SYNOPSIS
Objectives.
This study provided a population-based estimate of the prevalence of smoking during pregnancy by combining information from two data sources: birth certificates (BCs) and a self-administered questionnaire.
Methods.
We analyzed data from 39,345 women who delivered live births in one of 24 states and responded to a questionnaire from the Pregnancy Risk Assessment Monitoring System (PRAMS), an ongoing, state- and population-based surveillance system. We compared prevalence of smoking during pregnancy based on the BC, the PRAMS questionnaire, and the two data sources combined. Data were weighted to represent all women delivering live births in each of the 24 states during 2004.
Results.
The combined estimate indicated that 15.1% of women reported smoking during pregnancy, whereas the BCs alone reported 10.4% and the PRAMS questionnaires alone reported 13.4%.
Conclusions.
Based on the combined BC and PRAMS questionnaire data, the number of infants exposed to tobacco in-utero may be 31% higher than is currently reported on the BCs. Combining the data from the two different sources led to higher ascertainment of prenatal smoking.
While the prevalence of smoking during pregnancy declined 38% from 1990 to 2002,1 it continues to be a major cause of adverse pregnancy outcomes. Infants born to mothers who smoke have an estimated 40% increased rate of mortality.2 These infants have an increased risk of premature birth, low birth weight, and Sudden Infant Death Syndrome (SIDS).3 An estimated 30% of small-for-gestational-age infants, 10% of preterm infants, and 5% of infant deaths are attributable to prenatal smoking.2 In addition to adverse effects on pregnancy outcomes, in-utero exposure to cigarette smoke is associated with increased risk of obesity4 and becoming a smoker later in life.5
Prevalence of prenatal smoking is routinely monitored nationally using data from birth certificates (BCs) and, for 30 states and New York City, using the Pregnancy Risk Assessment Monitoring System (PRAMS). For BCs, maternal smoking history is obtained from maternal medical records/information obtained from the mother at delivery. BCs have high sensitivity (0.89–0.98) but low specificity (0.65–0.72) for prenatal smoking when using maternal smoking documented in the mother's medical record as the gold standard.6–9 In PRAMS, information on maternal tobacco use is collected from the mother at three to six months postpartum through a mailed questionnaire or by telephone for the small percentage of late responders. A comparison of prenatal smoking captured on BCs and PRAMS questionnaires in six states from 1993 to 1996 suggested that the PRAMS questionnaires ascertained more prenatal smokers than the BCs, and that both data sources underestimated the true prevalence of smoking during pregnancy.10 To our knowledge, more recent comparisons of prenatal smoking using BCs and PRAMS questionnaires have not been conducted.
The aim of this study was to update and expand the previous study by including data from 24 states for pregnancies ending in live births in 2004. We estimated the prevalence of prenatal smoking by combining the data from the BCs and the PRAMS questionnaires for the same sample of women. In addition, we assessed differences in characteristics of prenatal smokers identified by each of the data sources.
METHODS
Sample design
PRAMS, which is sponsored by the Centers for Disease Control and Prevention (CDC), was operational in 30 states during 2004. Based on the inclusion criterion of at least a 70% response rate, our analysis was restricted to the following 24 states: Alaska, Arkansas, Colorado, Georgia, Hawaii, Illinois, Louisiana, Maine, Maryland, Michigan, Minnesota, Mississippi, North Carolina, Nebraska, New Jersey, New Mexico, Oklahoma, Oregon, Rhode Island, South Carolina, Utah, Vermont, Washington, and West Virginia. Although Florida met the criteria of a 70% response rate for PRAMS, data from Florida was excluded because it did not collect maternal smoking information on the BC in the standard format. State-specific response rates ranged from 70% to 89%, and sample sizes ranged from 770 to 2,291.
In each PRAMS state, a monthly random, stratified, systematic sample of 100 to 200 women, selected from BCs, were mailed a self-administered questionnaire approximately two to three months after delivery. For women who did not respond to the mailed questionnaires, attempts were made to conduct an interview by telephone. BC and PRAMS questionnaire data were combined into one dataset (n=39,345). The data were weighted to adjust for survey design, noncoverage, and nonresponse and were representative of women delivering a live infant in each respective state. Detailed information about the PRAMS methodology can be found at http://www.cdc.gov/prams/methodology.htm. PRAMS has been approved by CDC's Institutional Review Board.
