conjunction with previous studies using gadolinium or streptomycin (Kockskamper et al., 2008; von Lewinski et al., 2004), that under our experimental conditions SACs do not contribute to the SFR. A possible explanation for this finding is that local deformation of the membrane, rather than end-to-end stretch, might be required for activation of SACs (Isenberg et al., 2003).
In summary, in human atrium stretch activates at least two signalling pathways, a positive inotropic and a negative inotropic mechanism. The former involves release and autocrine/paracrine actions of angiotensin II and endothelin (but not stimulation of the NHE-[Na+]i-NCX axis) and MLCK-dependent phosphorylation of MLC2a with a subsequent increase in myofilament Ca2+ responsiveness. As in ventricle, the negative inotropic mechanism needs to be characterised in detail in future studies.
4.3. The SFR in human heart — friend or foe?
The SFR makes a significant contribution to the stretch-induced increase in contractile force in both atrium and ventricle. Its physiological function, therefore, may be to increase cardiac output under conditions of elevated load. Such a mechanism is probably not essential in a healthy heart with normal atrial and ventricular function, but it may become particularly important in a diseased heart with depressed contractility and cardiac output. In a failing heart with increased pre- and/or afterload, the extra increase in contractile force gained by the SFR may decide about compensated or decompensated heart failure. Another clinically relevant example is conversion of atrial fibrillation. Atria from patients with chronic atrial fibrillation undergo substantial remodelling processes that cumulate in a profound depression of atrial contractility (Allessie et al., 2002). After conversion of atrial fibrillation to sinus rhythm, atrial contraction is still impaired for some time. Under these conditions, changes in atrial load may recruit the SFR and thereby help increase ventricular filling and improve cardiac function.
On the other hand, the stretch-induced decline in force subsequent to the SFR may limit the beneficial effects of the SFR and even adversely affect cardiac function. Furthermore, the (positive inotropic) signalling pathways activated by stretch are known to be involved in the development of hypertrophy and its progression to heart failure. Increased activity of NHE has been implicated in a variety of cardiac diseases (Avkiran and Haworth, 2003). It contributes to hypertrophy, fibrosis, and reperfusion injury. Inhibition of NHE can prevent or revert these adverse effects. Moreover, MLCK is involved in sarcomere organisation during hypertrophic growth of cardiac myocytes (Aoki et al., 2000) and prevention of MLC phosphorylation can induce atrial hypertrophy and dilatation in mice (Sanbe et al., 1999). The increases in [Na+]i and SR Ca2+ load observed in ventricular myocardium can impair diastolic function (Pieske et al., 2002) and facilitate the development of triggered arrhythmias (Sipido et al., 2007). Thus, stretch-dependent activation of these pathways may exhibit adverse effects in the long run.
In conclusion, the stretch-induced SFR is a universal phenomenon in both human atrium and ventricle. It is mediated by stretch-dependent activation of various signalling pathways and may exert both beneficial and adverse effects, depending on the physiological and pathophysiological conditions of the heart (e.g. cardiac disease) and the duration, magnitude, and frequency of the increases in load.
