Abstract
Background
The alanine allele of P12A polymorphism in PPARG gene in a few studies has been associated with a reduced or increased risk of acute myocardial infarction (AMI). Yet, the risk relation has not been confi rmed, and data on ischemic stroke (IS) is scarce. We therefore investigated the role of this polymorphism on occurrence of AMI, coronary heart disease (CHD) and IS.
Methods and fi ndings
We performed a case-cohort study in 15,236 initially healthy Dutch women and applied a Cox proportional hazards model to study the relation of the P12A polymorphism and AMI (n = 71), CHD (n = 211), and IS (n = 49) under different inheritance models. In addition, meta-analyses of published studies were performed. Under the dominant inheritance model, carriers of the alanine allele compared with those with the more common genotype were not at increased or decreased risk of CHD (hazard ratio [HR] = 0.82; 95% confi dence interval [CI], 0.58 to 1.17) and of IS (HR = 1.03; 95% CI, 0.14 to 7.74). In addition no relations were found under the recessive and additive models. Our meta-analyses corroborated these fi ndings by showing no signifi cant association. For AMI we found a borderline signifi cant association under dominant (HR = 0.49; 95% CI, 0.26 to 0.94), and additive (HR = 0.51; 95% CI, 0.26 to 1.00) models which could be due to chance, because of small cases in this subgroup. The meta-analysis did not show any association between the polymorphism and risk of AMI under the different genetic models.
Conclusions
Our study in healthy Dutch women in combination with the meta-analyses of previous reports does not provide support for a role of P12A polymorphism in PPARG gene in MI and CHD risk. Also our study shows that the polymorphism has no association with IS risk.
Keywords: genetics, myocardial infarction, polymorphism, PPARG gene, risk factors, population-based
Introduction
The most prevalent human PPARG gene mutation is a cytosine to guanine substitution in exon B (codon 12) of this gene (Knouff and Auwerx 2004), resulting in an exchange of proline (P) to alanine (A) at amino acid (Temelkova-Kurktschiev et al 2004). Initially recognized to play a role only in adipogenesis and glucose homeostasis, recent works have shown associations with regulation of cell growth, migration and infl ammation (Schiffrin et al 2003; Youssef et al 2004). Also, PPARG2 has a role in insulin signaling, insulin resistance, and development of type 2 diabetes (Memisoglu et al 2003).
There is some evidence that P12A polymorphism in PPARG gene is related to vascular risk factors (Deeb et al 1998; Altshuler et al 2000; Meirhaeghe et al 2000; Masud and Ye 2003; Ostgren et al 2003; Doney et al 2004). A meta-analysis showed a 21% risk reduction for type2 of diabetes (Altshuler et al 2000). Moreover, A12 allele carriers have signifi cantly higher body mass index (BMI) (Masud and Ye 2003), lower insulin resistance (Deeb et al 1998; Meirhaeghe et al 2000) and reduced blood pressure (Ostgren et al 2003; Doney et al 2004). These fi ndings suggest that a possible role in atherosclerosis development. This is supported by recent fi ndings showing a relation of A12A genotype to reduced common carotid intima-media thickness (Temelkova-Kurktschiev et al 2004; Al Shali et al 2004b).
However, information on the relation with acute myocardial infarction (AMI), coronary heart disease (CHD) and ischemic stroke (IS) as the clinical endpoints is scarce, and inconsistent (Vos et al 2000; Ridker et al 2003; Doney et al 2004; Tobin et al 2004; Pischon et al 2005; Li et al 2006) for CHD. A reduced risk for ischemic stroke has been reported (Lee et al 2006). We set out to investigate the relation of P12A polymorphism in PPARG gene on occurrence of AMI, CHD and ischemic stroke in middle-aged Dutch women. To expand the evidence further, we performed meta-analyses using published data from observational studies.
Methods
Prospect-EPIC study
Study design, general questionnaire, anthropometric and Laboratory measurements have been described in detail elsewhere (Zafarmand et al in Press). Briefl y, the study population consisted of participants of the Prospect-EPIC cohort. Participants were recruited between 1993 and 1997 among women living in Utrecht and vicinity who attended the regional population-based breast cancer-screening program. A total of 17,357 women aged 49–70 were included. Follow-up event information was obtained from the Dutch Centre for Health Care Information, which holds a standardized computerized register of hospital discharge diagnoses. Using the International Classifi cation of Diseases, ninth Revision (ICD-9) codes for the main discharge reason, we categorized cardiovascular disease (codes 390–459) as CHD (codes 410–414), including acute myocardial infarction (code 410), as ischemic cerebrovascular disease (codes 433–435), and other cardiovascular diseases. Whenever multiple events occurred, the fi rst diagnosis was taken as endpoint of interest. All women signed an informed consent form prior to study inclusion. The study was approved by the Institutional Review Board of the University Medical Center Utrecht.
