Abstract
It is proposed that CCR2+ monocytes are specifically recruited to inflammatory sites, whereas CCR2− monocytes are recruited to normal tissue to become resident macrophages. Whether these subsets represent separate lineages, how differential trafficking is regulated and whether monocytes undergo further differentiation is uncertain. Using a mouse model of autoimmune uveoretinitis we examined monocyte trafficking to the inflamed retina in vivo. We show that bone marrow-derived CD11b+ F4/80− monocytes require 24 to 48 h within the circulation and lymphoid system before acquiring the CCR2+ phenotype and trafficking to the inflamed retina is enabled. This phenotype, and the capacity to traffic were lost by 72 h. Monocyte CCR2 expression followed a similar time course in normal mice indicating that differentiation to an inflammatory phenotype is a constitutive, time-limited property, independent of local inflammatory mediators. Phenotypic analysis of adoptively transferred cells indicated that circulating inflammatory monocytes also differentiate into CD11c+ and B220+ dendritic cells and F4/80+ tissue macrophages in vivo. Our data supports the hypothesis of continuous extravasation and progressive differentiation over time of inflammatory monocytes in the circulation rather than replication within the actively inflamed tissue, and supports the concept of myeloid dendritic cell differentiation from trafficking monocytes under physiological conditions in vivo.
Mononuclear phagocytes are critical mediators both of innate and adaptive immunity, and are potential therapeutic tools for delivering immunosuppressive cytokines or as vaccines, but the successful deployment of such therapeutic strategies requires knowledge of the mechanisms governing successful trafficking and recruitment of these cells to specific tissues, and their potential for further functional differentiation in vivo. Under steady-state conditions in mice, half of the circulating monocytes leave the bloodstream each day (1, 2) entering all tissues of the body. There, they may differentiate into tissue macrophages (1, 2) or myeloid dendritic cells (DC)3 (3-5). They may also differentiate into more specialized forms in particular tissues, examples being osteoclasts in bone (6, 7) or microglia in the CNS, (8, 9) where the process of myeloid monocyte turnover is believed to be very slow. Experimental data on monocyte migration, differentiation and function in steady state and in inflammation in vivo are scarce and there is speculation as to whether maintenance of tissue myeloid cells is achieved by self-renewal, proliferation of precursors in peripheral tissue, or continuous extravasation and differentiation (9-11). In addition, there is controversy as to whether myeloid cells that differentiate into DC are derived from separate lineages or represent differentiation stages from common precursors (12-15).
Innate activation of tissue resident myeloid cells by infection or injury initiates phagocytosis and migration of Ag-bearing cells to draining lymph nodes where lymphocytes are activated (16-18) and a local inflammatory response is initiated. Although myeloid cell-driven inflammation is a protective response to control infection and promote tissue repair within the inflamed tissue, monocytes are also thought to be the primary cell type responsible for cellular pathology and tissue damage, due to their ability to phagocytose foreign particles and apoptotic bodies, act as APCs, secrete cytokines, and release proteolytic enzymes and oxygen radicals (19-21). Are these different functions affected by different subsets of mononuclear phagocytes or are they functional consequences of differentiation and maturation? It is known that chemokines derived from inflammatory sites recruit blood monocytes into the draining lymph nodes, (22, 23) but little is known about how monocytes are recruited to the inflammatory site itself. Some reports have shown the importance of CCR2+ monocytes for the development of inflammation (24-26) and recently two subsets of circulating monocytes have been identified in mice (27). One population corresponds to the main monocyte population of humans, known as CD14+CD62L+CCR2+, and is characterized by recruitment to the inflamed peritoneum. The second subset is similar to human CD16+CCR2− monocytes and is proposed to be a resident cell population recruited to tissues independently of inflammatory stimuli. Although this indicates that specific recruitment of inflammatory and resident monocyte subsets occurs, whether these subsets represent separate lineages and how differential trafficking is regulated requires more thorough investigation.
The experiment of choice would be the adoptive transfer of marked blood monocytes into congenic recipient mice. However, the source of monocytes within the circulation is uncertain and the difficulty of isolating mouse monocytes due to their relative rarity, phenotypic heterogeneity, and potential for functional maturation during extended isolation protocols have hampered in vivo transfer experiments (28). In this study, we compared three different populations of mouse monocytes that can be obtained in larger quantities, including in vitro cultured bone marrow monocytes, resident inactivated peritoneal monocytes, and freshly isolated bone marrow monocyte precursors. These were used to investigate the trafficking of monocytes to the site of inflammation in a model of experimental autoimmune uveoretinitis (EAU) (29, 30). We found that only bone-marrow-derived CD11b+ monocytic cells circulated freely and trafficked efficiently to the retina, and that the inflammatory CCR2+ phenotype was also acquired in normal mice. Even in the presence of an established inflammatory vasculature, trafficking to the site of inflammation occurred over a limited time window. A 24- to 48-h period within the circulation was required before inflammatory monocyte phenotype was acquired and monocytes entered the retina in significant numbers. This phenotype and trafficking ability was short lived, and lost by 72 h. Phenotypic analysis of adoptively transferred cells also indicated that circulating monocytes differentiated into CD11c+ and B220+DC and F4/80+ macrophages. Our data supports the hypothesis of continual influx of monocytes from the circulation at a defined stage of differentiation rather than replication within the actively inflamed tissue, and also supports the concept of myeloid DC differentiation from trafficking monocytes within inflamed tissues.
Materials and Methods
Animals and retinal inflammation model
Eight- to 12-wk-old wild-type C57BL/6 mice were supplied by the Medical Research Facility (University of Aberdeen, Aberdeen, U.K.). Homozygous C57BL/6 mice expressing enhanced GFP (EGFP) under the control of a chicken β-actin promoter and CMV enhancer were obtained from Prof. M. Okabe, Genome Information Research Center (Osaka University, Osaka, Japan) and maintained in the Medical Research Facility at University of Aberdeen. Procedures were approved by the Home Office Regulations for Animal Experimentation (U.K.). Retinal inflammation was induced in wild-type C57BL/6 mice as described (31). Briefly, 500 μg of interphotoreceptor retinoid binding protein peptides 1–20 (GPTHLFQPSLVLD-MAKVLLD; Sigma-Genosys) emulsified in CFA (2.5 mg/ml Mycobacterium tuberculosis) was injected s.c. into one thigh. Mice were administered an additional i.p. injection of 100 μl (1.5 μg) of Bordetella pertussis toxin. Retinal inflammation starts at day 16–18 postinfection with most mice showing mild to moderate disease by day 21 and severe disease by day 28 postinfection (31).
Monocyte preparation
Three different monocyte populations derived from transgenic mice expressing EGFP+ on the β-actin promoter were used in this study. 1) Cultured bone marrow macrophage (CuBM-Mφ) were generated using a protocol developed in our laboratories (32). Briefly, cells were aspirated from the femurs of mice, filtered (30-μm mesh) to form a single cell suspension, and washed and RBC removed by ammonium chloride cell lysis. Cells were plated at 2.5 × 106/ml in 75-cm flasks at 37°C in medium containing M-CSF (10% L929 cell conditioned medium) (32), and at day 6 clusters of developing monocyte/macrophages were harvested. 2) Freshly isolated, lymphocyte-depleted bone marrow monocyte (BM-Mo) were collected from bone marrow cells as described and contaminating T cells and B cells removed by magnetic depletion using CD45R (B220), CD4, and CD8 microbeads (Miltenyi Biotec) according to manufacturer's instructions. Separations were conducted in the cold using ice-cold solutions and 10 μl of beads per 107 cells. Harvested myeloid cells remaining were then used immediately. 3) Lymphocyte-depleted noninduced peritoneal macrophage (P-Mφ) were collected by lavage using RPMI 1640 injected into peritoneal cavities of normal EGFP+ transgenic mice. Lymphocytes were removed by immunomagnetic depletion method previously described. Remaining cells were used for in vivo cell trafficking.
