Abstract
Background
In older adults, there is often substantial undiagnosed chronic disease detectable on noninvasive testing, not accounted for by most comorbidity indices. We developed a simple physiologic index of comorbidity by scoring five noninvasive tests across the full range of values. We examined the predictive validity of this index for mortality and disability.
Methods
There were 2928 (mean age 74.5 years, 60% women, 85% white, and 15% black) participants in the Cardiovascular Health Study (1992–1993) who had carotid ultrasound, pulmonary function testing, brain magnetic resonance scan, serum cystatin-C, and fasting glucose. These were combined into a single physiologic index of comorbid chronic disease on a scale of 0–10. Cox proportional hazard models were used to predict mortality, mobility limitation, and activities of daily living (ADL) difficulty after a maximum of 9 years.
Results
The range of the physiologic index was quite broad, with very few individuals having total scores of either 0 or 10. Those with an index of 7–10 had a hazard ratio of 3.80 (95% confidence interval, 2.82–5.13) for mortality compared to those with scores of 0–2, after adjustment for demographics, behavioral risk factors, and clinically diagnosed conditions. Associations with mobility limitation and ADL difficulty were also significant. The index explained about 40% of the age effect on mortality risk.
Conclusion
Older adults with low levels of markers of chronic disease are rather rare but have remarkably good health outcomes. The ability of such an index to distinguish usual from low risk might provide an opportunity to better understand optimal health in old age.
Keywords: Disability, Mortality, Comorbidity
In older adults, chronic health conditions are quite common and are heterogeneous in patterns of co-occurrence, duration, and severity (1). Indices of comorbid conditions have been developed to account for overall disease burden when examining correlates and outcomes of an index condition. For example, differences in mortality in patients with coronary artery disease are largely explained by the extent of other concomitant morbid health conditions (2). Many investigators have successfully used simple counts of the number of common chronic conditions to summarize comorbidity (3,4). The Charlson Index is a more detailed method that is notable for accounting for severity as well as number of conditions (4).
Most comorbidity indices are based on medical records, claims data (5), or self-report of a physician diagnosis of disease, and thus represent clinically recognized comorbidity. It is well known that, with advancing age, the prevalence of many common chronic diseases increases markedly (3). Less well recognized is that chronic disease is often quite extensive well before clinical diagnosis is made. In community-dwelling older adults who do not report disease, the extent of subclinical disease can be substantial (6–9). Several epidemiologic studies have incorporated noninvasive tests of disease to better quantify early pathology. For a number of measures, such as vascular ultrasound, pulmonary function testing, and kidney function testing, the extent of measurable disease in older adults has been shown to range from extremely low levels to very high levels that can be as high as or higher than levels found in clinically diagnosed patient populations (10–12). Although the term “subclinical” is often used to describe this chronic disease pathology, it can be asymptomatic, presymptomatic, atypically symptomatic, or simply undiagnosed. Regardless of the reason for a lack of clinical diagnosis, such disease would not be counted in most clinical comorbidity indices. Because these noninvasive tests detect such a wide range of disease in the “undiagnosed,” it is possible that comorbidity assessment could be extended with noninvasive testing to further distinguish a very high versus very low level of disease in a more continuous fashion than can be done by using clinical diagnosis.
In the Cardiovascular Health Study (CHS), several of the most common chronic conditions were assessed using noninvasive testing. Using these data, we developed a simple physiologic index of comorbidity by scoring these tests across the full range of values. We examined the predictive validity of this index for mortality as well as for disability. We hypothesized that such an index might be a better determinant of risk than a clinical comorbidity index and would better identify those at medium to low risk. We also examined whether a composite index of these assessments might explain part of the contribution of age itself to risk.