We applied the case-cohort design introduced by Prentice (1986). In this design, data is collected on all subjects, but the data would only be analyzed on cases and sub-cohort members. Cases are those emerging in the total cohort; controls are subjects in the sub-cohort. The sub-cohort is a randomly selected sample of 10% (n = 1736) from the 17,357 women in the total cohort. Women who did not consent to linkage with vital status registries or who were not traceable (cases n = 3/sub-cohort n = 38) were not included. Women who reported a diagnosis of cardiovascular disease (ICD-9; 390–459) at baseline, who had missing questionnaires or blood or DNA samples were excluded. This resulted in 15,236 women in the total cohort and 1519 women in the sub-cohort at baseline (as the control group). All fi rst fatal and non-fatal CHD and ischemic stroke events that arose during follow-up until January 1st 2000 were selected as cases. These were 211 CHD cases, including 71 AMIs, and 49 ischemic cerebrovascular events. For all case subjects follow up ended at the date of diagnosis or at the date of death due to cardiovascular disease.
Genetic analysis
Genetic analysis was performed at the Cardiovascular Genotyping (CAGT) laboratory of the Department of Internal Medicine of the University Hospital Maastricht. Genomic DNA was extracted from buffy coats with the use of the QIAamp® Blood Kit (Qiagen Inc., Valencia, California, USA). Genotyping of the polymorphisms was performed using a multilocus genotyping assay for candidate markers of cardiovascular disease risk (Roche Molecular Systems Inc., Pleasanton, CA, USA) (Cheng et al 1999). Genotyping was preformed blinded to the case-control status. A random double-check was performed to detect potential genotyping errors.
Data analysis
To assess the relation of P12A polymorphism with the outcome, we used a Cox proportional hazards model with an estimation procedure adapted for case-cohort designs. We used the unweighted method by Prentice, which is incorporated in a SAS macro at http://lib.stat.cmu.edu/general/robphreg.
Baseline characteristics of sub-cohort by genotypes (P12P, P12A and A12A) is given. Hardy-Weinberg equilibrium (HWE) was evaluated with the χ2 test. Frequencies of A12 allele and P12 allele were determined. We assessed the association between the polymorphism and events under different genetic models. The dominant genetic model compares individuals with one or more polymorphic alleles (P12A and A12A genotypes combined) with a group with no polymorphic alleles (P12P). The recessive genetic model compares the A12A genotype with the combined P12P and P12A genotypes. The additive genetic model assumes that there is a linear gradient in risk between the P12P, P12A and A12A genotypes (P12P genotype baseline). This is equivalent to a comparison of the A12 allele versus the P12 allele (baseline). All analyses were performed for AMI, CHD, ischemic stroke and total ischemic events. A value of p < 0.05 (2-sided) was considered signifi cant.
Meta-analysis
Search strategy and data extraction
For the meta-analysis, published data was used concerning the P12A polymorphism in PPARG gene and MI, CHD and IS. The search was done on November 15, 2006. In addition, our own data were included. Studies were found with PubMed/Medline, ISI Web of Knowledge, and Embase using the following text search string: (Pro12Ala OR P12A) AND (“Peroxisome proliferator-activated receptor gamma” OR PPARG) AND (coronary disease OR coronary heart disease OR CHD OR myocardial infarction OR MI OR myocardial infarct OR coronary artery disease OR CAD OR ischemic heart disease OR IHD OR cardiovascular disease OR heart disease OR angina OR ischemic stroke OR CVA OR stroke OR cerebrovascular accident). The following constraints were applied to the search: (1) only published articles in journals or their supplements (English); and (2) studies only in human subjects. Manual bibliography review was added. This search (done by MHZ and MLB) identifi ed 36 potentially relevant articles. Studies were included if they reported the relative risks, ORs or HRs and 95% confi dence intervals [CIs] for events related to PPARG2 P12A polymorphism or provided raw data that allowed estimation of these values. We excluded 24 studies because of other endpoints (such as vascular risk factors); one repeated publication; one study which did not provide suffi cient data; two review papers; and one study with carotid intima-media thickness as endpoint. Since only one paper had been found for ischemic stroke, we excluded ischemic stroke from the meta-analysis. Hence, data were available for these analyses from 8 original reports (6 studies found with databases, one additional article identifi ed by a hand search and our data) involving 2793 cases and 7680 controls (Table 4). As Pischon and colleagues (2005) had provided data from two different studies (Nurses’ Health Study [NHS] in women and Health Professionals Follow-up Study [HPFS] in men), we consider them in the analysis as two studies. The following information was extracted from each study: fi rst author, study design, year of publication, geographical location, defi nition and number of cases and controls, mean age of cases and controls, gender, genotype frequency, genotyping methods and consistency of genotype frequencies with Hardy-Weinberg equilibrium.
Table 4.