Variables
In this analysis, 22 of the 24 states used the 1989 revised BC, and two states (South Carolina and Washington) used the 2003 revised BC. The 1989 revised BC included a check box to capture smoking at anytime during pregnancy.11 A woman was considered a prenatal smoker if “Yes” was checked and a nonsmoker if “No” was checked. During 2003, the BC was revised; the new version included items on smoking before pregnancy and during each trimester of pregnancy. For this analysis, prenatal smoking was defined as any answer other than zero for number of cigarettes smoked during any trimester of pregnancy.
The PRAMS smoking question captures smoking during the last three months of pregnancy, and for this analysis, prenatal smoking was defined as any response from “less than one cigarette per day” to “41 or more cigarettes per day.” Women who responded “none (0 cigarettes)” were considered nonsmokers.
For the combined BC and PRAMS questionnaire estimate, smoking status during pregnancy was defined in the following way: women were considered prenatal smokers if they had reported smoking on either source (BC or PRAMS questionnaire) or on both sources. Women were considered nonsmokers when neither source indicated prenatal smoking. If smoking status was missing from one data source, then the information was used from the other data source. If smoking status was missing from both data sources, it was coded as missing for the combined estimate.
We compared characteristics of prenatal smokers identified on the BC, the PRAMS questionnaire, or both data sources. The following maternal characteristics were assessed: age, education, race/ethnicity, marital status, entry into prenatal care and parity (obtained from the BC), and insurance status and Women, Infants, and Children (WIC) program participation (obtained from the PRAMS questionnaire). Infant birth weight from the BC also was investigated.
Statistical analysis
To achieve the primary aim, we computed the combined prevalence of smoking during pregnancy with 95% confidence intervals (CIs) for the entire sample and by state. A kappa statistic was calculated to compare agreement of BC and PRAMS questionnaire estimates of the prevalence of smoking during pregnancy. Two-way t-tests were conducted to evaluate differences in the estimation of prevalence of smoking between the two data sources. Chi-square tests were used to investigate differences in maternal characteristics by reporting source and smoking status. SAS12 and SUDAAN13 were used for the analysis.
RESULTS
Smoking status by data source
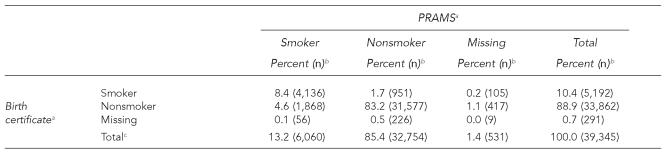
Of the 7,116 women who reported smoking during pregnancy on at least one data source, 4,136 (58.1%) reported smoking on both data sources, 1,056 (14.8%) reported smoking only on the BC, and 1,924 (27.0%) reported smoking only on the PRAMS questionnaire (Table 1). Overall, there was substantial agreement between the self-reported smoking status of the two data sources (kappa = 0.70; 95% CI 0.69, 0.71).
Table 1.
Smoking status during pregnancy by birth certificate and PRAMS questionnaire, 2004
aKappa = 0.70 (0.69, 0.71)
bThe percentages were weighted to adjust for survey design, noncoverage, and nonresponse.
cThe total sample included 39,345 women, of which 7,116 reported smoking during pregnancy on at least one data source (i.e., birth certificate or PRAMS questionnaire).