Acknowledgements
The authors’ studies were supported by grants from the Deutsche Forschungsgemeinschaft (to JK & BP: PI 414/1 and PI 414/2, Klinische Forschergruppe 155, TP 6), the German Ministry for Education and Research (BMBF, Kompetenznetz Herzinsuffizienz, TP 8, to TE and BP), and the NIH and the Oshei Foundation (to FS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen DG, Kurihara S. The effects of muscle length on intracellular calcium transients in mammalian cardiac muscle. J. Physiol. 1982;327:79–94. doi: 10.1113/jphysiol.1982.sp014221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc. Res. 2002;54:230–246. doi: 10.1016/s0008-6363(02)00258-4. [DOI] [PubMed] [Google Scholar]
- Alvarez BV, Perez NG, Ennis IL, Camilion de Hurtado MC, Cingolani HE. Mechanisms underlying the increase in force and Ca2+ transient that follow stretch of cardiac muscle: a possible explanation of the Anrep effect. Circ. Res. 1999;85:716–722. doi: 10.1161/01.res.85.8.716. [DOI] [PubMed] [Google Scholar]
- Andersen GO, Qvigstad E, Schiander I, Aass H, Osnes JB, Skomedal T. Alpha1-AR-induced positive inotropic response in heart is dependent on myosin light chain phosphorylation. Am. J. Physiol. 2002;283:H1471–1480. doi: 10.1152/ajpheart.00232.2002. [DOI] [PubMed] [Google Scholar]
- Aoki H, Sadoshima J, Izumo S. Myosin light chain kinase mediates sarcomere organization during cardiac hypertrophy in vitro. Nat. Med. 2000;6:183–188. doi: 10.1038/72287. [DOI] [PubMed] [Google Scholar]
- Avkiran M, Haworth RS. Regulatory effects of G protein-coupled receptors on cardiac sarcolemmal Na+/H+ exchanger activity: signalling and significance. Cardiovasc. Res. 2003;57:942–952. doi: 10.1016/s0008-6363(02)00782-4. [DOI] [PubMed] [Google Scholar]
- Bluhm WF, Lew WY. Sarcoplasmic reticulum in cardiac length-dependent activation in rabbits. Am. J. Physiol. 1995;269:H965–972. doi: 10.1152/ajpheart.1995.269.3.H965. [DOI] [PubMed] [Google Scholar]
- Bluhm WF, Lew WY, Garfinkel A, McCulloch AD. Mechanisms of length history-dependent tension in an ionic model of the cardiac myocyte. Am. J. Physiol. 1998;274:H1032–1040. doi: 10.1152/ajpheart.1998.274.3.H1032. [DOI] [PubMed] [Google Scholar]
- Bowman CL, Gottlieb PA, Suchyna TM, Murphy YK, Sachs F. Mechanosensitive ion channels and the peptide inhibitor GsMTx-4: history, properties, mechanisms and pharmacology. Toxicon. 2007;49:249–270. doi: 10.1016/j.toxicon.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell KM, Molenaar P, Dawson PJ, Kaumann AJ. Contractile and arrhythmic effects of endothelin receptor agonists in human heart in vitro: blockade with SB 209670. J. Pharmacol. Exp. Ther. 2000;292:449–459. [PubMed] [Google Scholar]
- Calaghan S, White E. Activation of Na+-H+ exchange and stretch-activated channels underlies the slow inotropic response to stretch in myocytes and muscle from the rat heart. J. Physiol. 2004;559:205–214. doi: 10.1113/jphysiol.2004.069021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calaghan SC, Colyer J, White E. Cyclic AMP but not phosphorylation of phospholamban contributes to the slow inotropic response to stretch in ferret papillary muscle. Pflügers Arch. 1999;437:780–782. doi: 10.1007/s004240050846. [DOI] [PubMed] [Google Scholar]
- Calaghan SC, White E. Contribution of angiotensin II, endothelin 1 and the endothelium to the slow inotropic response to stretch in ferret papillary muscle. Pflügers Arch. 2001;441:514–20. doi: 10.1007/s004240000458. [DOI] [PubMed] [Google Scholar]
- Chuck LH, Parmley WW. Caffeine reversal of length-dependent changes in myocardial contractile state in the cat. Circ. Res. 1980;47:592–598. doi: 10.1161/01.res.47.4.592. [DOI] [PubMed] [Google Scholar]