Monocytes prepared for adoptive transfer were phenotyped by LSR flow cytometry (BD Biosciences) as described below. Abs used included CD11b (M1/70), CD11c (HL3), B220 (RA3-6B2), CD3∊ (145-2C11), and DC marker 33D1 were all from BD Biosciences; F4/80 (CI:A3-1), CD115 (MCA1898), and CD205 (DEC205) were from Serotec; murine plasmacytoid DC Ag-1 (mPDCA-1; JF05-1C2) is from Miltenyi Biotec; and CCR2 (33). These Abs were conjugated to FITC, PE, allophycocyanin, PerCP, PerCP-Cy5.5, or biotin as required. Biotin-labeled Abs were detected by addition of streptavidin-allophycocyanin or streptavidin-PE (1/400; BD Biosciences). Negative isotype controls and single positive controls were preformed to allow breakthrough compensation. Gates and instrument settings were set according to forward and side scatter characteristics. For analysis, a single large gate was set to exclude fragmented or clumped cells but include lymphocytes as well as larger more granular myeloid cells. Fig. 1 shows that all three populations of cells were mainly CD11b+. However, F4/80 expression varied among those different types of cells, and up to 25% of peritoneal macrophages (P-Mφ) also expressed CD11c (Fig. 1). CD115 was expressed by 20% of fresh BM-Mo cells and nearly 50% of P-Mφ cells. Few CD3+ were detected in the bone marrow preparations and significant numbers (>1%) of DC-specific markers CD205, 33D1, or murine PDCA-1 were not found (data not shown). Both monocytes and polymorphonuclear cells (PMN) express CD11b and GR1, and due to a lack of monocyte-specific markers it was not possible to physically remove PMN from the BM-Mo preparations. Flow cytometric analysis of CD11b and GR1 expression of BM-Mo preparations indicated that 42% of CD11b+ cells were GR1med consistent with monocyte phenotype and 22% were GR1high, consistent with PMN phenotype (Fig 1b). The CD11b−Gr1− cells (16%) included CD115+ monocytic precursors as well as residual CD3+ and B220+ cells. Confocal analysis of the same population stained with DRAQ5 anthraquinone DNA reagent (Alexis) also showed that the majority of the cells had a mononuclear appearance, the remaining cells having polymorphic nuclei (Fig. 1c). Twenty-four hours after infusion, 57% of transferred EGFP+ BM-Mo cells recovered from the blood of normal recipient mice showed the classical kidney-shaped nucleus associated with monocyte morphology, and other EGFP+ cells showed a more indented nucleus appearing in confocal section as “doughnut” shaped (Fig. 1d). Very few remaining EGFP+ cells displayed polymorphic nuclei, probably due to the short lifespan of PMN cells in the circulation (<24 h). We therefore focused our analysis on EGFP+ BM-Mo cells remaining in the circulation at 24, 48, and 72 h posttransfer.
FIGURE 1.

Phenotype of monocyte populations used in the experiments. a, Different monocyte preparations from EGFP+ mice were immunostained for monocyte/macrophage and DC markers and analyzed by flow cytometry immediately before adoptive transfer. Expression of DC-specific markers CD205, 33D1, and PDCA-1 were not found. Data expressed as a percentage ± SEM of positively stained gated viable cells (n = 3). b, Expression of CD11b and GR1 on freshly prepared BM-Mo cells showing 42% are GR1med and 22% are GR1high. c, Confocal image of freshly prepared BM-Mo cells stained with DRAQ5 anthraquinone DNA reagent showing mononuclear cells, with kidney or doughnut shaped morphology (arrows) or PMN (arrowheads). d, Confocal image of blood cells recovered from a mouse injected with EGFP BM-Mo cells 24 h before. Cytospin of blood leukocytes were stained with DRAQ5 anthraquinone DNA reagent and observed by confocal microscopy. A EGFP+ cell shows a typical monocyte nuclear morphology (*). Bar = 10 μm.
In vivo monocyte trafficking
Scanning laser ophthalmoscopy (SLO)
In vivo monocyte trafficking in mouse retina and ear was studied using our SLO technique (available online at 〈http://bjo.bmjjournals.com/misc/eyemov.shtml〉) (34, 35) with slight modifications. Briefly, mice were anesthetized with an i.m. injection of 0.4 ml/kg Hypnorm (fentanyl/fluanisone; Janssen-Cilag) and 1 ml/kg diazepam (Phoenix Pharmaceuticals) i.p. Eight million (8 × 106) EGFP+ cells in 100 μl of PBS together with 50 μl of 0.05% (w/v) sodium fluorescein (Sigma-Aldrich) were injected via the tail vein. SLO images were recorded simultaneously on videotape (S-VHS) and digitally at 25 frames per second as previously described (35) at different times after cell injection. For each eye, three regions of interest containing one to three veins/venules were recorded for at least 30 min. To image mouse ear, fur was removed with hair removal gel, an extra lens (+8 diopter) was placed 5 mm above the ear to obtain clear image view. In some experiments, trafficking of endogenous myeloid cells in inflamed retina was imaged by injection of 30 μl of FITC-conjugated rat anti-mouse CD11b mAb through the tail vein 10 min before SLO. Due to the weak signal of CD11b+ cells in vivo, high laser power of 1.2 mW was used in some experiments and the images were recorded for a shorter time of 15 min. This power level is lower than the threshold limit of tissue damage (36).
Image analysis
Video analysis was conducted offline as described (35). Rolling leukocytes and those not interacting with the endothelium were counted in each venule. Rolling cells were defined as those cells with a velocity below the critical velocity (37, 38). The rolling efficiency was calculated as the percentage of rolling fluorescent cells (either adoptively transferred EGFP+ cells or endogenous anti-mouse CD11b Ab labeled cells) among the total number of fluorescent cells entering a venule.
Tracking EGFP+ monocytes in the retina and other tissues
A total of 8 × 106 (150 μl) EGFP+ cells were injected via the tail vein into normal or day 21–28 postinterphotoreceptor retinoid binding protein peptide immunized EAU mice. In some experiments, EGFP+ cells were injected i.p. or s.c. At different times after cell infusion, 50 μl of 2% Evans blue (Sigma-Aldrich) was injected via the tail vein and allowed to bind for 5–10 min. The eyes, spleen, mesenteric and cervical lymph nodes, liver, thymus, kidney, lung, heart and muscles tissues were then harvested and fixed in 2% (w/v) paraformaldehyde (Agar Scientific). Retinal whole mounts were prepared as described elsewhere (39, 40). Other tissues were cryoembedded in OCT compound and frozen sections prepared. Both retinal whole mounts and tissue sections were observed using a confocal scanning laser microscope (LSM 510 META; Carl Zeiss). For tissue sections both He-Ne laser 488 and 543 nm wavelengths were used to distinguish between autofluorescence and EGFP+ cells. For statistical analysis, three sections were obtained from each tissue and three images were taken randomly from each section using ×20 objective lens. Migrating cells in the tissue were expressed as the number of cells per square millimeter.
In some experiments spleens were removed from recipient mice either 24 h before or 24 h after BM-Mo cell transfer. Control mice were sham operated.
Flow cytometry
At different times after cell infusion, EGFP+ cells were recovered from the spleen and blood. Single cell suspensions were blocked with 1% normal rat serum and stained for CD11b (M1/70), CD11c (HL3), CD11a (M17/4), B220 (RA3-6B2), all from BD Biosciences; F4/80 (CI:A3-1) and CD115 (MCA1898) from Serotec; and CCR2 (33). Samples were detected by LSR flow cytometry (BD Biosciences). These were conjugated to FITC, PE, allophycocyanin, PerCP, PerCP-Cy5.5, or biotin as required. Biotin-labeled Abs were detected by addition of streptavidin-allophycocyanin or streptavidin-PE (1/400; BD Biosciences). Negative isotype controls and single positive controls were performed to allow accurate breakthrough compensation. Gates and instrument settings were set according to forward and side scatter characteristics and populations gated to exclude dead or clumped cells. Data were collected from at least three individual animals in each group and expressed as means and SEM and compared using an unpaired Student t test.