PRAMS = Pregnancy Risk Assessment Monitoring System
Prevalence of smoking during pregnancy
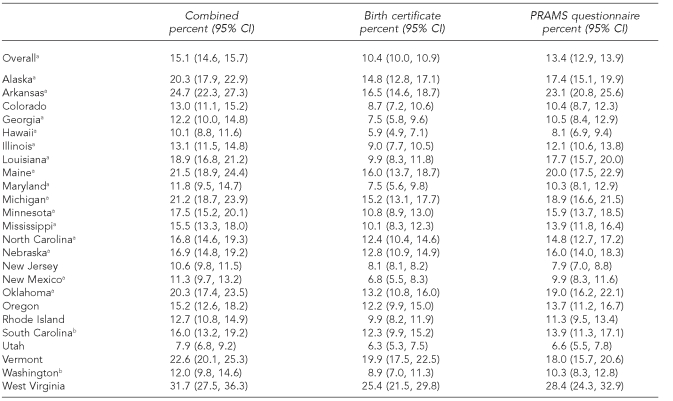
When combining smoking status from both data sources, 15.1% (95% CI 14.6, 15.7) of the women were identified as prenatal smokers in the 24 states (Table 2). When using the BC, 10.4% (95% CI 10.0, 10.9) of the women were identified as prenatal smokers, approximately 31% lower than the combined estimate. While using the PRAMS questionnaire, 13.4% (95% CI 12.9, 13.9) were identified as prenatal smokers, approximately 11% lower than the combined estimate.
Table 2.
Prevalence of smoking during pregnancy using birth certificate and PRAMS questionnaire data by state, 2004
aThe difference between birth certificates and the PRAMS questionnaire as assessed by a t-test, p<0.05
bStates with the 2003 revised birth certificate
PRAMS = Pregnancy Risk Assessment Monitoring System
CI = confidence interval
The state-specific combined estimates of prenatal smoking ranged from a low of 7.9% (95% CI 6.8, 9.2) in Utah to a high of 31.7% (95% CI 27.5, 36.3) in West Virginia. Except in two states, New Jersey and Vermont, the PRAMS questionnaire estimates were higher than the BC estimates; for 15 states, this difference was statistically significant (p<0.05). The two states using the 2003 revised BC (South Carolina and Washington) did not have significantly different BC and PRAMS questionnaire estimates than the other states.
Demographic and birth outcome variables by self-reported smoking status
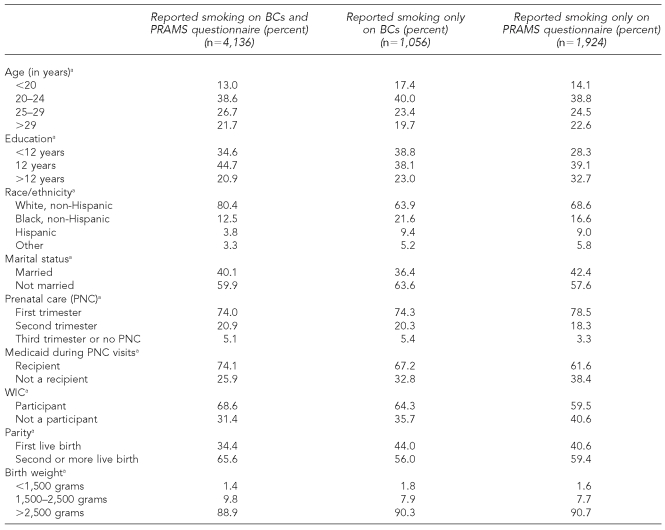
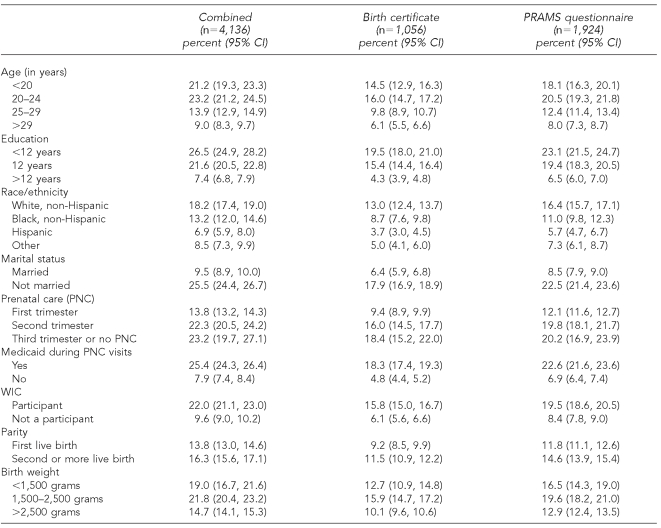
Women with a greater number of years of education and those who were not on Medicaid during pregnancy were significantly more likely to report smoking only on the PRAMS questionnaire (Table 3). Women younger than 20 years of age were more likely to be identified as smokers only on the BC. Women who were identified as smokers only on the BC and women who were identified as smokers only on the PRAMS questionnaire had similar proportions of infants weighing 1,500 to 2,500 grams (7.9% and 7.7%, respectively). Women who were identified on both sources had a higher proportion (9.8%) of infants weighing 1,500 to 2,500 grams. However, the two data sources identified the same groups of women with the highest prevalence of smoking during pregnancy (Table 4). These groups included the following: age younger than 25 years, less than 12 years of education, white non-Hispanic, unmarried, prenatal care initiated after first trimester, Medicaid recipient, WIC recipient, and multiparity.