- Dassouli A, Sulpice JC, Roux S, Crozatier B. Stretch-induced inositol trisphosphate and tetrakisphosphate production in rat cardiomyocytes. J. Mol. Cell. Cardiol. 1993;25:973–982. doi: 10.1006/jmcc.1993.1109. [DOI] [PubMed] [Google Scholar]
- Ennis IL, Garciarena CD, Perez NG, Dulce RA, Camilion de Hurtado MC, Cingolani HE. Endothelin isoforms and the response to myocardial stretch. Am. J. Physiol. 2005;288:H2925–2930. doi: 10.1152/ajpheart.01202.2004. [DOI] [PubMed] [Google Scholar]
- Grimm M, Haas P, Willipinski-Stapelfeldt B, Zimmermann WH, Rau T, Pantel K, Weyand M, Eschenhagen T. Key role of myosin light chain (MLC) kinase-mediated MLC2a phosphorylation in the alpha 1-adrenergic positive inotropic effect in human atrium. Cardiovasc. Res. 2005;65:211–220. doi: 10.1016/j.cardiores.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Grimm M, Mahnecke N, Soja F, El-Armouche A, Haas P, Treede H, Reichenspurner H, Eschenhagen T. The MLCK-mediated alpha1-adrenergic inotropic effect in atrial myocardium is negatively modulated by PKCepsilon signaling. Br. J. Pharmacol. 2006;148:991–1000. doi: 10.1038/sj.bjp.0706803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, McBride DW., Jr. The pharmacology of mechanogated membrane ion channels. Pharmacol. Rev. 1996;48:231–252. [PubMed] [Google Scholar]
- Hibberd MG, Jewell BR. Calcium- and length-dependent force production in rat ventricular muscle. J. Physiol. 1982;329:527–540. doi: 10.1113/jphysiol.1982.sp014317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holubarsch C, Hasenfuss G, Schmidt-Schweda S, Knorr A, Pieske B, Ruf T, Fasol R, Just H. Angiotensin I and II exert inotropic effects in atrial but not in ventricular human myocardium. An in vitro study under physiological experimental conditions. Circulation. 1993;88:1228–1237. doi: 10.1161/01.cir.88.3.1228. [DOI] [PubMed] [Google Scholar]
- Hongo K, White E, Le Guennec JY, Orchard CH. Changes in [Ca2+]i, [Na+]i and Ca2+ current in isolated rat ventricular myocytes following an increase in cell length. J. Physiol. 1996;491:609–619. doi: 10.1113/jphysiol.1996.sp021243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G, Kazanski V, Kondratev D, Gallitelli MF, Kiseleva I, Kamkin A. Differential effects of stretch and compression on membrane currents and [Na+]c in ventricular myocytes. Prog. Biophys. Mol. Biol. 2003;82:43–56. doi: 10.1016/s0079-6107(03)00004-x. [DOI] [PubMed] [Google Scholar]
- Kamkin A, Kiseleva I, Wagner KD, Bohm J, Theres H, Gunther J, Scholz H. Characterization of stretch-activated ion currents in isolated atrial myocytes from human hearts. Pflügers Arch. 2003;446:339–346. doi: 10.1007/s00424-002-0948-0. [DOI] [PubMed] [Google Scholar]
- Katz AM. Ernest Henry Starling, his predecessors, and the “Law of the Heart”. Circulation. 2002;106:2986–2992. doi: 10.1161/01.cir.0000040594.96123.55. [DOI] [PubMed] [Google Scholar]
- Kentish JC, ter Keurs HE, Ricciardi L, Bucx JJ, Noble MI. Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Influence of calcium concentrations on these relations. Circ. Res. 1986;58:755–768. doi: 10.1161/01.res.58.6.755. [DOI] [PubMed] [Google Scholar]
- Kentish JC, Wrzosek A. Changes in force and cytosolic Ca2+ concentration after length changes in isolated rat ventricular trabeculae. J. Physiol. 1998;506:431–444. doi: 10.1111/j.1469-7793.1998.431bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kockskamper J, Khafaga M, Grimm M, Elgner A, Kockskamper A, von Lewinski D, Post H, Grossmann M, Dorge H, Gottlieb P, Sachs F, Eschenhagen T, Schondube F, Pieske B. Angiotensin II and myosin light chain phosphorylation contribute to the stretch-induced slow force response in human atrial myocardium. 2008 doi: 10.1093/cvr/cvn126. in Revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratev D, Gallitelli MF. Increments in the concentrations of sodium and calcium in cell compartments of stretched mouse ventricular myocytes. Cell Calcium. 2003;34:193–203. doi: 10.1016/s0143-4160(03)00084-8. [DOI] [PubMed] [Google Scholar]