Methods
Population
The CHS is an ongoing observational cohort study of cardiovascular risk in 5888 men and women from four regions of the United States (13). The cohort was 65 years old or older at enrollment in 1989–1990 and was supplemented with added minority recruitment in 1992–1993. Participants and eligible household members were identified from a random sample of Medicare enrollees at each field center. To be eligible, participants were 65 years old or older, did not have cancer under active treatment, could not be wheelchair- or bed-bound in the home, and did not plan to move out of the area within 3 years. We used data from the 1992–1993 examination to include all of the minority participants and to include the brain magnetic resonance imaging (MRI) scan conducted at that time. Of 3660 individuals with a brain MRI scan, a total of 2928 men and women had a clinical examination with complete data for the other major components used in the analysis.
Physiologic Index of Comorbidity
The clinical examination conducted in 1992–1993 included cardiovascular and pulmonary function tests, blood tests for kidney function and glucose tolerance, and a brain MRI. The choice of tests used in the analysis was based on previous reports that each is individually an important predictor of mortality (13,18), and that each represents a major, common age-related chronic disease. Additional tests available in the CHS were considered, but were not assessed at this same time point (bone density, e.g.). Others were nonspecific risk factors for mortality (such as C-reactive protein or interleukin-6), that is, they were not measures of chronic disease. Preliminary analyses confirmed that each was an independent predictor of mortality in the CHS. All noninvasive tests had been obtained and examined independently and were not used to diagnose or confirm clinical conditions.
Carotid ultrasound was obtained in the left and right internal and common carotid arteries to assess near and far wall thicknesses and Doppler flow. The mean of the maximum of the internal carotid artery was used in this analysis to represent the extent of atherosclerotic vascular disease (14). Spirometry, including forced vital capacity (FVC) and forced expiratory volume in one second (FEV1), was conducted using a water-sealed Collins Survey II spirometer (WE Collins, Braintree, MA) according to the standards of the American Thoracic Society (13). Fasting glucose levels were measured on a Kodak Ektachem 700 Analyzer (Ektachem Test Methodologies, Eastman Kodak, Rochester, NY) and assayed within 30 days. Average monthly coefficient of variation was 0.93% (15). Kidney function was assessed using a BNII nephelometer (Dade Behring Inc., Deerfield, IL) that used a particle-enhanced immunonephelometric assay (N Latex Cystatin-C) (16). Brain MRI was assessed on General Electric or Picker 1.5-T scanners at three field centers and on a 0.35-T Toshiba instrument at the fourth. The scanning protocol included a series of axial spin density, T1- and T2-weighted scans. Standardized sagittal T1-weighted spin-echo images, axial spin density/T2-weighted and T1-weighted images were acquired, and scanned data were interpreted at a central MRI Reading Center by a neurologist trained in a standardized protocol (17). The white matter grade score was used to indicate small-vessel vascular disease in the brain (18).
To construct the physiologic index of comorbidity, each of the five measures was divided into three groups with the best values classified as 0 and the worst as 2. Individual scores were summed for a total score ranging from 0 to 10. Tertile cut points for FVC on pulmonary function testing were sex-specific. (Women: 0 = 2.6–3.8 L, 1 = 2.2–2.6 L, 2 = 0.6–2.2 L; Men: 0 = 3.9–6.5 L, 1 = 3.2–3.9, 2 = 0.3–3.2 L). For the carotid wall thickness, tertile cut points were scored as 0 =0.60–1.06 mm, 1 =1.06–1.53 mm, 2 = 1.53–3.94 mm. Similarly, tertile cut points were used for cystatin-C, scored as 0 = 0.6–1.0 mg/dL, 1 = 1.0–1.1 mg/dL, 2 = 1.1–3.5 mg/dL. For white matter grade, tertile cut points were scored as 0 = 0–1 units, 1 = 2 units, 2 = 3–9 units on the 0–9 ordinal scale. Fasting glucose was the only measure not classified by tertile. Although results were similar, for clinical interpretation, this presentation uses cut points classified according to clinical cut points defined by the American Diabetes Association (0 = <100, 1 = 100–126, 2 = >126) (19). Although the choice of cut points was arbitrary, the best score of “0” was generally found to represent a healthy, young normal value, and values of “2” were in the range of individuals with diagnosed chronic disease. Of note, the Spearman correlations between each pair of individual measures were low (all between 0 and 0.15), although most of these correlations were statistically significant. Alternative combinatorial techniques, such as principal components analysis, did not reveal any useful groupings of these variables.