Table 3.
Demographic characteristics of self-reported smokers by data source, 2004
NOTE: Homogeneity of distribution was assessed with Chi-square tests.
ap<0.0001
BC = birth certificate
PRAMS = Pregnancy Risk Assessment Monitoring System
WIC = Women, Infants, and Children program
Table 4.
Prevalence of smoking during pregnancy by demographic and birth outcome variables, 2004
PRAMS = Pregnancy Risk Assessment Monitoring System
CI = confidence interval
WIC = Women, Infants, and Children program
DISCUSSION
This study provided an estimate of prenatal smoking based on data from two population-based sources for which smoking information was gathered for the same women at two different time periods and using two different methods. When examining the two data sources separately, the prenatal smoking estimate derived from the PRAMS questionnaires was higher than the BC's estimate; however, the combined estimate identified even more smokers than did either data source alone. These results are consistent with a prior study conducted in six states approximately 10 years earlier.10
The combined estimate of 15.1% is likely to be lower than the true prevalence because it is based on self-reported smoking. Studies using biochemical validation have found a wide range in nondisclosure rates, from 6% in a population of pregnant women attending a prenatal clinic in Sweden14 to 73% in a population of pregnant women attending one of four publicly funded clinics in Philadelphia.15 A study conducted in New Zealand using data from the medical record combined with maternal self-reports from a mailed questionnaire found that 22% of the women in the sample wrongly classified themselves as nonsmokers when using biochemical validation.16 Windsor et al. recommended applying a 20% smoking misclassification rate to pregnant women in the U.S.17 If this rate were applied to our sample, the true prevalence of smoking during pregnancy would be 18.1%. The accuracy of self-reported smoking status has been shown to be influenced by many factors, including “characteristics of the individual respondents,” “method and setting of encounter,” “cognitive demands imposed by the question,” and “the motivation of the respondents as mediated by the social desirability of the subject of inquiry.”18 Additional research is needed to investigate how to improve the accuracy of self-reported measures of smoking during pregnancy.
We found systematic differences in the reporting of prenatal smoking by data source. For example, women who were older and more educated were more likely to be identified as smokers on the PRAMS questionnaire and not on the BC. This finding may indicate that these women were more likely to admit smoking in a confidential self-administered questionnaire than to a provider. However, the systematic differences in reporting by data source are unlikely to result in meaningful biases due to their small magnitude. In fact, each data source independently identified the same subgroups of women with the highest prenatal smoking prevalence.
The 2003 revised BC questions were based on research indicating that when women were asked these questions directly, the trimester-specific question had a significantly higher sensitivity and a lower smoking misclassification rate.19 However, in some hospitals, this information is gathered from the medical record, and in these cases, it would be unlikely that these revisions to the BC questions would affect reporting. In these situations, the revised BC may even pose some disadvantages, because smoking status during each trimester is not typically recorded in the medical record. In a separate sub-analysis, we examined the prevalence of prenatal smoking before and after South Carolina and Washington State began using the 2003 revised BC, and we found the prevalence unchanged: South Carolina, 11.8% (95% CI 9.3, 14.4) to 12.3% (95% CI 9.9, 15.2); Washington State, 12.8% (95% CI 10.3, 15.7) to 9.2% (95% CI 7.2, 11.7). As more states adopt the 2003 revised BC, further evaluation of these questions and how the rates compare between the old and new BC questions will be important.