- Konhilas JP, Irving TC, de Tombe PP. Frank-Starling law of the heart and the cellular mechanisms of length-dependent activation. Pflügers Arch. 2002;445:305–310. doi: 10.1007/s00424-002-0902-1. [DOI] [PubMed] [Google Scholar]
- Luers C, Fialka F, Elgner A, Zhu D, Kockskamper J, von Lewinski D, Pieske B. Stretch-dependent modulation of [Na+]i, [Ca2+]i, and pHi in rabbit myocardium-a mechanism for the slow force response. Cardiovasc. Res. 2005;68:454–463. doi: 10.1016/j.cardiores.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Mackenzie L, Bootman MD, Laine M, Berridge MJ, Thuring J, Holmes A, Li WH, Lipp P. The role of inositol 1,4,5-trisphosphate receptors in Ca2+ signalling and the generation of arrhythmias in rat atrial myocytes. J. Physiol. 2002;541:395–409. doi: 10.1113/jphysiol.2001.013411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Lehnart S, Pieske B, Schlottauer K, Munk S, Holubarsch C, Just H, Hasenfuss G. Influence of endothelin 1 on human atrial myocardium--myocardial function and subcellular pathways. Basic Res. Cardiol. 1996;91:86–93. doi: 10.1007/BF00788869. [DOI] [PubMed] [Google Scholar]
- Morano I. Tuning the human heart molecular motors by myosin light chains. J. Mol. Med. 1999;77:544–555. doi: 10.1007/s001099900031. [DOI] [PubMed] [Google Scholar]
- Niederer SA, Smith NP. A mathematical model of the slow force response to stretch in rat ventricular myocytes. Biophys. J. 2007;92:4030–4044. doi: 10.1529/biophysj.106.095463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrow KL, Mammoser A, Suchyna T, Sachs F, Oswald R, Kubo S, Chino N, Gottlieb PA. cDNA sequence and in vitro folding of GsMTx4, a specific peptide inhibitor of mechanosensitive channels. Toxicon. 2003;42:263–274. doi: 10.1016/s0041-0101(03)00141-7. [DOI] [PubMed] [Google Scholar]
- Oswald RE, Suchyna TM, McFeeters R, Gottlieb P, Sachs F. Solution structure of peptide toxins that block mechanosensitive ion channels. J. Biol. Chem. 2002;277:34443–34450. doi: 10.1074/jbc.M202715200. [DOI] [PubMed] [Google Scholar]
- Palomeque J, Sapia L, Hajjar RJ, Mattiazzi A, Vila Petroff M. Angiotensin II-induced negative inotropy in rat ventricular myocytes: role of reactive oxygen species and p38 MAPK. Am. J. Physiol. 2006;290:H96–106. doi: 10.1152/ajpheart.00324.2005. [DOI] [PubMed] [Google Scholar]
- Parmley WW, Chuck L. Length-dependent changes in myocardial contractile state. Am. J. Physiol. 1973;224:1195–1199. doi: 10.1152/ajplegacy.1973.224.5.1195. [DOI] [PubMed] [Google Scholar]
- Perez NG, de Hurtado MC, Cingolani HE. Reverse mode of the Na+-Ca2+ exchange after myocardial stretch: underlying mechanism of the slow force response. Circ. Res. 2001;88:376–382. doi: 10.1161/01.res.88.4.376. [DOI] [PubMed] [Google Scholar]
- Pieske B, Maier LS, Piacentino V, 3rd, Weisser J, Hasenfuss G, Houser S. Rate dependence of [Na+]i and contractility in nonfailing and failing human myocardium. Circulation. 2002;106:447–453. doi: 10.1161/01.cir.0000023042.50192.f4. [DOI] [PubMed] [Google Scholar]
- Sanbe A, Fewell JG, Gulick J, Osinska H, Lorenz J, Hall DG, Murray LA, Kimball TR, Witt SA, Robbins J. Abnormal cardiac structure and function in mice expressing nonphosphorylatable cardiac regulatory myosin light chain 2. J. Biol. Chem. 1999;274:21085–21094. doi: 10.1074/jbc.274.30.21085. [DOI] [PubMed] [Google Scholar]