Demographic, Behavioral Health, and Clinical Disease Variables
Other variables included age, sex, and race (black, white, or other), which were ascertained by self-report. Physical activity (20), smoking (21), and physical function (22) were assessed by standardized interview. Blood pressure, height, and weight were assessed by standardized protocols. Body mass index (BMI) was calculated as kilograms per meter squared. Clinically diagnosed pulmonary disease, diabetes, kidney disease, and arthritis were assessed by self-report of physician diagnosis. Depression was defined on the basis of a score > 10 on a modified 10-item Center for Epidemiologic Studies Short Depression Scale (CES-D) score (23,24). Reports of cardiovascular disease and stroke were confirmed by review of medications and medical records. Using this information, a clinical comorbidity count was constructed for each person with a maximum of 7 for these chronic health conditions: cardiovascular disease, stroke, pulmonary disease, diabetes, kidney disease, arthritis, and depression.
Outcomes
Total mortality, incident mobility disability, and incident disability for activities of daily living (ADL) were the primary outcomes. For the disability outcomes, individuals with disability at the time of the noninvasive testing were excluded, resulting in a sample size of 2376 for mobility disability and 2700 for ADL disability outcome. Total and cause-specific mortality, cardiovascular events, and self-reported disability were assessed every 6 months via telephone interview alternating with clinic interview. Death was confirmed with death certificate, obituaries, proxy interview, review of hospital records, and a search of Medicare records. Mortality follow-up was virtually complete. Self-reported physical functioning was assessed by standard interview. Incident disability was defined as new onset of difficulty walking 1/2; mile (mobility limitation) and also by the new onset of difficulty with any one of six ADLs (25). Maximum follow-up was up to 9 years after the 1992–1993 examination.
Analysis
Crude event rates per person-year were calculated, and hazard ratios using Cox proportional hazards models were estimated for each point of the physiologic index treated as a continuous variable, as well as by groupings of the physiologic index score (0–3, 4–5, 6–7, 8–10). Time to event or censor in the Cox models was calculated from the date of the 1992–1993 clinical examination. Models were first examined without adjustment for other factors, then with adjustment, first for age, sex, and race, then with multivariate adjustment for behavioral risk factors (smoking, physical activity, BMI) and either the clinical comorbidity index or for each individual chronic health condition. Interactions between the physiologic index and age, sex, and race were assessed; none were significant. Proportional hazards assumptions were confirmed for all models. The Schwartz Bayesian Criterion, which penalizes overfitting, was used as a measure of goodness of fit when comparing models (26). The integrated average of areas under time-dependent receiver operating characteristic curves was used to compare the predictive accuracy of different survival models (27,28). All analyses were conducted using SAS version 8.0 (Cary, NC).
Results
The 2928 participants’ mean age was 74.5 years (Table 1). There were 1691 (57.7%) women and 423 (14.4%) blacks. BMI was similar in men and women, whereas smoking was more common in men, and they reported more physical activity. Prevalence of most chronic health conditions varied, with depression, chronic obstructive pulmonary disease, and arthritis reported more often by women, and vascular disease and diabetes reported more often by men. Mean values for the physiologic index were somewhat lower for women than for men, whereas the number of chronic conditions was slightly higher in women.
Table 1.