Characteristics of studies included in the meta - analysis
| Author | Year of publication | Country | Study design | Cases | Controls | Effect measurement (With 95% CI; P value) | Mean age ± SD (years) | Sex | End point | Allele frequency 12Ala | P (HWE) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P/P | P/A | A/A | P/P | P/A | A/A | Dominant | Recessive | Additive | Cases | Controls | |||||||||
| 1 | Vos et al | 2000 | Netherlands | Case-control | 437 | 105 | 21 | 512 | 122 | 12 | 1.1 (0.84–1.45; 0.49) | 2.0 (1.0–4.2; 0.049) | 1.2 (0.9–1.5; 0.19) | No data | No data | M | MI | 0.113 | 0.14 |
| 2 | Bluher et al | 2002 | Germany | Cross-sectional | 174 | 23 | 4 | 140 | 22 | 2 | 0.91 (0.50–1.6; 0.74) | 1.6 (0.3–9.09; 0.56) | 0.97 (0.56–1.7; 0.91) | 67.1 (43–91)a | 63.3 (33–87)a | M/F | CHD | 0.079 | 0.30 |
| 3 | Ridker et al | 2003 | USA | Nested Case-control | 425 | 92 | 6 | 1610 | 451 | 31 | 0.77 (0.60–0.98; 0.03) | 0.76 (0.32–1.8; 0.55) | 0.79 (0.63–0.99; 0.04) | 58.3 | 58.4 | M | MI | 0.123 | 0.93 |
| 4 | Tobin et al | 2004 | UK | Case-control | 434 | 103 | 10 | 381 | 120 | 4 | 0.80 (0.60–1.07; 0.13) | 2.33 (0.73–7.5; 0.14) | 0.87 (0.67–1.14; 0.31) | 61.9 ± 9.2 | 58.6 ± 10.7 | M/F | MI | 0.127 | 0.09 |
| 5 | Doney et al | 2004 | Scotland | Cohort | 35 | 1141 | 0.21 (0.06–0.69; 0.01) | No data | No data | No data | 64.4 ± 11.6 | M/F | MI | 0.111 | 0.69 | ||||
| 6 | Pischon et al (NHS study) | 2005 | USA | Cohort | 187 | 54 | 4 | 386 | 93 | 6 | 1.2 (0.84-1.7; 0.31) | 1.3 (0.37–4.7; 0.66) | 1.19 (0.85–1.7; 0.30) | 60.4 ± 0.4 | 60.3 ± 0.3 | F | CHD | 0.108 | 0.88 |
| 7 | Pischon et al (HPFS study) | 2005 | USA | Cohort | 187 | 59 | 4 | 407 | 91 | 4 | 1.44 (1.0-2.07; 0.05) | 2.02 (0.5–8.16; 0.31) | 1.4 (1.01–1.97; 0.042) | 65.2 ± 0.5 | 65.1 ± 0.4 | M | CHD | 0.099 | 0.66 |
| 8 | Li et al | 2006 | China | Case-control | 195 | 23 | 0 | 588 | 36 | 2 | 1.83 (1.06-3.1; 0.03) | 0.57 (0.0–4.9; 0.41) | 1.69 (0.98–2.8; 0.057) | 65.0 ± 11 | 62.1 ± 8.2 | M/F | MI | 0.032 | 0.08 |
| 9 | Zafarmand et al (present study) | 2007 | Netherlands | Case-cohort | 167 | 41 | 3 | 1143 | 346 | 30 | 0.82 (0.58–1.17; 0.27) | 0.72 (0.22–2.37; 0.58) | 0.81 (0.59–1.12; 0.20) | 61 ± 6 | 57± 6 | F | CHD, MI | 0.134 | 0.52 |
Abbreviations: CHD, coronary heart disease; MI, myocardial infarction; HWE, Hardy – Weinberg equilibrium.
Notes: Range of age (year).
Data analysis
For the meta-analysis, Mantel-Haenszel was used as fi xed effects model and the DerSimonian-Laird method was used as random-effects model, all under different genetic models. The Egger’s test with 95% CI was used for evaluating publication bias. In each study, we tested for HWE by using an asymptomatic χ2 test or an exact test among the controls (Trikalinos et al 2006). We used Cochran’s χ2 – based Q statistic for between-study heterogeneity (Lau et al 1997), which is considered signifi cant for p < 0.10, as well as the I2 statistic for estimation of inconsistency in meta-analyses. I2 represents the percentage of the observed between-study variability due to heterogeneity rather than to chance and ranges from 0 to 100 percent where a value of 0% indicates no observed heterogeneity, and larger values an increasing degree of heterogeneity. Values above 75 percent imply high heterogeneity (Higgins et al 2003). Meta-analysis was carried out using STATA 9.1.
Results
Prospect-EPIC study results
General characteristics of the randomly sampled participants of the cohort (n = 1519) are given in Table 1. Of the participants 1143 (75.2%) had the common type allele (P12P), 346 (22.8%) were heterozygous for the A12 allele (P12A), and 30 (2%) were homozygous for the A12 allele. The genotype distribution was in HWE.
Table 1.