- Sipido KR, Bito V, Antoons G, Volders PG, Vos MA. Na/Ca exchange and cardiac ventricular arrhythmias. Ann. N. Y. Acad. Sci. 2007;1099:339–348. doi: 10.1196/annals.1387.066. [DOI] [PubMed] [Google Scholar]
- Suchyna TM, Besch SR, Sachs F. Dynamic regulation of mechanosensitive channels: capacitance used to monitor patch tension in real time. Phys. Biol. 2004;1:1–18. doi: 10.1088/1478-3967/1/1/001. [DOI] [PubMed] [Google Scholar]
- Suchyna TM, Johnson JH, Hamer K, Leykam JF, Gage DA, Clemo HF, Baumgarten CM, Sachs F. Identification of a peptide toxin from Grammostola spatulata spider venom that blocks cation-selective stretch-activated channels. J. Gen. Physiol. 2000;115:583–598. doi: 10.1085/jgp.115.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavi P, Han C, Weckstrom M. Mechanisms of stretch-induced changes in [Ca2+]i in rat atrial myocytes: role of increased troponin C affinity and stretch-activated ion channels. Circ. Res. 1998;83:1165–1177. doi: 10.1161/01.res.83.11.1165. [DOI] [PubMed] [Google Scholar]
- Tavi P, Han C, Weckstrom M. Intracellular acidosis modulates the stretch-induced changes in E-C coupling of the rat atrium. Acta Physiol. Scand. 1999;167:203–213. doi: 10.1046/j.1365-201x.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- Tavi P, Weckstrom M, Ruskoaho H. cAMP- and cGMP-independent stretch-induced changes in the contraction of rat atrium. Pflügers Arch. 2000;441:65–68. doi: 10.1007/s004240000403. [DOI] [PubMed] [Google Scholar]
- Todaka K, Ogino K, Gu A, Burkhoff D. Effect of ventricular stretch on contractile strength, calcium transient, and cAMP in intact canine hearts. Am. J. Physiol. 1998;274:H990–1000. doi: 10.1152/ajpheart.1998.274.3.H990. [DOI] [PubMed] [Google Scholar]
- Vila Petroff MG, Kim SH, Pepe S, Dessy C, Marban E, Balligand JL, Sollott SJ. Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+ release in cardiomyocytes. Nat. Cell Biol. 2001;3:867–873. doi: 10.1038/ncb1001-867. [DOI] [PubMed] [Google Scholar]
- von Lewinski D, Stumme B, Fialka F, Luers C, Pieske B. Functional relevance of the stretch-dependent slow force response in failing human myocardium. Circ. Res. 2004;94:1392–1398. doi: 10.1161/01.RES.0000129181.48395.ff. [DOI] [PubMed] [Google Scholar]
- von Lewinski D, Stumme B, Maier LS, Luers C, Bers DM, Pieske B. Stretch-dependent slow force response in isolated rabbit myocardium is Na+ dependent. Cardiovasc. Res. 2003;57:1052–1061. doi: 10.1016/s0008-6363(02)00830-1. [DOI] [PubMed] [Google Scholar]
- White E, Boyett MR, Orchard CH. The effects of mechanical loading and changes of length on single guinea-pig ventricular myocytes. J. Physiol. 1995;482:93–107. doi: 10.1113/jphysiol.1995.sp020502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm J, Kondratev D, Christ A, Gallitelli MF. Stretch induced accumulation of total Ca and Na in cytosol and nucleus: a comparison between cardiac trabeculae and isolated myocytes. Can. J. Physiol. Pharmacol. 2006;84:487–498. doi: 10.1139/y05-134. [DOI] [PubMed] [Google Scholar]
- Zeng T, Bett GC, Sachs F. Stretch-activated whole cell currents in adult rat cardiac myocytes. Am. J. Physiol. 2000;278:H548–557. doi: 10.1152/ajpheart.2000.278.2.H548. [DOI] [PubMed] [Google Scholar]
- Zima AV, Blatter LA. Inositol-1,4,5-trisphosphate-dependent Ca2+ signalling in cat atrial excitation-contraction coupling and arrhythmias. J. Physiol. 2004;555:607–615. doi: 10.1113/jphysiol.2003.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer HG. Who discovered the Frank-Starling mechanism? News Physiol. Sci. 2002;17:181–184. doi: 10.1152/nips.01383.2002. [DOI] [PubMed] [Google Scholar]