Characteristics of Study Participants: The Cardiovascular Health Study, 1992–1993 Examination
| Characteristics | Men (N = 1237) | Women (N = 1691) | Total (N = 2928) | p* |
|---|---|---|---|---|
| Demographics | ||||
| Age, y, mean (SD) | 75.0 (5.0) | 74.2 (4.8) | 74.5 (4.9) | <.001 |
| Race | ||||
| White, n (%) | 1071 (86.6) | 1420 (84.0) | 2491 (85.1) | .138 |
| Black, n (%) | 160 (12.9) | 263 (15.6) | 423 (14.5) | |
| Others, n (%) | 6 (0.5) | 8 (0.5) | 14 (0.5) | |
| Behavioral risk factors | ||||
| Body mass index, kg/m2 mean (SD) | 26.4 (3.6) | 26.8 (5.0) | 26.6 (4.4) | .018 |
| Smoking status | ||||
| Current, n (%) | 114 (9.2) | 154 (9.1) | 268 (9.2) | <.001 |
| Past, n (%) | 743 (60.1) | 568 (33.6) | 1311 (44.8) | |
| Never, n (%) | 380 (30.7) | 969 (57.3) | 1349 (46.1) | |
| Physical activity, kcal/wk, mean (SD) | 1850.4 (2002.8) | 1295.1 (1541.8) | 1529.3 (1772.2) | <.001 |
| Chronic health conditions | ||||
| Coronary heart disease, n (%) | 341 (27.6) | 284 (16.8) | 625 (21.4) | <.001 |
| Cerebrovascular disease, n (%) | 105 (8.5) | 75 (4.4) | 180 (6.2) | <.001 |
| Diabetes, n (%) | 207 (16.7) | 190 (11.2) | 397 (13.6) | <.001 |
| Chronic obstructive lung disease, n (%) | 259 (20.9) | 536 (31.7) | 795 (27.2) | <.001 |
| Kidney disease, n (%) | 11 (0.9) | 20 (1.2) | 31 (1.1) | .443 |
| Arthritis, n (%) | 451 (36.5) | 936 (55.4) | 1387 (47.4) | <.001 |
| Depression (CES-D > 10), n (%) | 135 (10.9) | 275 (16.3) | 410 (14.0) | <.001 |
| No. of chronic health conditions, 0–7, mean (SD) | 1.2 (1.1) | 1.4 (1.1) | 1.3 (1.1) | <.001 |
| Physiologic index, 0–10, mean (SD) | 5.1 (2.1) | 5.7 (2.1) | 5.5 (2.1) | <.001 |
Notes: Chi-square or t test p value.
SD = standard deviation; CES-D = Center for Epidemiologic Studies Short Depression Scale.
The distribution of the physiologic index was compared to that of a comorbidity count (Figure 1). The range of the physiologic index was quite broad. Very few individuals had total scores of either 0 or 10, although there was a slight skewing toward better (lower) values. The comorbidity count was also skewed toward lower values, showing little discrimination at the low end of disease.
Figure 1.
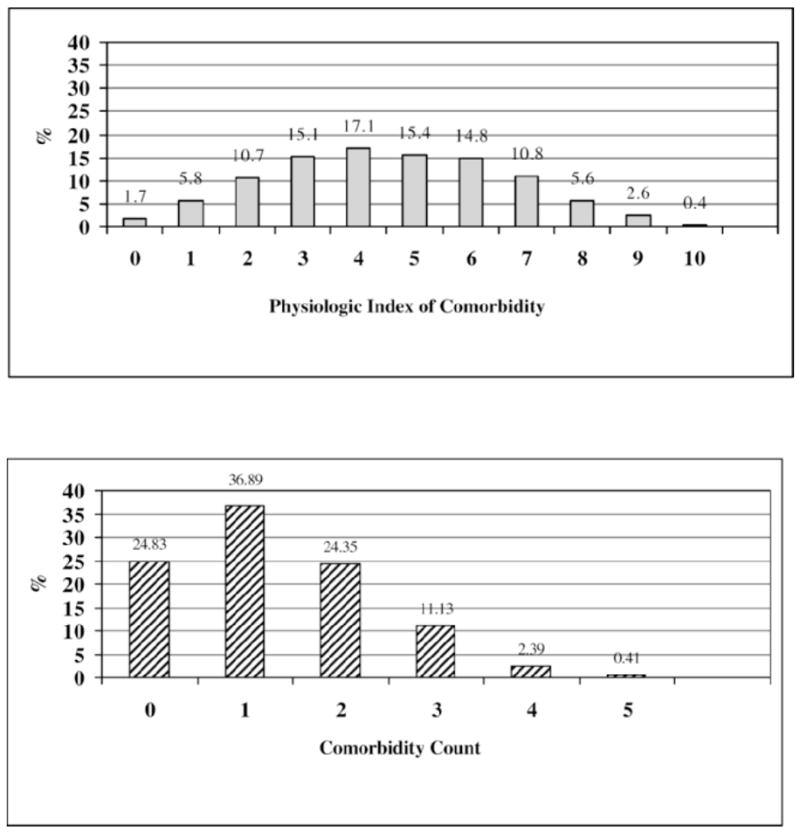
Distribution of physiologic index of comorbidity versus comorbidity count in 2928 Cardiovascular Health Study participants, 1992–1993. (Lower scores indicate less disease burden for both measures.)