Baseline characteristics of the sub-cohort according to genotype, and clinical characteristics of CHD cases and controls in the Prospect – Epic cohort
| sub-cohort (N = 1519) | p-valueb | CHD cases | Sub-cohort | p-valuec | |||
|---|---|---|---|---|---|---|---|
| P12P | P12A | A 12A | |||||
| N total | 1143 | 346 | 30 | - | 211 | 1519 | - |
| Age at intake (yr)a | 57.2 ± 6.1 | 57.1 ± 6.0 | 56.1 ± 5.4 | 0.63 | 60.5 ± 5.9 | 57.1 ± 6.1 | <0.01 |
| Body mass index (kg/m2)a | 25.8 ± 3.9 | 25.8 ± 4.1 | 26.4 ± 3.6 | 0.69 | 26.8 ± 3.9 | 25.8 ± 4.0 | <0.01 |
| Weight (kg)a | 69.5 ±11.2 | 69.9 ±11.8 | 72.7 ±11.6 | 0.28 | 71.1 ± 11.3 | 69.7 ± 11.3 | 0.08 |
| Height (cm)a | 164.2 ± 6 | 164.5 ± 5 | 165.8 ± 6 | 0.24 | 162.8 ± 6 | 164.3 ± 6 | 0.01 |
| Waist to hip ratioa | 0.78 ± 0.05 | 0.78 ± 0.05 | 0.79 ± 0.06 | 0.56 | 0.81 ± 0.06 | 0.79 ± 0.06 | <0.01 |
| Hypertension (%)d | 34.6 | 29.8 | 33.3 | 0.25 | 51.7 | 33.4 | <0.01 |
| Systolic blood pressure (mm Hg)a | 133.1 ± 20.2 | 131.3 ± 19.3 | 132.9 ± 19.5 | 0.35 | 143.3 ± 22.3 | 132.7 ± 20.0 | <0.01 |
| Diastolic blood pressure (mm Hg)a | 79.1 ± 10.6 | 78.7 ± 10.5 | 79.8 ± 10.6 | 0.80 | 81.6 ± 10.7 | 79.0 ± 10.6 | <0.01 |
| Presence of diabetes (%) | 2.3 | 2.0 | 3.3 | 0.88 | 5.7 | 2.2 | <0.01 |
| Presence of hypercholesterolemia (%) | 4 | 4 | 0 | 0.53 | 11.4 | 3.9 | <0.01 |
| Current alcohol consumption (%) | 87.9 | 88.3 | 88.9 | 0.97 | 80.9 | 88.0 | <0.01 |
| Smoking status (%) Past | 34.6 | 35.3 | 33.3 | 0.96 | 26.1 | 34.8 | <0.01 |
| Current | 23.1 | 21.4 | 33.3 | 0.31 | 34.1 | 22.9 | <0.01 |
| Pack- yearse | 6.8 ± 9.5 | 6.1 ± 9.1 | 7.9 ± 11.3 | 0.38 | 9.8 ± 11.4 | 6.7 ± 9.5 | <0.01 |
| Total cholesterol (mmol/L)a | 5.9 ± 1 | 5.8 ± 1 | 5.9 ± 1.1 | 0.14 | 6.4 ± 1 | 5.9 ± 1 | <0.01 |
| HDL cholesterol (mmol/L)a | 1.6 ± 0.4 | 1.6 ± 0.4 | 1.5 ± 0.4 | 0.70 | 1.4 ± 0.3 | 1.6 ± 0.4 | <0.01 |
| LDL cholesterol (mmol/L)a | 3.9 ± 0.9 | 3.9 ± 0.9 | 4.0 ± 1.1 | 0.28 | 4.4 ± 1 | 3.9 ± 0.9 | <0.01 |
| Serum glucose (mmol/L)a | 4.5 ± 1.4 | 4.5 ± 1.5 | 4.2 ± 1.5 | 0.31 | 5.1 ± 2.5 | 4.5 ± 1.4 | <0.01 |
Notes:
Mean ± standard deviation;
Comparison of risk factors across genotypes, using the ANOVA F test (continuous variables) and the χ2 statistic (categorical variables);
Comparison of risk factors across disease status, using the independent samples t-test (continuous variables) and the χ2 statistic (categorical variables);
Defi ned as a systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg and/or questionnaire positive;
The number of packs of cigarettes smoked per day by the number of years the person has smoked.
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; CHD, coronary heart disease (ICD 410–414).
None of conventional risk factors were statistically signifi cantly related to the P12A polymorphism (Table 1). Median follow up time for the sub-cohort was 4.3 years, with a total of 6,525 person years. The actual follow-up in the baseline cohort of 15,236 women was 64,768 person years. Due to the case-cohort design, 23 women in the sub-cohort eventually were CHD cases and 5 of them were ischemic stroke cases (totally 28 cases). Clinical characteristics of CHD cases and controls are presented in Table 1.
Comparing allele frequencies in cases and control groups separately did not show signifi cant difference between them, except for myocardial infarction, which showed a borderline signifi cant relation (Table 2).
Table 2.