Confocal microscopy of differentiated cells
In some EAU recipient mice, 24 and 48 h after adoptive transfer of EGFP+ BM-Mo cells, retinas were dissected (39, 40) and fixed with 2% (w/v) paraformaldehyde (Agar Scientific). Retinal tissues were permeabilized with 0.3% (w/v) Triton X-100 and then double-stained with either anti-mouse F4/80 (1/20, PE-conjugated; Serotec) and CD11c (1/20, allophycocyanin-conjugated; BD Biosciences) or CD11c and B220, or CD11c and CD8α (1/20; all from BD Biosciences). After thorough washing to remove excess fluorescent label, whole retinal tissues were mounted on slides, vitreous side up, and observed by confocal microscopy (LSM510 META).
Image analysis and statistics
Confocal images were analyzed using Image-Pro Plus system (Media Cybernetics). Infiltrating cells in the tissue at different time points were compared using one-way ANOVA Tukey test. Difference between the populations of different monocyte subsets at 24 and 48 h were compared using Student's t test. Cell velocities and shear stress at the ear and retinal circulation were compared with Student's t test. Probability values of p < 0.05 were considered statistically significant.
Results
Monocyte trafficking in normal mice
Monocyte trafficking in retinal and ear circulation
In vivo trafficking of the three different monocyte populations was analyzed by SLO (35) at different time points posttransfer. In normal mice, immediately after adoptive transfer, large numbers of either BM-Mo or P-Mφ cells could be observed circulating in both the neural vasculature of the retina (Fig. 2, a and c) and the normal, fenestrated vasculature of the ear (Fig. 2b). In contrast, few CuBM-Mφ circulated in the blood stream of normal mice (Fig. 2c). Twenty-four hours after cell infusion, only adoptively transferred BM-Mo cells continued to circulate in significant numbers (>100 cells/min), very few P-Mφ cells or CuBM-Mφ cells (<1 cell/min) could be detected (Fig. 2c).
FIGURE 2.

In vivo monocyte trafficking in normal mice using SLO. Eight million monocytes from EGFP+ mice were adoptively transferred into normal C57BL/6 mice. Monocytes were tracked using SLO in the retina (a) and ear (b). Moving cells have a striped appearance due to raster effects of scanning image collection (arrowheads) whereas stationary cells are bright (*). OD, optic disc; A, arterioles; V, venules. Bar = 100 μm. c, The number of adoptively transferred EGFP+ cells per minute counted in the retinal circulation 30 min and 24 h after cell infusion. Data shown are the mean ± SEM from three animals.
Immediately after transfer, both BM-Mo and P-Mφ were observed moving fast in the retinal blood vessels of normal mice. No cells were observed to roll in any retinal vessels. Occasionally cells would abruptly stop for a few seconds in retinal venules or capillaries without rolling and then move away (supplementary video 1).4 However, in normal mouse ear, an average of 10.58% adoptively transferred circulating BM-Mo cells rolled on the venule endothelium (supplementary video 2). Some cells were found sticking in the venules and capillaries. These rolling and sticking cells may have been contaminating neutrophils in our preparations at this time point. As neutrophils have a very short half-life in the circulation, neutrophils would be predicted to been marginated and removed by 24 h postadoptive transfer. The velocities of BM-Mo cells in normal retinal arterioles and venules were 21.63 ± 1.12 mm/sec and 17.18 ± 1.05 mm/sec, respectively, similar to splenocytes observed previously by us in normal B10.RIII mice (35, 41, 42). In the ear, cell velocities were significantly slower, 18.55 ± 2.47 mm/sec in the arterioles and 13.39 ± 1.14 mm/sec in the venules (p < 0.05 compared with the velocities in retinal vessels). The corresponding shear stress in the arterioles and the venules of the ear were 30.45 ± 3.55 dyn/cm2 and 10.98 ± 0.98 dyn/cm2, respectively, which is significantly lower than that of retinal vessels (35, 41).
Monocyte trafficking in other tissues
Confocal microscopy was used to assess numbers of cells entering the retina (40, 43) or other tissues. EGFP+-CuBM-Mφ cells that were lost from the blood circulation immediately after i.v. adoptive transfer were detected in large numbers in the lung 1 h after cell infusion (Fig. 3a). Few cells were detected in the liver and spleen, and no cells were detected in the lymph nodes, heart, thymus, kidney or retina. Twenty-four hours later they were largely absent from all tissues suggesting that these cultured macrophages were rapidly cleared from the system soon after transfer. Small numbers (2–3/mm2) of EGFP+ P-Mφ cells were detected in the spleen, liver, and lung at 24 h (Fig. 3b), but these were cleared from the tissues by 48 h postadoptive transfer. A proportion of adoptively transferred EGFP+ P-Mφ lost from the circulation after 24 h were detected trafficking back into the peritoneal cavity (3.06% of total peritoneal cells, data not shown).
FIGURE 3.

Recruitment of monocytes to the tissues in the absence of inflammation. Eight million different types of EGFP+ monocytes were adoptively transferred into normal C57BL/6 mice. At the indicated time points, organs were collected from the recipient mice and cryosections prepared. EGFP+ cells were detected using confocal microscopy. a, Adoptive transfer of CuBM-Mφ cells. Large numbers of fluorescent monocytes were observed in lung 1 h posttransfer. Few cells trafficked to lymphoid tissues. Virtually all fluorescent cells were cleared from tissues by 24 h. b, Adoptive transfer of resident (unstimulated) P-Mφ. Small numbers of fluorescent cells observed in lymphoid tissue and lung 24 h posttransfer. No fluorescent cells were detected in tissue examined at later time points. c, Adoptive transfer of freshly isolated BM-Mo cells. Substantial numbers of transferred cells localized within lymphoid tissues. Numbers of transferred cells per square millimeter remained relatively stable over time, large numbers of viable fluorescent cells persisting at 48 and 72 h posttransfer. Data expressed as mean ± SEM of numbers of fluorescent cells per square millimeter of tissue (n = 3). *, p < 0.05; **, p < 0.01, compared with previous time point measured, using multiple Tukey test.
In contrast, EGFP+ BM-Mo cells survived well in the tissues, the majority being found within the spleen and lymph nodes. Forty-eight hours after adoptive transfer a significant number of EGFP+ cells were also detected in the recipient bone marrow (0.01% of total recipient BM-Mo, data not shown). Although adoptively transferred EGFP+ BM-Mo cells were present within the blood circulation for >72 h, many cells were found in the spleen and lymph nodes (Fig. 3c). The number of cells detected in the lymphoid tissues reached a peak at 48 h (30–50/mm2) (Fig. 3c). Few cells were detected in the lung or liver (Fig. 3c). No EGFP+ cells were detected in the normal retina in any of these experiments.
Monocyte trafficking in EAU mice
When T cells are adoptively transferred into mice with EAU, T cell adhesion to retinal venules occurs within a few minutes and within 1 h T cells have crossed the endothelium and can be found within the retinal parenchyma (43). By comparison, in this study we found that adoptively transferred monocytes took much longer to infiltrate the inflamed retina even when transferred to mice with well-established disease and in which blood-retina barrier breakdown in venules could be demonstrated by extensive Evan's blue leakage. This implies that monocyte infiltration is critically dependent on differentiation to an inflammatory phenotype as proinflammatory chemokine gradients and permissive endothelial cell changes were already well established. When EGFP+ BM-Mo cells (8 × 106 cells/150 μl/mouse) were adoptively transferred into day 21–28 postimmunization EAU mice via the tail vein significant numbers of monocytes were not observed within the inflammatory tissue until 16 h posttransfer. By 48 h as many as 348.0 ± 90 BM-Mo cells had infiltrated the inflamed retina, but homing to the inflamed retina by the other monocyte cell populations was much less efficient with only 85.6 ± 20.2 P-Mφ cells and 7.8 ± 0.5 CuBM-Mφ cells per retina counted. For this reason, only EGFP+ BM-Mo cells were used in subsequent experiments.