Limitations
Our study has several limitations. First, as mentioned previously, our estimate of the prevalence of prenatal smoking is likely an underestimation of the true prevalence, because both the BC and the PRAMS questionnaire rely on maternal self-reporting of smoking status, which is known to be underreported. Errors in self-reporting can occur for several reasons. Respondents may not fully understand the questions being asked, possibly due to language barriers or poorly written questions. Respondents may fail to report the information to their doctors or on a questionnaire because of the social stigma associated with smoking during pregnancy. Finally, there may be errors in data entry, which could lead to an under- or overreporting of smoking.
Second, PRAMS is a retrospective survey, and recall bias may have affected self-reported smoking. Next, because of the different reference time frames, the BC estimate may include women who smoked during their first two trimesters of pregnancy then quit for the last three months of pregnancy, whereas the PRAMS estimate would not include these women. In a separate sub-analysis of the South Carolina and Washington State estimates, we found that 11 (<0.1%) women reported smoking during the first two trimesters and abstinence during the third trimester; subsequently, these women were included on the BC estimate but not captured on the PRAMS estimate. Lastly, our findings are generalizeable only to the 24 states included in this analysis and not to the U.S. as a whole.
CONCLUSION
Combining smoking status from BCs and PRAMS questionnaires resulted in identification of a greater number of prenatal smokers and, therefore, is likely to generate a more accurate estimate of the prevalence of prenatal smoking than using either data source alone.
Acknowledgments
The authors acknowledge the Pregnancy Risk Assessment Monitoring System (PRAMS) Working Group, which includes the following state collaborators: Albert Woolbright (Alabama), Kathy Perham-Hester (Alaska), Mary McGehee (Arkansas), Alyson Shupe (Colorado), Charlon Kroelinger (Delaware), Jamie Fairclough (Florida), Carol Hoban (Georgia), Sharon Sirling (Hawaii), Theresa Sandidge (Illinois), Joan Wightkin (Louisiana), Kim Haggan (Maine), Diana Cheng (Maryland), Hafsatou Diop (Massachusetts), Violanda Grigorescu (Michigan), Jan Jernell (Minnesota), Vernesia Wilson (Mississippi), Venkata Garikapaty (Missouri), JoAnn Dotson (Montana), Jennifer Severe-Oforah (Nebraska), Lakota Kruse (New Jersey), Eirian Coronado (New Mexico), Anne Radigan-Garcia (New York State), Candace Mulready-Ward (New York City), Paul Buescher (North Carolina), Sandra Anseth (North Dakota), Lily Tatham (Ohio), Dick Lorenz (Oklahoma), Kenneth Rosenberg (Oregon), Kenneth Huling (Pennsylvania), Sam Viner-Brown (Rhode Island), Jim Ferguson (South Carolina), Christine Rinki (South Dakota), Fouad Derrahou (Texas), David Law (Tennessee), Laurie Baksh (Utah), Peggy Brozicevic (Vermont), Michelle White (Virginia), Linda Lohdefinck (Washington), Melissa Baker (West Virginia), Katherine Kvale (Wisconsin), Angi Crotsenberg (Wyoming), and the CDC PRAMS Team, Applied Sciences Branch, Division of Reproductive Health.
Footnotes
This work was completed at The Centers for Disease Control and Prevention (CDC) while the first author had an Association of Schools of Public Health fellowship.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of CDC.