Genotype and allele frequencies of the polymorphism among AMI, CHD, and ischemic stroke cases and sub-cohort of the Prospect – Epic cohort
| Genotype/allele | Acute myocardial infarction | Coronary heart disease | Ischemic stroke | Sub-cohort | ||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| No. of subjects | 71 | 211 | 49 | 1519 | ||||
| Genotype | ||||||||
| P/P | 61 | 85.9 | 167 | 79.1 | 38 | 77.6 | 1143 | 75.2 |
| P/A | 10 | 14.1 | 41 | 19.4 | 10 | 20.4 | 346 | 22.8 |
| A/A | 0 | 0.0 | 3 | 1.4 | 1 | 2 | 30 | 2 |
| Allele | ||||||||
| Pro | 132 | 93.0 | 375 | 88.9 | 86 | 87.8 | 2632 | 86.6 |
| Ala | 10 | 7.0 | 47 | 11.1 | 12 | 12.2 | 406 | 13.4 |
| χ2 = 4.77 | χ2 = 1.61 | χ2 = 0.10 | ||||||
| Df = 1 | df = 1 | df = 1 | ||||||
| p = 0.03 | p = 0.20 | p = 0.75 | ||||||
A lower risk of AMI with only borderline effects was found under dominant (OR = 0.51; 95% CI, 0.26 to 1.00; p = 0.05) and additive models (OR = 0.49; 95% CI, 0.26 to 0.94; p = 0.03) but not under recessive inheritance mode (Table 3). The analyses were repeated for CHD and ischemic stroke as primary outcomes and also for all ischemic events (CHD and ischemic stroke). No statistically signifi cant association was seen for risks of CHD and ischemic stroke (Table 3). None of the risk factors was statistically signifi cantly associated with genotypes (Table 1) and we did not consider them as confounders in our data. The rationale for not adjusting for the risk factors is that we did not want to adjust in a primary analysis for intermediates in a potential causal pathway and that because genes are randomly assigned at conception (Mendelian randomization) confounding by lifestyle related factors (or intermediate phenotypes) should not be a problem in genetic epidemiology studies (Smith and Ebrahim 2004).
Table 3.
Hazard ratios of cardiovascular events under different genetic models for P12A polymorphism in PPARG gene in the Prospect – Epic cohort
| Different events Model | Inheritance Model | Hazard ratio | 95% CI | p-value |
|---|---|---|---|---|
| Acute myocardial infarction | Dominant | 0.51 | 0.26–1.00 | 0.05 |
| Recessive | 0.34 | 0.00–2.68 | 0.47 | |
| Additive | 0.49 | 0.26–0.94 | 0.03 | |
| Coronary heart disease | Dominant | 0.82 | 0.58–1.17 | 0.27 |
| ecessive | 0.72 | 0.22–2.37 | 0.58 | |
| Additive | 0.81 | 0.59–1.12 | 0.20 | |
| Ischemic stroke | Dominant | 0.90 | 0.46–1.78 | 0.77 |
| Recessive | 1.03 | 0.14–7.74 | 0.97 | |
| Additive | 0.90 | 0.49–1.67 | 0.75 | |
| All ischemic envents | Dominant | 0.85 | 0.62–1.17 | 0.31 |
| Recessive | 0.78 | 0.27–2.22 | 0.63 | |
| Additive | 0.83 | 0.62–1.11 | 0.21 |
We examined the interaction between the P12A polymorphism and risk factors for each of the events separately by introduction of risk factor*A12 allele carriers term in the logistic regression models. No signifi cant interactions between P12A and risk factors in AMI, CHD and total ischemic events were seen, apart from smoking (current) and A12 allele carriers (P = 0.027). In the light of the many associations we studied, this may actually be a chance fi nding.