SLO analysis of in vivo BM-Mo trafficking within inflamed vasculature
In the 30 min following adoptive transfer, very few cells were observed rolling in inflamed retinal vessels (see supplementary video 3). However, 24 h later many more EGFP+ cells rolled and the rolling EGFP+ cells reached a peak at 48 h, but by 72 h after adoptive transfer, the number of rolling cells was reduced significantly (Fig. 4a). To determine whether the treatment of the adoptively transferred cells had altered rolling efficiency, we compared them with fluorescent Ab labeled endogenous CD11b+ monocytes trafficking in inflamed retina. The rolling efficiency of endogenous CD11b+ cells was 31.84%, similar to that of adoptively transferred EGFP+ BM-Mo cells at 48 h. Almost all of the rolling cells were observed in retinal venules. However, occasionally, adoptively transferred EGFP+ BM-Mo cells were also observed rolling in the arterioles of EAU mice (1–3 cells/30 min).
FIGURE 4.
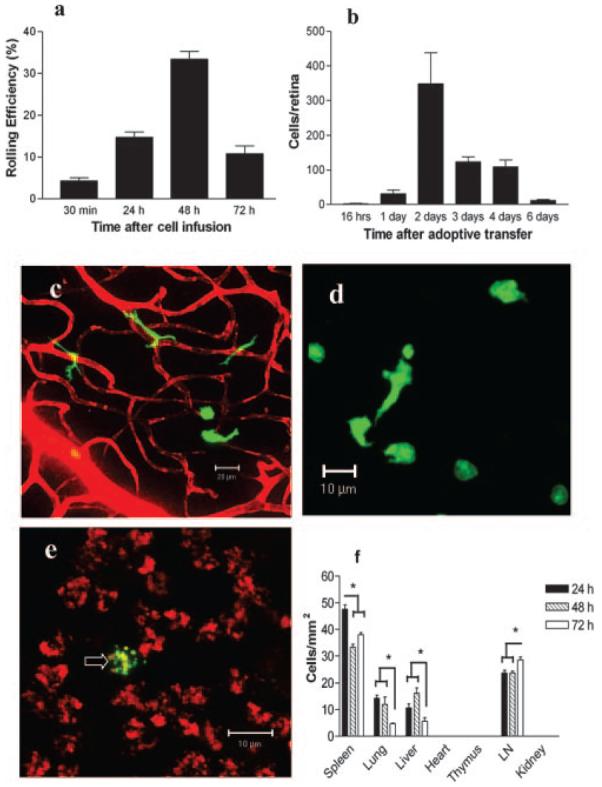
Monocyte trafficking in retina inflamed mice. Eight million EGFP+ BM-Mo cells were adoptively transferred into mice with EAU (day 21–28 postinfection). Cell trafficking in the retina was studied by SLO (a) and confocal microscopy of retinal whole mounts (b–e) and tissue sections (f). a, Rolling efficiency of adoptively transferred EGFP+ BM-Mo cells in retinal venules at different time points. Data are expressed as a percentage of fluorescent cells passing through the vessel observed to roll on the venule endothelium. b, Absolute number of EGFP+ cells numbers infiltrating the inflamed retina at different time points. c, Infiltrating EGFP+ cells within the retina 24 h after transfer. Note dendriform morphology of infiltrating cells. Retinal vessels and areas of venule leaking visualized using Evans blue dye. d, Infiltrating EGFP+ cells at the inflamed retina/vitreous surface 48 h after transfer. Note more rounded morphology of these cells. e, Infiltrating EGFP+ cells in the inflamed retina 6 days after transfer. The extent of retinal inflammation is apparent from leaked Evans blue staining of endogenous infiltrating cells; a fragmented EGFP+ cell is arrowed. f, Trafficking of EGFP+ to other tissues in mice with EAU at different times after cell infusion. Data expressed as mean ± SEM of numbers of fluorescent cells per square millimeter tissue (n = 3). *, p < 0.05.
Ability of monocytes to migrate into the inflamed retina
Infiltration of adoptively transferred EGFP+ BM-Mo cells in the inflamed retina was not observed until at least 16 h after cell infusion (Fig. 4b). Numbers increased rapidly and peaked at 48 h, then declined rapidly. By day 6 few cells remained. Infiltrating EGFP+ BM-Mo cells displayed dendriform shape around the blood vessels (Fig. 4c), but EGFP+ cells migrating into the vitreous lost their dendriform shape, adopting a round or irregular shape (Fig. 4d). The proportion of large dendriform EGFP+ cells among the total EGFP+ cells detected in the inflamed retina reduced significantly from 24 h (73.27 ± 3.14%) to 48 h (37.55 ± 2.20%) (n = 6, p < 0.01) post cell injection. By day 6, only a few EGFP+ cells could be detected in the inflamed retina. They appeared as round, condensed and fragmented cells consistent with the morphology of apoptotic cells (Fig. 4e). No dendriform EGFP+ cells were observed at that time point.
To investigate whether infiltrating monocytes in inflamed retina could also be recruited from other tissue sites, we injected EGFP+ BM-Mo cells s.c. and EGFP+ P-Mφ cells i.p. and s.c. Very few BM-Mo cells migrated into the inflamed retina from the skin (1.2 ± 0.5, n = 6) or the peritoneal cavity (8.2 ± 2.2, n = 6), consistent with the paradigm that monocytes within peripheral tissues do not re-enter the blood circulation. Small numbers of P-Mφ cells also migrated to the inflamed retina from the peritoneal cavity (9.2 ± 2.1, n = 6) and from the skin (13.7 ± 4.2, n = 6). This result suggests that during inflammation some monocyte/macrophages from other tissue sites might be able to traffic back into the blood circulation and migrate into inflammatory sites. However numbers were very small compared with numbers migrating from the blood and may represent cells re-entering circulation at the injection site via blood vessels breached during s.c. injection.
Trafficking of EGFP+ BM-Mo cells into nonlymphoid tissues was largely unaffected by retinal inflammation at 48 h (Fig. 4f). In the spleen a significant reduction in the numbers of EGFP+ cells was observed at both 48 and 72 h time points (p < 0.05). Although in the draining lymph nodes, there was a significant increase in the EGFP+ cell numbers at 72 h (p < 0.05). This is consistent with increased traffic of monocytes from the blood during inflammation (2) and from the inflammatory site to a draining node (5). No dividing EGFP+ cells were observed in lymphoid tissue or eye (see below) and in other studies using CFSE as labeling for transferred cells, no significant diminution of fluorochrome, consistent with cell division was noted by flow cytometry (data not shown).
Phenotype of trafficking EGFP+ BM-Mo cells in EAU mice
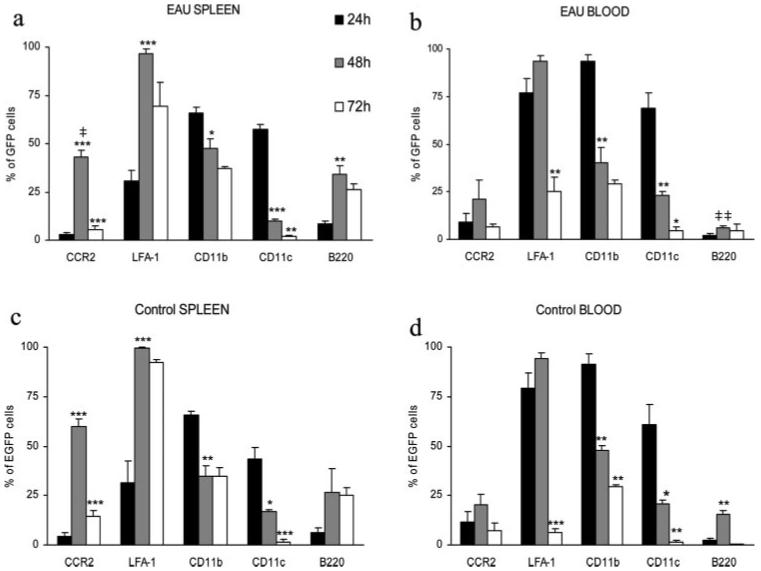
Fig. 5 shows the phenotype of EGFP+ BM-Mo cells recovered from the blood and spleens of control mice and mice with EAU at different time points after adoptive transfer. With the exception of CCR2 expression by spleen monocytes and B220 expression by blood monocytes, no real differences were found between data from EAU mice (Fig. 5, a and b) and control mice (Fig. 5, c and d). In spleen from control mice, CCR2 was expressed by 60 ± 3.8% recovered EGFP+ cells compared with 43.1 ± 3.3% cells from mice with EAU. This reduction was significant (p < 0.03). Fig. 5 also shows that although cells were CD11b+CD11clow/− at time of transfer (Fig. 1b), by 24 h, around 50% of EGFP+ cells in both the spleen and the blood were also expressing CD11c. This phenotype was rapidly lost however, and by 48 h numbers of cells expressing CD11c were very significantly reduced both in the spleen (p < 0.001) and the blood (p < 0.01) in both control and diseased mice. At 48 h numbers of EGFP+ cells expressing B220 were also increased in the spleen of EAU mice (p < 0.01). At 24 h, levels of CCR2 were very low in EAU spleen and blood but by 48 h, the time point coinciding with maximum cell rolling and peak numbers of EGFP+ cells within the inflamed retina, CCR2 was up-regulated and this was very significant in spleen (p < 0.001). LFA-1 expression was also maximal at this time point, and the increased expression on spleen EGFP+ cells was again highly significant (p < 0.001). In contrast, CD11b expression steadily decreased over time with significant reduction on recovered EGFP+ cells at 48 h in spleen and blood (Fig. 5, a and b).