Figure 2 shows crude mortality rates for each index. The crude mortality rates were highest in those with the highest physiologic index of comorbidity, but were also very high for those with a comorbidity count of ≥5. Of note, the mortality rate at the low end of the physiologic index was extremely low. Across this full range, the crude risk gradient was 20-fold higher for a score of 10 compared to a score of 0.
Figure 2.
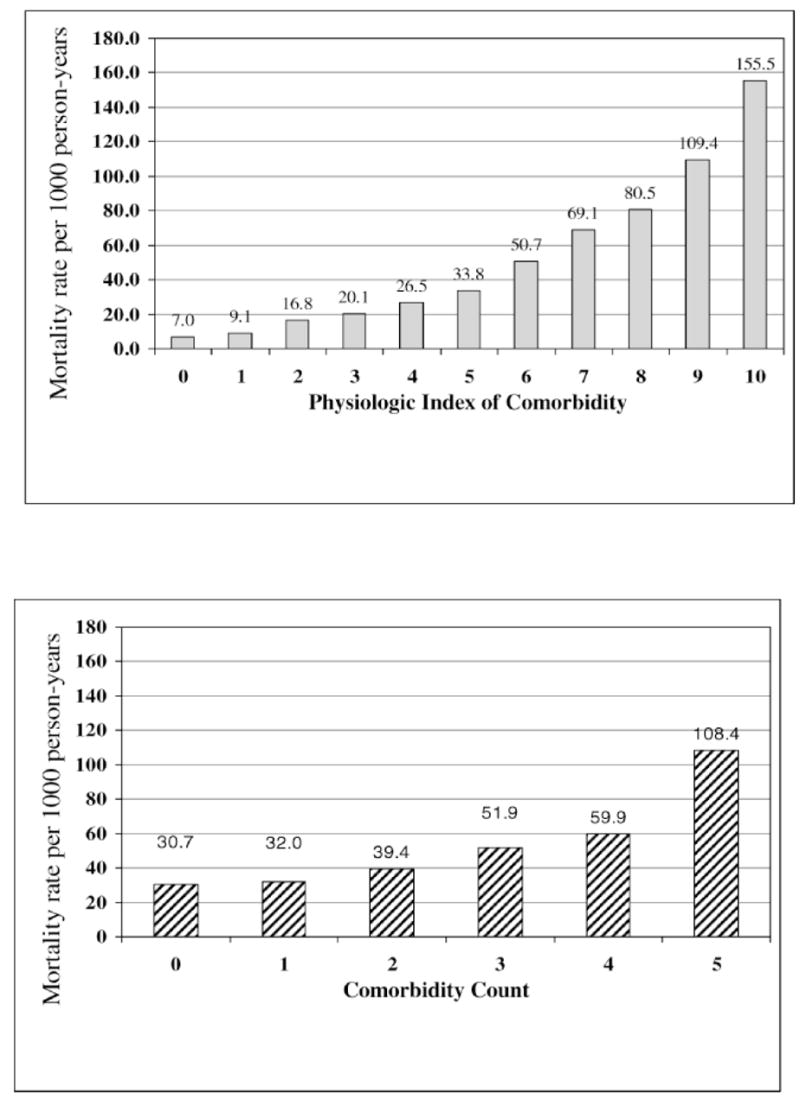
Crude mortality rates by physiologic index of comorbidity versus comorbidity count.