Meta-analysis results
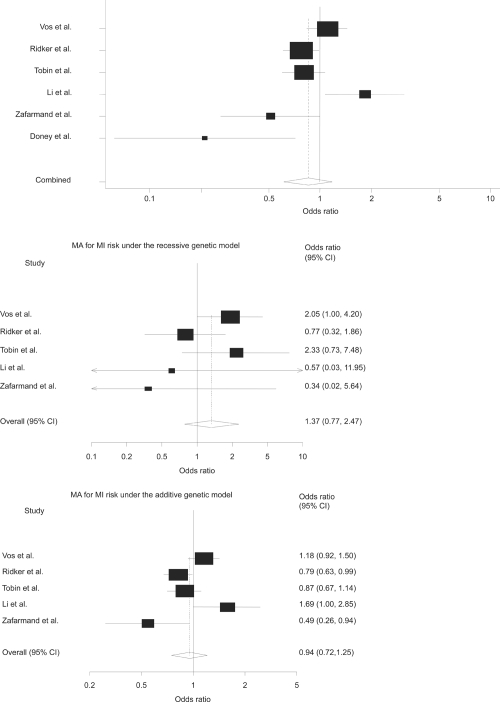
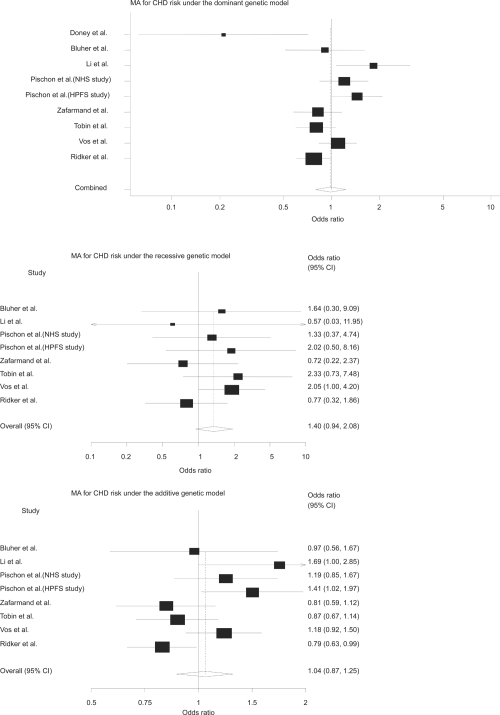
Table 4 shows the characteristics of studies included in this meta-analysis. The genotype frequencies in the studies were consistent with HWE. The meta-analyses did not show a signifi cant association under dominant genetic model (OR = 0.85; 95% CI, 0.61 to 1.17; p = 0.32), recessive model (OR = 1.37; 95% CI, 0.77 to 2.47; p = 0.29) and additive model (OR = 0.94; 95% CI, 0.72 to 1.25; p = 0.69) (Figure 1). Furthermore, pooled estimate (Figure 2) did not show a signifi cant association between the polymorphism and CHD, under dominant genetic model (OR = 0.99; 95% CI, 0.79 to 1.23; p = 0.92), recessive model (OR = 1.40; 95% CI, 0.94 to 2.08; p = 0.10) and additive model (OR = 1.04; 95% CI, 0.87 to 1.25; p = 0.64). There was evidence for heterogeneity under dominant and additive genetic models for MI (p = 0.002, p = 0.006) and CHD (p = 0.002, p = 0.01), respectively. To deal with heterogeneity we used random-effect model (the DerSimonian-Laird method) for pooling data. There was not evidence for signifi cant publication bias (Egger’s test = 1.85; 95% CI, −4.86 to 8.56; p = 0.54).
Figure1. Meta-analyses of AMI risk under the different genetic models.
These forest plots show the overall odds ratio for 6 studies included in the meta-analysis under the dominant, recessive, and additive models respectively. Doney and colleagues (2004) did not provide data for recessive and additive models. Size of cubes represents weight of each study.
Figure 2. Meta-analyses of CHD risk under the different genetic models.
These forest plots show the overall odds ratio for 9 studies included in the meta-analysis under the dominant, recessive, and additive models respectively. Doney and colleagues (2004) did not provide data for recessive and additive models. Size of cubes represents weight of each study.
Discussion
In this prospective study among healthy Dutch women aged 49 to 70 years, no statistically signifi cant association for exchanging proline with alanine in PPARG gene was seen with CHD and ischemic stroke risk under different genetic models. We found a borderline effect for AMI risk under the dominant and additive models, which could be a chance fi nding. In the meta-analyses of published observational studies we did not fi nd any signifi cant association for the polymorphism and AMI and CHD risks under different inheritance models.
In this study, prevalent cases of CHD and cerebrovascular disease were excluded to prevent introducing bias due to potentially selective survival. The Prospect study is a population-based cohort, which makes it less susceptible to selection bias. Additional strengths are the comprehensive data and sample collection, complete hospital admission and mortality follow-up, and the case-cohort design which combines the advantages of cohort studies (multiple outcomes and time-dependent covariates) with those of case-control analyses (fewer subjects), thus being more effi cient. Since the genotypes were in the Hardy-Weinberg equilibrium, we did not have misclassifi cation of exposure (genotypes). Limitations are the relative short period of follow-up and the relative small number of cases. The latter in general reduces the power to show a statistical signifi cant relations.
A new aspect is that we conducted meta-analyses on the relation between the P12A polymorphism and MI, CHD and ischemic stroke under different genetic models. Yet, due to the genotype frequency, its power is limited for most studies especially under recessive genetic model. The results of these meta-analyses indicate no association between the polymorphism and risk of MI and CHD under different genetic models. However it must be noted that an important issue in every meta-analysis is publication bias as negative studies are less likely to be submitted or accepted for publication, especially when this concerns smaller studies. Although publication bias was not present based on Egger’s test, the performance of this test and the usual funnel plot have been challenged (Peters et al 2006) and so we can not completely rule out low probability for missing of small negative studies. Another limitation may be that our meta-analysis was based on published results and that we did not have access to original individual data. Fi nally, the case control studies included in the meta-analysis could be potentially prone to selection bias if survival post-MI varies by genotype.