REFERENCES
- 1.Smoking during pregnancy—United States, 1990–2002. MMWR Morb Mortal Wkly Rep. 2004;53(39):911–5. [PubMed] [Google Scholar]
- 2.Salihu HM, Aliyu MH, Pierre-Louis BJ, Alexander GR. Levels of excess infant deaths attributable to maternal smoking during pregnancy in the United States. Matern Child Health J. 2003;7:219–27. doi: 10.1023/a:1027319517405. [DOI] [PubMed] [Google Scholar]
- 3.Department of Health and Human Services (US) The health consequences of smoking: a report of the Surgeon General. Atlanta: DHHS, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health (US); 2004. DHHS 576. [Google Scholar]
- 4.Li C, Goran MI, Kaur H, Nollen N, Ahluwalia JS. Developmental trajectories of overweight during childhood: role of early life factors. Obesity (Silver Spring) 2007;15:760–71. doi: 10.1038/oby.2007.585. [DOI] [PubMed] [Google Scholar]
- 5.Al Mamun A, O'Callaghan FV, Alati R, O'Callaghan M, Najman JM, Williams GM, et al. Does maternal smoking during pregnancy predict the smoking patterns of young adult offspring? A birth cohort study. Tob Control. 2006;15:452–7. doi: 10.1136/tc.2006.016790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiGiuseppe DL, Aron DC, Ranbom L, Harper DL, Rosenthal GE. Reliability of birth certificate data: a multi-hospital comparison to medical records information. Matern Child Health J. 2002;6:169–79. doi: 10.1023/a:1019726112597. [DOI] [PubMed] [Google Scholar]
- 7.Zollinger TW, Przybylski MJ, Gamache RE. Reliability of Indiana birth certificate data compared to medical records. Ann Epidemiol. 2006;16:1–10. doi: 10.1016/j.annepidem.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Roohan PJ, Josberger RE, Acar J, Dabir P, Feder HM, Gagliano PJ. Validation of birth certificate data in New York State. J Community Health. 2003;28:335–46. doi: 10.1023/a:1025492512915. [DOI] [PubMed] [Google Scholar]
- 9.Northam S, Knapp TR. The reliability and validity of birth certificates. J Obstet Gynecol Neonatal Nurs. 2006;35:3–12. doi: 10.1111/j.1552-6909.2006.00016.x. [DOI] [PubMed] [Google Scholar]
- 10.Dietz PM, Adams MM, Kendrick JS, Mathis MP. Completeness of ascertainment of prenatal smoking using birth certificates and confidential questionnaires: variations by maternal attributes and infant birth weight. Am J Epidemiol. 1998;148:1048–54. doi: 10.1093/oxfordjournals.aje.a009581. PRAMS Working Group. Pregnancy Risk Assessment Monitoring System. [DOI] [PubMed] [Google Scholar]
- 11.Tolson GC, Barnes JM, Gay GA, Kowaleski JL. The 1989 revision of the U.S. standard certificates and reports. Vital Health Stat. 1991;4:1–34. [PubMed] [Google Scholar]
- 12.SAS Institute Inc. SAS: Version 9.1.3. Cary (NC): SAS Institute Inc; 2002–2003. [Google Scholar]
- 13.Research Triangle Institute. SUDAAN: Version 9.0. Research Triangle Park (NC): Research Triangle Institute; 2005. [Google Scholar]
- 14.Lindqvist R, Lendahls L, Tollbom O, Aberg H, Hakansson A. Smoking during pregnancy: comparison of self-reports and cotinine levels in 496 women. Acta Obstet Gynecol Scand. 2002;81:240–4. doi: 10.1034/j.1600-0412.2002.810309.x. [DOI] [PubMed] [Google Scholar]
- 15.Webb DA, Boyd NR, Messina D, Windsor RA. The discrepancy between self-reported smoking status and urine cotinine levels among women enrolled in prenatal care at four publicly funded clinical sites. J Public Health Manag Pract. 2003;9:322–5. doi: 10.1097/00124784-200307000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Ford RP, Tappin DM, Schluter PJ, Wild CJ. Smoking during pregnancy: how reliable are maternal self-reports in New Zealand? J Epidemiol Community Health. 1997;51:246–51. doi: 10.1136/jech.51.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Windsor RA, Woodby LL, Miller TM, Hardin JM, Crawford MA, DiClemente CC. Effectiveness of Agency for Health Care Policy and Research clinical practice guideline and patient education methods for pregnant smokers in Medicaid maternity care. Am J Obstet Gynecol. 2000;182:68–75. doi: 10.1016/s0002-9378(00)70492-3. 1 Pt 1. [DOI] [PubMed] [Google Scholar]
- 18.Shaffer HJ, Eber GB, Hall MN, Vanderbilt J. Smoking behavior among casino employees: self-report validation using plasma cotinine. Addict Behav. 2000;25:693–704. doi: 10.1016/s0306-4603(00)00076-9. [DOI] [PubMed] [Google Scholar]
- 19.Kharrazi M, Epstein D, Hopkins B, Kreutzer R, Doebbert G, Hiatt R, et al. Evaluation of four maternal smoking questions. Public Health Rep. 1999;114:60–70. doi: 10.1093/phr/114.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]