FIGURE 5.
Phenotype of adoptively transferred EGFP+ BM-Mo cells recovered from spleen and blood. Eight million EGFP+ BM-Mo cells were adoptively transferred into EAU (a and b) or control (c and d) C57BL/6 recipient mice. At the indicated time points, EGFP+ cells were recovered from the spleen (a and c) and blood (b and d) and their phenotype analyzed by flow cytometry. Gates were set to include only viable lymphoid cells and then transferred cells identified and gated by plotting EGFP+ population against forward scatter or side scatter parameters. No significant difference was found between data acquired on either forward or side scatter gated EGFP+ populations. Data expressed as a percentage of positively stained cells ± SEM in side scatter gated EGFP+ population (n = 3) (*, p < 0.05; **, p < 0.01; ***, p < 0.001, using unpaired Student's t test compared with previous time point. ‡, p < 0.03; ‡‡, p < 0.01 compared with data from control group at the same time point.
To determine whether the spleen was essential for inflammatory monocyte differentiation and trafficking capability we repeated our experiments using immunized mice with EAU that had their spleens removed either 1 day before or 1 day after adoptive transfer of EGFP+ BM-Mo. Control mice had no treatment or were sham operated. No significant differences in the number of cells infiltrating the retina were observed (data not shown) and EGFP+ cells recovered from the blood showed no differences in phenotype compared with controls (Table I).
Table I.
Splenectomy has no effect on inflammatory monocyte differentiation
| CCR2 | LFA-1 | CD11b | CD11c | |
|---|---|---|---|---|
| Controla | 28.0 ± 10.0b | 26.7 ± 7.1 | 73.7 ± 7.1 | 25.3 ± 2.8 |
| EAU controlc | 26.7 ± 4.7 | 50.7 ± 11.6 | 67.7 ± 7.1 | 21.3 ± 7.0 |
| EAU splenectomy Ad | 30.0 ± 13.5 | 23.0 ± 8.0 | 39.7 ± 10.0 | 22.0 ± 0.6 |
| EAU splenectomy Be | 24.5 ± 5.7 | 23.3 ± 8.3 | 49.5 ± 3.2 | 23 ± 6.8 |
Normal mice, sham operated.
Percent of EGFP+ cells gated from blood positively expressing Ag. Data from three mice expressed as means ± SEM.
Mice with EAU, sham operated.
Mice with EAU, spleens removed 1 day before adoptive transfer of EGFP cells.
Mice with EAU, spleens removed 1 day after adoptive transfer of EGFP cells.
EGFP+ BM-Mo differentiation in inflamed retina
Migrating monocytes were analyzed from different levels within the retina. At the retina-vitreous interface (inner limiting membrane of the retina), endogenous F4/80+ or CD11c+ cells were round or spindle shaped (Fig. 6, a and b), whereas in retinal photoreceptor layers (the target tissue of the autoimmune reaction) endogenous F4/80+ and CD11c+ monocytes were mostly in dendriform shape (Fig. 6, c and d). This morphology was consistent with observed morphology of infiltrating EGFP+ monocytes in similar anatomical locations at 24 h postadoptive transfer (Fig. 4, c and d). Confocal analysis of retinal whole mounts for tissue macrophage marker F4/80 and the DC subset markers CD11c, CD8α, or B220 was conducted to further define phenotype of EGFP+ cells within the inflamed retina at 24 and 48 h posttransfer. In addition to F4/80+ macrophages and single positive CD11c+ classical DC, some evidence for interstitial DC (CD11c+ F4/80+) (44) and plasmacytoid DC (CD11c+B220+) (45) was also found (Fig. 7a). Small numbers of CD8α+ have been observed in the retina during EAU (46) but no EGFP+ CD8α+ cells were identified in this study.
FIGURE 6.
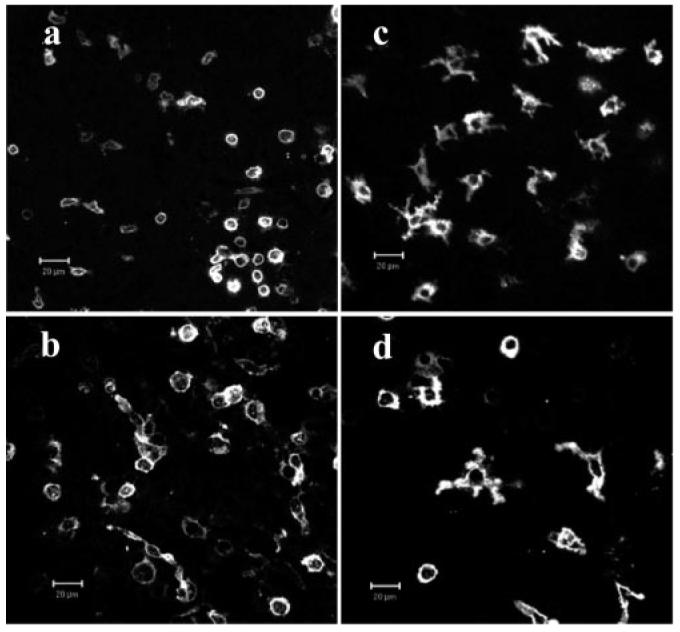
Endogenous DC and macrophages in inflamed retina. Retinal whole mounts from mice with EAU were immunostained with PE-conjugated mAb to CD11c+ (a and c) or F4/80+ (b and d) and imaged at retinal-vitreous interface (a and b) or within photoreceptor layer (c and d) of the retina by confocal microscopy. Endogenous CD11c+ DC (a) and F4/80+ macrophages (b) display small round or spindle shapes in the retinal surface-vitreous interface (a and b) and dendriform shape in the photoreceptor layer (c and d). Bar = 20 μm.
FIGURE 7.