Hazard ratios were calculated for total mortality, incident mobility limitation, and incident ADL difficulty (Table 2). Risk ratios were expressed for each unit of the physiologic index and also for grouping of the index into approximate quartiles. Without adjustment, each unit of the physiologic index was associated with a 12%–27% higher risk of all of these adverse outcomes. This was somewhat attenuated but not fully explained by age adjustment and by full adjustment for other risk factors. In an alternate analysis, based on approximate quartiles of the score with smaller groups at the extremes collapsed, individuals with scores of 7–10 had a relative risk for mortality that was more than 6-fold higher than those with scores of 0–2. In all models, the physiologic index remained a strong and independent predictor of all of these outcomes. Figure 3, a–c, illustrates these differences in risk in survival curves for mortality, incident mobility limitation, and incident ADL disability. In all cases, there is a clear separation of risk throughout the follow-up period.
Table 2.
Hazard Ratios for Mortality, Incident Mobility Limitation and Incident ADL Difficulty by Physiologic Index of Comorbidity (0–10)
| Outcomes | Events per 1000 Person-Years | HR (95% CI) Unadjusted | HR (95% CI) Age, Sex, Race | HR (95% CI) Multivariate* |
|---|---|---|---|---|
| Mortality (N = 2928) | ||||
| HR per unit of Index | 1.37 (1.32–1.42) | 1.28 (1.24–1.33) | 1.26 (1.21–1.31) | |
| 0–2 (referent) | 13.3 | 1.00 | 1.00 | 1.00 |
| 3–4 | 23.5 | 1.78 (1.33–2.40) | 1.55 (1.16–2.09) | 1.50 (1.11–2.02) |
| 5–6 | 41.8 | 3.26 (2.46–4.32) | 2.46 (1.85–3.27) | 2.27 (1.70–3.03) |
| 7–10 | 78.8 | 6.54 (4.95–8.65) | 4.28 (3.21–5.72) | 3.80 (2.82–5.13) |
| Mobility limitation (N = 2376) | ||||
| HR per unit of Index | 1.21 (1.17–1.25) | 1.19 (1.15–1.23) | 1.11 (1.07–1.15) | |
| 0–2 (referent) | 51.1 | 1.00 | 1.00 | 1.00 |
| 3–4 | 78.2 | 1.55 (1.26–1.91) | 1.49 (1.21–1.84) | 1.31 (1.07–1.62) |
| 5–6 | 109.0 | 2.17 (1.76–2.66) | 2.10 (1.63–2.48) | 1.52 (1.23–1.89) |
| 7–10 | 159.6 | 3.31 (2.66–4.11) | 2.92 (2.33–3.68) | 1.93 (1.52–2.45) |
| ADL difficulty (N = 2700) | ||||
| HR per unit of Index | 1.16 (1.12–1.19) | 1.14 (1.11–1.78) | 1.06 (1.03–1.10) | |
| 0–2 (referent) | 49.8 | 1.00 | 1.00 | 1.00 |
| 3–4 | 73.4 | 1.49 (1.22–1.83) | 1.45 (1.18–1.78) | 1.29 (1.05–1.59) |
| 5–6 | 92.0 | 1.87 (1.52–2.29) | 1.73 (1.40–2.13) | 1.37 (1.11–1.69) |
| 7–10 | 123.5 | 2.58 (2.08–3.20) | 2.32 (1.85–2.90) | 1.48 (1.17–1.88) |
Notes: Adjusted for age, sex, race, physical activity, smoking, body mass index, and clinical comorbidity index.
ADL = activities of daily living; HR = hazard ratio; CI = confidence interval.
Figure 3.