The Physician’s Health Study (Ridker et al 2003) reported a modest protective effect of P12A polymorphism in PPARG gene for incidence of AMI among 523 individuals who subsequently developed myocardial infarction and 2092 individuals who remained free of reported cardiovascular disease in a prospective cohort of 14916 initially healthy American white men (Physician’s Health Study cohort) aged 40 to 84 years over a mean follow-up period of 13.2 years. The Ala12 allele was associated with 23% reduction in myocardial infarction risk (OR 0.77; 95% CI, 0.60 to 0.98). In a recent case-control study of 844 subjects including 218 patients, increased risk of MI was seen under dominant mode of inheritance (OR 1.83; 95% CI, 1.06–3.1) (Li et al 2006). Under dominant and additive modes of inheritance we found a statistically signifi cant association (p = 0.05 and 0.03, respectively) for risk of AMI, but it must be considered that the number of AMI cases was 71. Since it has been documented that very large sample sizes are required to provide suffi ciently precise estimates of genotype–disease associations (Smith and Ebrahim 2004), the power in our study was low (under 20%) which means that the probability for having a false positive fi nding was around 80%. Therefore, we conducted a meta-analysis of 6 studies with 1739 AMI cases and 5903 controls to obtain a more precise estimate. The meta-analysis did not show a signifi cant association under dominant, recessive and additive genetic models. Moreover, in a very recent prospective population-based study of multi-locus candidate gene polymorphisms by a group of investigators who had previously published a part of their results (Ridker et al 2003), showed that neither these three polymorphisms nor the others were predictors of MI (Zee et al 2006).
Our fi ndings with respect to CHD are in accordance with results from Nurses’ Health Study and Health Professionals Follow-up Study respectively in women and men of 245 cases of nonfatal MI or fatal CHD in women (compared with 485 controls) and 250 in men (compared with 502 controls) during 8 and 6 years of follow-up that the P12A polymorphism is not associated with decreased risk of CHD (Pischon et al 2005). In a cross-sectional study of patients with diabetes mellitus type 2 in Germany (in 201 patients with and 164 without CHD) that the A12 allele was not related to CHD risk (Bluher et al 2002). Our fi ndings and the meta analyses fi ndings agree with these fi ndings.
Recently, P12A polymorphism has been related to a reduced risk for ischemic stroke in patients with type 2 diabetes (Lee et al 2006). We found no association between the polymorphism and risk of occurrence of ischemic stroke under different genetic models. As these two studies are the only ones available, further studies are needed.
In conclusion, this study in healthy women free from previous cardiovascular disease and the meta-analyses show that, the P12A polymorphism in PPARG gene is not associated with future risk of AMI, CHD, and ischemic stroke.
Acknowledgments
We are grateful to the participants of the Prospect-EPIC study. We thank all fi eld workers, laboratory technicians and skillful contributions to the data collection and all those who contributed to this study.
Funding Dr Zafarmand has been supported by a grant from the Iranian Ministry of Health and Medical Education (FN12265). The Prospect-EPIC study was funded by "Europe Against Cancer" Programme of the European Commission (SANCO).
Footnotes
Declaration of interests Authors declare that no competing interests exist.
References
- Al Shali KZ, House AA, Hanley AJ, et al. Genetic variation in PPARG encoding peroxisome proliferator-activated receptor gamma associated with carotid atherosclerosis. Stroke. 2004;35:2036–40. doi: 10.1161/01.STR.0000138784.68159.a5. [DOI] [PubMed] [Google Scholar]
- Altshuler D, Hirschhorn JN, Klannemark M, et al. The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet. 2000;26:76–80. doi: 10.1038/79216. [DOI] [PubMed] [Google Scholar]
- Bluher M, Klemm T, Gerike T, et al. Lack of association between peroxisome proliferator-activated receptor-gamma-2 gene variants and the occurrence of coronary heart disease in patients with diabetes mellitus. Eur J Endocrinol. 2002;146:545–51. doi: 10.1530/eje.0.1460545. [DOI] [PubMed] [Google Scholar]
- Cheng S, Grow MA, Pallaud C, et al. A multilocus genotyping assay for candidate markers of cardiovascular disease risk. Genome Res. 1999;9:936–49. doi: 10.1101/gr.9.10.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeb SS, Fajas L, Nemoto M, et al. A Pro12Ala substitution in PPARgamma2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat Genet. 1998;20:284–7. doi: 10.1038/3099. [DOI] [PubMed] [Google Scholar]