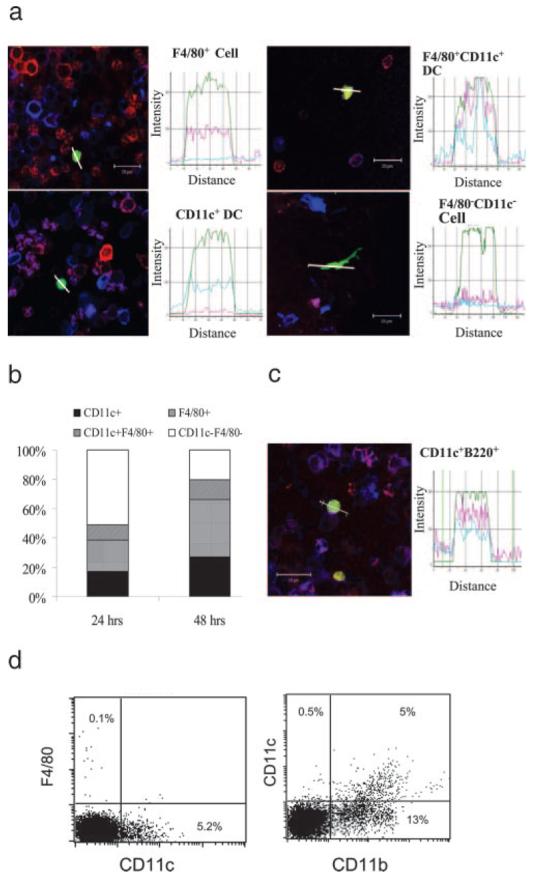
Phenotype of differentiating monocytes in inflamed retina. a and b, Infiltrating adoptively transferred EGFP+ BM-Mo in inflamed retina differentiate into DC and tissue macrophages. At 24 and 48 h after adoptive transfer, retinal whole mounts were stained with R-PE anti-mouse F4/80 and allophycocyanin anti-mouse CD11c and observed by confocal microscopy. a, Merged confocal images of infiltrating EGFP+ cells analyzed using Image-Pro Plus software. Histograms show the fluorescent intensity in the line paths of the EGFP+ cells in the confocal images. The x-axis represents the distance measured across the cell, represented by the white line on the image, whereas the y-axis indicates fluorescence intensity. b, Composite bar graph shows proportion of phenotypic subsets of EGFP+ cells in the retina at 24 and 48 h posttransfer. c, Confocal image shows an infiltrating EGFP+ cell is positively stained for CD11c+-PE and B220+-allophycocyanin. Bar = 20 μm. d, FACS analysis of blood from EAU mouse showing that F4/80 expression on circulating monocytes and DC was negligible (0.1%), whereas 5% CD11b+ cells coexpressed CD11c.
Coexpression of PE and allophycocyanin fluorescence intensities by EGFP+ cells was confirmed using Image-Pro Plus software analysis of merged confocal digital images, and percentage of single- or dual-labeled cells calculated. At least 200 cells per retina were included in the analysis. Fig. 7b shows that the number of F4/80+ or CD11c+ cells increased significantly at 48 h compared with 24 h post cell infusion (p < 0.05). It is of note that the proportion of EGFP CD11c+ cells within the retina at 24 h (17% CD11c+, 10% CD11c+ and F4/80+) is lower than EGFP CD11c+ cells in EAU blood (>70%) (Fig. 5), and as F4/80 was not expressed by circulating EGFP+ cells suggests that CD11c+ cells do not traffic freely from the blood into the retina. Lack of F4/80 expression by blood monocytes in general was confirmed by flow cytometry. Using mouse serum blocked samples to avoid nonspecific labeling of via FcR binding and directly conjugated Abs, flow cytometric analysis of F4/80 expression by all blood leukocytes was found to be <0.5%, whereas CD11c was expressed by 5% of all blood leukocytes (Fig. 7d). This is in accordance with other studies that show F4/80+ expression is consistent with a mature phenotype and normally restricted to tissue monocyte/macrophages (5, 11). At 48 h posttransfer the proportion of DC within the retina increased slightly (27% CD11c+, 13% CD11c+ F4/80+). This was in excess of EGFP CD11c+ circulating cells in EAU blood at same time point (25%) (Fig. 5) indicating either further maturation and differentiation of transferred cells within the inflamed retina or accumulation of CD11c+ cells over time within the lesion.
Our data therefore suggests that infiltrating, adoptively transferred monocytes continued to differentiate and mature within the retina. CD11c+ B220+ adoptively transferred cells were also present in significant numbers at 48 h in the inflamed retina (12.1% in total EGFP+ CD11c+ cells) (Fig. 7c). Few EGFP+ B220+ cells were present in blood at either 24 or 48 h posttransfer (Fig. 5b), suggesting that this phenotype was also acquired within the retinal tissue.
Discussion
Much of the extensive research into leukocyte trafficking has focused on neutrophils and T cells (47, 48). Intrinsic mononuclear myeloid cell diversity has made specific studies on monocyte trafficking more difficult (28, 49). Recently it has become clear that circulating monocytes recruited to the tissues can undergo differentiation into diverse tissue resident macrophage populations or myeloid DC (17, 21, 27). Other studies have tracked monocyte migration from blood or from normal or inflamed peripheral tissue to draining lymph nodes where differentiation into functional DC can occur (5, 22). Much of this information has been derived from in vitro experiments or experiments using endogenous monocyte populations, and many questions concerning the trafficking and differentiation of monocytes under physiological conditions in vivo remain. In particular, studies on the interactions between monocyte and blood endothelial cells controlling trafficking to normal or inflamed tissue in vivo are required for therapeutic applications (50, 51).
In this study we have developed an in vivo monocyte trafficking model to address these questions and shown that only freshly isolated CD11b+ bone marrow cells transferred i.v. recirculated freely. This population also included CD11b+ GR1high PMN, but these were predicted to be marginalized and largely removed from the circulation within 24 h and therefore unlikely to have unduly influenced our data collected at later time points. The transferred cells also exhibited similar rolling efficiency to endogenous CD11b+ mononuclear cells and migrated into inflamed tissues adopting similar morphology to endogenous myeloid cells. The life span of these cells in the circulation and lymphoid tissue was ∼3 days, in agreement with seminal studies by van Furth and Cohn (1) conducted >30 years ago, but up to 6 days within the inflammatory lesion. Monocytes from other sources that expressed F4/80 Ag or were passively transferred s.c. or via the peritoneal cavity were unable to recirculate and traffic freely. Our data therefore supports the concept of time-dependent constitutive differentiation of monocytes within the circulation into inflammatory monocytes and CD11c+ DC, with further differentiation into F4/80+ macrophages or B220+ DC within inflamed tissue.
Monocytes normally do not cross the blood-brain barrier, but pathogenic mononuclear phagocytes that have trafficked from the blood are the main cell type found in most CNS inflammatory reactions. We therefore studied the ability of BM-derived monocytes to traffic to a localized inflammatory site within the retina where leukocyte-endothelial cell reactions can be imaged noninvasively (35, 41). We found that monocytes required 24 – 48 h within the circulation before acquiring the ability to roll on neural microvascular endothelium at an established site of inflammation and migrate into the inflammatory site. This migratory capacity correlated with maximum LFA-1 expression and the acquisition of the “inflammatory” CCR2+ phenotype (27, 52). The CCR2+ phenotype and migratory capacity was lost by 72 h postadoptive transfer. As monocytes were transferred to mice with well-established retinal inflammation where blood–retina barrier breakdown had occurred, this implies that critical changes permitting monocyte infiltration occurs at the level of the monocyte rather than at the level of a permissive endothelium, further supporting the concept of a distinct inflammatory monocyte phenotype.
Mice deficient in CCR2 or CCL2 are largely resistant to experimental autoimmune encephalitis suggesting that neural inflammation is CCR2- or CCL2-dependent and CCR2 expression is indeed consistent with the ability of monocytes to traffic to inflammatory sites (53). We found maximum CCR2 expression (60%) on transferred monocytes within the spleen of normal mice 2 days postadoptive transfer. This was significantly reduced in spleens of mice with ocular inflammation (40%; p < 0.03). Our data therefore suggests that differentiation to a CCR2+ inflammatory phenotype is a constitutive, time-limited property of monocytes, independent of inflammatory cytokines or chemokines. The significantly reduced numbers of CCR2+ cells within the spleen and blood of animals with EAU is also consistent with inflammatory monocyte recruitment to inflamed tissue (22). In common with other mouse models of inflammation, (40) CCR2 is widely expressed within the retina in EAU and is readily detectable using the Ab used in this study in acetone-fixed cryosections (I. J. Crane, unpublished observations) but is less effective in paraformaldehyde fixed retinal whole mounts where epitopes are less well preserved. This precluded detailed confocal analysis of retinal CCR2 distribution in this study. Blood monocytes can differentiate into tissue macrophages or myeloid DC. We therefore examined the potential of our trafficking monocytes to differentiate in vivo by analyzing the phenotype of adoptively transferred cells recovered from the blood and spleen and in retinal whole mounts from EAU mice. On isolation <5% of CD11b+ bone marrow were CD11c+, but after 24 h in the circulation over 50% of cells became CD11c+. Whether these DC differentiated directly from monocytes is uncertain. It is probable that our initial BM monocyte population contained both myeloid derived DC precursors as well as true monocyte precursors and expression of fms-related tyrosine kinase 3 by these cells will be used to confirm this (14). As no increased accumulation of EGFP+ cells was found in lungs or liver at 48 h, the interpretation is that these CD11c+ DC also circulated freely and then trafficked rapidly to peripheral tissues. By 48 h very few CD11c+ EGFP+ cells remained in the circulation. It is also possible that our bone marrow preparations also contained common lymphoid precursors, but no EGFP+ CD8α+CD11c+ cells were found within the circulation or retinal tissue in this study. The B220 phenotype defines plasmacytoid DC in mice, and the spleens of EAU mice at 48 h contained significantly elevated numbers of EGFP+ B220+ cells. EGFP+ CD11c+B220+ cells were also found in the inflamed retina. In humans, plasmacytoid DC arise from lymphoid lineage DC, but the origin of murine plasmacytoid cells is less clear, (54) and Shigematsu et al. (55) have shown that plasmacytoid DC can develop from both the common lymphoid progenitor and common myeloid progenitor lineages. Further functional characterization of these cells will be required to confirm or refute their significance within the lesions.
More convincing evidence for myeloid DC subset differentiation from monocytes was found within the inflamed retina. The F4/80 Ag is typical of tissue resident monocyte/macrophages, so F4/80+ EGFP+ cells in the retina could represent differentiation in situ. It is of note that a proportion of these F4/80+ cells also expressed CD11c, a phenotype associated with interstitial DC in mice (44). Further work is required to confirm these observations. Recovery and purification of these subsets from the retina for functional studies will not be a trivial undertaking, and is beyond the scope of the present study.
In conclusion, we have developed and characterized a physiological in vivo monocyte trafficking system and examined the trafficking and differentiation of monocytes in the context of an organ-specific autoimmune disease. Our data supports the concept of CCR2+ inflammatory phenotype that is a constitutive, time-limited property of blood monocytes and is independent of local inflammatory mediators. Adoptively transferred monocytes also possessed the potential for further differentiation in vivo into diverse macrophage and DC subsets within the tissues.
Acknowledgment
We thank Dr. Jarmila Plskova for helping with mouse splenectomy.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
This work was supported by Grant Reference 068109 from the Wellcome Trust.
Abbreviations used in this paper: DC, dendritic cell; BM-Mo, bone marrow-derived monocyte; CuBM-Mφ, cultured bone marrow-derived macrophage; P-Mφ, peritoneal macrophage; EAU, experimental autoimmune uveoretinitis; EGFP, enhanced GFP; SLO, scanning laser ophthalmoscopy; PMN, polymorphonuclear cell; PDCA, plasmacytoid DC Ag.
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflict of interest.
References
- 1.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J. Exp. Med. 1968;128:415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Furth R, Diesselhoff-den Dulk MC, Mattie H. Quantitative study on the production and kinetics of mononuclear phagocytes during an acute inflammatory reaction. J. Exp. Med. 1973;138:1314–1330. doi: 10.1084/jem.138.6.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Muller WA, Randolph GJ. Migration of leukocytes across endothelium and beyond: molecules involved in the transmigration and fate of monocytes. J. Leukocyte Biol. 1999;66:698–704. doi: 10.1002/jlb.66.5.698. [DOI] [PubMed] [Google Scholar]
- 5.Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11:753–761. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- 6.Ash P, Loutit JF, Townsend KM. Osteoclasts derived from haematopoietic stem cells. Nature. 1980;283:669–670. doi: 10.1038/283669a0. [DOI] [PubMed] [Google Scholar]
- 7.Akagawa KS, Takasuka N, Nozaki Y, Komuro I, Azuma M, Ueda M, Naito M, Takahashi K. Generation of CD1+RelB+ dendritic cells and tartrate-resistant acid phosphatase-positive osteoclast-like multinucleated giant cells from human monocytes. Blood. 1996;88:4029–4039. [PubMed] [Google Scholar]
- 8.Kennedy DW, Abkowitz JL. Kinetics of central nervous system microglial and macrophage engraftment: analysis using a transgenic bone marrow transplantation model. Blood. 1997;90:986–993. [PubMed] [Google Scholar]
- 9.Kennedy DW, Abkowitz JL. Mature monocytic cells enter tissues and engraft. Proc. Natl. Acad. Sci. USA. 1998;95:14944–14949. doi: 10.1073/pnas.95.25.14944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawson LJ, Perry VH, Gordon S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience. 1992;48:405–415. doi: 10.1016/0306-4522(92)90500-2. [DOI] [PubMed] [Google Scholar]
- 11.Hume DA, Ross IL, Himes SR, Sasmono RT, Wells CA, Ravasi T. The mononuclear phagocyte system revisited. J. Leukocyte Biol. 2002;72:621–627. [PubMed] [Google Scholar]
- 12.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 13.Traver D, Akashi K, Manz M, Merad M, Miyamoto T, Engleman EG, Weissman IL. Development of CD8α-positive dendritic cells from a common myeloid progenitor. Science. 2000;290:2152–2154. doi: 10.1126/science.290.5499.2152. [DOI] [PubMed] [Google Scholar]
- 14.D'Amico A, Wu L. The early progenitors of mouse dendritic cells and plasmacytoid predendritic cells are within the bone marrow hemopoietic precursors expressing Flt3. J. Exp. Med. 2003;198:293–303. doi: 10.1084/jem.20030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Garra A, Trinchieri G. Are dendritic cells afraid of commitment? Nat. Immunol. 2004;5:1206–1208. doi: 10.1038/ni1204-1206. [DOI] [PubMed] [Google Scholar]
- 16.Cumberbatch M, Dearman RJ, Griffiths CE, Kimber I. Langerhans cell migration. Clin. Exp. Dermatol. 2000;25:413–418. doi: 10.1046/j.1365-2230.2000.00678.x. [DOI] [PubMed] [Google Scholar]
- 17.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 18.Liu L, MacPherson GG. Dendritic cells “in vivo”: their role in the initiation of intestinal immune responses. Adv. Exp. Med. Biol. 1995;371A:271–274. doi: 10.1007/978-1-4615-1941-6_56. [DOI] [PubMed] [Google Scholar]