- Doney AS, Fi scher B, Leese G, et al. Cardiovascular risk in type 2 diabetes is associated with variation at the PPARG locus: a Go-DARTS study. Arterioscler Thromb Vasc Biol. 2004;24:2403–7. doi: 10.1161/01.ATV.0000147897.57527.e4. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knouff C, Auwerx J. Peroxisome proliferator-activated receptor-gamma calls for activation in moderation: lessons from genetics and pharmacology. Endocr Rev. 2004;25:899–918. doi: 10.1210/er.2003-0036. [DOI] [PubMed] [Google Scholar]
- Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–6. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- Lee BC, Lee HJ, Chung JH. Peroxisome proliferator-activated receptor-gamma2 Pro12Ala polymorphism is associated with reduced risk for ischemic stroke with type 2 diabetes. Neurosci Lett. 2006;410:141–5. doi: 10.1016/j.neulet.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Li L, Cheng LX, Nsenga R, et al. Association between Pro12Ala polymorphism of peroxisome proliferator-activated receptor-gamma 2 and myocardial infarction in the Chinese Han population. Clin Cardiol. 2006;29:300–4. doi: 10.1002/clc.4960290706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masud S, Ye S. Effect of the peroxisome proliferator activated receptor-gamma gene Pro12Ala variant on body mass index: a meta-analysis. J Med Genet. 2003;40:773–80. doi: 10.1136/jmg.40.10.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirhaeghe A, Fajas L, Helbecque N, et al. Impact of the Peroxisome Proliferator Activated Receptor gamma2 Pro12Ala polymorphism on adiposity, lipids and non-insulin-dependent diabetes mellitus. Int J Obes Relat Metab Disord. 2000;24:195–9. doi: 10.1038/sj.ijo.0801112. [DOI] [PubMed] [Google Scholar]
- Memisoglu A, Hu FB, Hankinson SE, et al. Prospective study of the association between the proline to alanine codon 12 polymorphism in the PPARgamma gene and type 2 diabetes. Diabetes Care. 2003;26:2915–17. doi: 10.2337/diacare.26.10.2915. [DOI] [PubMed] [Google Scholar]
- Ostgren CJ, Lindblad U, Melander O, et al. Peroxisome proliferator-activated receptor-gammaPro12Ala polymorphism and the association with blood pressure in type 2 diabetes: skaraborg hypertension and diabetes project. J Hypertens. 2003;21:1657–62. doi: 10.1097/01.hjh.0000084734.53355.0d. [DOI] [PubMed] [Google Scholar]
- Peters JL, Sutton AJ, Jones DR, et al. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676–80. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- Pischon T, Pai JK, Manson JE, et al. Peroxisome proliferator-activated receptor-gamma2 P12A polymorphism and risk of coronary heart disease in US men and women. Arterioscler Thromb Vasc Biol. 2005;25:1654–8. doi: 10.1161/01.ATV.0000171993.78135.7e. [DOI] [PubMed] [Google Scholar]
- Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73:1–11. [Google Scholar]
- Ridker PM, Cook NR, Cheng S, et al. Alanine for proline substitution in the peroxisome proliferator-activated receptor gamma-2 (PPARG2) gene and the risk of incident myocardial infarction. Arterioscler Thromb Vasc Biol. 2003;23:859–63. doi: 10.1161/01.ATV.0000068680.19521.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffrin EL, Amiri F, Benkirane K, et al. Peroxisome proliferator-activated receptors: vascular and cardiac effects in hypertension. Hypertension. 2003;42:664–8. doi: 10.1161/01.HYP.0000084370.74777.B6. [DOI] [PubMed] [Google Scholar]
- Smith GD, Ebrahim S. Mendelian randomization: prospects, potentials, and limitations. Int J Epidemiol. 2004;33:30–42. doi: 10.1093/ije/dyh132. [DOI] [PubMed] [Google Scholar]
- Temelkova-Kurktschiev T, Hanefeld M, Chinetti G, et al. Ala12Ala genotype of the peroxisome proliferator-activated receptor gamma2 protects against atherosclerosis. J Clin Endocrinol Metab. 2004;89:4238–42. doi: 10.1210/jc.2003-032120. [DOI] [PubMed] [Google Scholar]
- Tobin MD, Braund PS, Burton PR, et al. Genotypes and haplotypes predisposing to myocardial infarction: a multilocus case-control study. Eur Heart J. 2004;25:459–67. doi: 10.1016/j.ehj.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Trikalinos TA, Salanti G, Khoury MJ, et al. Impact of violations and deviations in Hardy-Weinberg equilibrium on postulated gene-disease associations. Am J Epidemiol. 2006;163:300–9. doi: 10.1093/aje/kwj046. [DOI] [PubMed] [Google Scholar]
- Vos HL, Doggen CJM, Rosendaal FR. Effect of the PPAR gamma2 Pro12Ala polymorphism on the risk of myocardial infarction. Blood. 2000;96:62B. [Google Scholar]
- Youssef J, Badr M. Role of peroxisome proliferator-activated receptors in infl ammation control. J Biomed Biotechnol. 2004;2004:156–166. doi: 10.1155/S1110724304308065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafarmand MH, van der Schouw YT, Grobbee DE, et al. T64A polymorphism in β3-adrenergic receptor gene (ADRB3) and coronary heart disease: a case-cohort study and meta-analysis. J Intern Med. 2007 doi: 10.1111/j.1365-2796.2007.01876.x. In Press. [DOI] [PubMed] [Google Scholar]
- Zee RYL, Cook NR, Cheng S, et al. Multi-locus candidate gene polymorphisms and risk of myocardial infarction: a population-based, prospective genetic analysis. J Thromb Haemost. 2006;4:341–8. doi: 10.1111/j.1538-7836.2006.01754.x. [DOI] [PubMed] [Google Scholar]