- 19.Kamradt T, Mitchison NA. Tolerance and autoimmunity. N. Engl. J. Med. 2001;344:655–664. doi: 10.1056/NEJM200103013440907. [DOI] [PubMed] [Google Scholar]
- 20.Davidson A, Diamond B. Autoimmune diseases. N. Engl. J. Med. 2001;345:340–350. doi: 10.1056/NEJM200108023450506. [DOI] [PubMed] [Google Scholar]
- 21.Gordon S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 22.Palframan RT, Jung S, Cheng G, Weninger W, Luo Y, Dorf M, Littman DR, Rollins BJ, Zweerink H, Rot A, von Andrian UH. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J. Exp. Med. 2001;194:1361–1373. doi: 10.1084/jem.194.9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janatpour MJ, Hudak S, Sathe M, Sedgwick JD, McEvoy LM. Tumor necrosis factor-dependent segmental control of MIG expression by high endothelial venules in inflamed lymph nodes regulates monocyte recruitment. J. Exp. Med. 2001;194:1375–1384. doi: 10.1084/jem.194.9.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fife BT, Huffnagle GB, Kuziel WA, Karpus WJ. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J. Exp. Med. 2000;192:899–905. doi: 10.1084/jem.192.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahad DJ, Ransohoff RM. The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) Semin. Immunol. 2003;15:23–32. doi: 10.1016/s1044-5323(02)00125-2. [DOI] [PubMed] [Google Scholar]
- 26.Daly C, Rollins BJ. Monocyte chemoattractant protein-1 (CCL2) in inflammatory disease and adaptive immunity: therapeutic opportunities and controversies. Microcirculation. 2003;10:247–257. doi: 10.1038/sj.mn.7800190. [DOI] [PubMed] [Google Scholar]
- 27.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 28.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J. Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 29.Forrester JV, Liversidge J, Dua HS, Dick A, Harper F, McMenamin PG. Experimental autoimmune uveoretinitis: a model system for immunointervention. A review. Curr. Eye Res. 1992;11(Suppl):33–40. doi: 10.3109/02713689208999509. [DOI] [PubMed] [Google Scholar]
- 30.Caspi RR, Chan CC, Wiggert B, Chader GJ. The mouse as a model of experimental autoimmune uveoretinitis (EAU) Curr. Eye Res. 1990;9(Suppl):169–174. doi: 10.3109/02713689008999438. [DOI] [PubMed] [Google Scholar]
- 31.Broderick C, Hoek RM, Forrester JV, Liversidge J, Sedgwick JD, Dick AD. Constitutive retinal CD200 expression regulates resident microglia and activation state of inflammatory cells during experimental autoimmune uveoretinitis. Am. J. Pathol. 2002;161:1669–1677. doi: 10.1016/S0002-9440(10)64444-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robertson MJ, Erwig LP, Liversidge J, Forrester JV, Rees AJ, Dick AD. Retinal microenvironment controls resident and infiltrating macrophage function during uveoretinitis. Invest. Ophthalmol. Visual Sci. 2002;43:2250–2257. [PubMed] [Google Scholar]
- 33.Mack M, Cihak J, Simonis C, Luckow B, Proudfoot AE, Plachy J, Bruhl H, Frink M, Anders HJ, Vielhauer V. Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J. Immunol. 2001;166:4697–4704. doi: 10.4049/jimmunol.166.7.4697. [DOI] [PubMed] [Google Scholar]
- 34.Xu H, Manivannan A, Daniels G, Liversidge J, Sharp PF, Forrester JV, Crane IJ. Evaluation of leukocyte dynamics in mouse retinal circulation with scanning laser ophthalmoscopy (video report) Br. J. Ophthalmol. 2001;85:765–890. [Google Scholar]
- 35.Xu H, Manivannan A, Goatman KA, Liversidge J, Sharp PF, Forrester JV, Crane IJ. Improved leukocyte tracking in mouse retinal and choroidal circulation. Exp. Eye Res. 2002;74:403–410. doi: 10.1006/exer.2001.1134. [DOI] [PubMed] [Google Scholar]
- 36.Mainster MA, Ham WT, Jr., Delori FC. Potential retinal hazards: instrument and environmental light sources. Ophthalmology. 1983;90:927–932. doi: 10.1016/s0161-6420(83)80019-0. [DOI] [PubMed] [Google Scholar]
- 37.Ley K, Gaehtgens P. Endothelial, not hemodynamic, differences are responsible for preferential leukocyte rolling in rat mesenteric venules. Circ. Res. 1991;69:1034–1041. doi: 10.1161/01.res.69.4.1034. [DOI] [PubMed] [Google Scholar]
- 38.Vajkoczy P, Laschinger M, Engelhardt B. α4-integrin-VCAM-1 binding mediates G protein-independent capture of encephalitogenic T cell blasts to CNS white matter microvessels. J. Clin. Invest. 2001;108:557–565. doi: 10.1172/JCI12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan-Ling T. Glial, vascular, and neuronal cytogenesis in whole-mounted cat retina. Microsc. Res. Tech. 1997;36:1–16. doi: 10.1002/(SICI)1097-0029(19970101)36:1<1::AID-JEMT1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 40.Xu H, Forrester JV, Liversidge J, Crane IJ. Leukocyte trafficking in experimental autoimmune uveitis: breakdown of blood-retinal barrier and up-regulation of cellular adhesion molecules. Invest. Ophthalmol. Visual Sci. 2003;44:226–234. doi: 10.1167/iovs.01-1202. [DOI] [PubMed] [Google Scholar]
- 41.Xu H, Manivannan A, Goatman KA, Jiang HR, Liversidge J, Sharp PF, Forrester JV, Crane IJ. Reduction in shear stress, activation of the endothelium, and leukocyte priming are all required for leukocyte passage across the blood–retina barrier. J. Leukocyte Biol. 2004;75:224–232. doi: 10.1189/jlb.1002479. [DOI] [PubMed] [Google Scholar]
- 42.Xu H, Manivannan A, Liversidge J, Sharp PF, Forrester JV, Crane IJ. Requirements for passage of T lymphocytes across non-inflamed retinal microvessels. J. Neuroimmunol. 2003;142:47–57. doi: 10.1016/s0165-5728(03)00258-3. [DOI] [PubMed] [Google Scholar]
- 43.Xu H, Manivannan A, Jiang HR, Liversidge J, Sharp PF, Forrester JV, Crane IJ. Recruitment of IFN-γ-producing (Th1-like) cells into the inflamed retina in vivo is preferentially regulated by P-selectin glycoprotein ligand 1:P/E-selectin interactions. J. Immunol. 2004;172:3215–3224. doi: 10.4049/jimmunol.172.5.3215. [DOI] [PubMed] [Google Scholar]
- 44.Weinlich G, Heine M, Stossel H, Zanella M, Stoitzner P, Ortner U, Smolle J, Koch F, Sepp NT, Schuler G, Romani N. Entry into afferent lymphatics and maturation in situ of migrating murine cutaneous dendritic cells. J. Invest. Dermatol. 1998;110:441–448. doi: 10.1046/j.1523-1747.1998.00161.x. [DOI] [PubMed] [Google Scholar]
- 45.Nakano H, Yanagita M, Gunn MD. CD11c+B220+Gr-1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J. Exp. Med. 2001;194:1171–1178. doi: 10.1084/jem.194.8.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang HR, Lumsden L, Forrester JV. Macrophages and dendritic cells in IRBP-induced experimental autoimmune uveoretinitis in B10RIII mice. Invest Ophthalmol. Visual Sci. 1999;40:3177–3185. [PubMed] [Google Scholar]
- 47.Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu. Rev. Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- 48.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 49.Imhof BA, Aurrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat. Rev. Immunol. 2004;4:432–444. doi: 10.1038/nri1375. [DOI] [PubMed] [Google Scholar]
- 50.Banchereau J, Paczesny S, Blanco P, Bennett L, Pascual V, Fay J, Palucka AK. Dendritic cells: controllers of the immune system and a new promise for immunotherapy. Ann. NY Acad. Sci. 2003;987:180–187. doi: 10.1111/j.1749-6632.2003.tb06047.x. [DOI] [PubMed] [Google Scholar]
- 51.Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat. Rev. Immunol. 2003;3:569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 52.Charo IF, Peters W. Chemokine receptor 2 (CCR2) in atherosclerosis, infectious diseases, and regulation of T-cell polarization. Microcirculation. 2003;10:259–264. doi: 10.1038/sj.mn.7800191. [DOI] [PubMed] [Google Scholar]
- 53.Izikson L, Klein RS, Luster AD, Weiner HL. Targeting monocyte recruitment in CNS autoimmune disease. Clin. Immunol. 2002;103:125–131. doi: 10.1006/clim.2001.5167. [DOI] [PubMed] [Google Scholar]
- 54.Ardavin C. Origin, precursors and differentiation of mouse dendritic cells. Nat. Rev. Immunol. 2003;3:582–590. doi: 10.1038/nri1127. [DOI] [PubMed] [Google Scholar]
- 55.Shigematsu H, Reizis B, Iwasaki H, Mizuno S, Hu D, Traver D, Leder P, Sakaguchi N, Akashi K. Plasmacytoid dendritic cells activate lymphoid-specific genetic programs irrespective of their cellular origin. Immunity. 2004;21:43–53. doi: 10.1016/j.immuni.2004.06.011. [DOI] [PubMed] [Google Scholar]