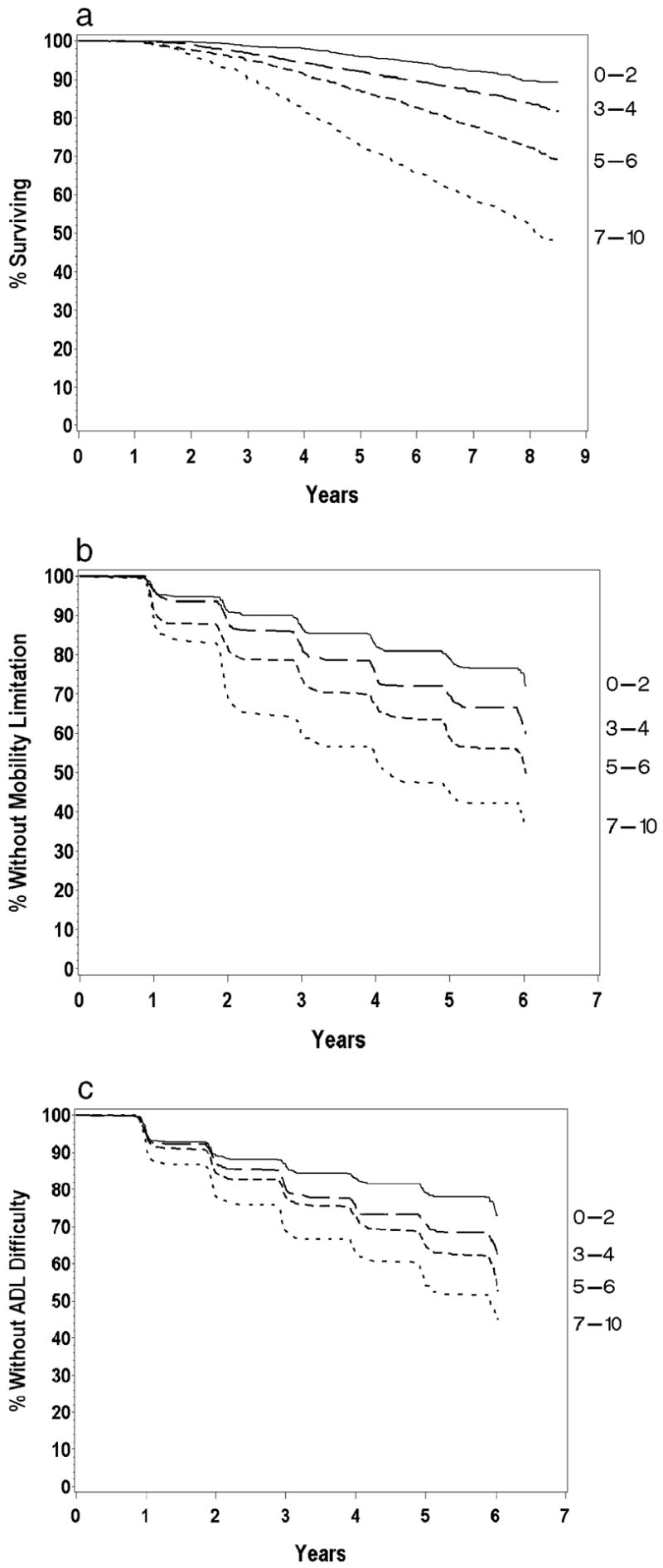
a, Survival curves according to physiologic index of comorbidity, grouped by 0–3, 4–5, 6–7, 8–10. b, Mobility limitation according to physiologic index of comorbidity, grouped by 0–3, 4–5, 6–7, 8–10. c, Incident activities of daily living (ADL) difficulty according to physiologic index of comorbidity, grouped by 0–3, 4–5, 6–7, 8–10.
To further evaluate relative predictive value of the physiologic index for mortality, we compared a model with age alone to another with the physiologic index alone and compared these to two additional models, one with age, sex, race, and the physiologic index and another where all variables including the components of the index, risk factors, and chronic health conditions were entered individually. Models were compared using the area under the curve (AUC). Age alone carried substantial predictive power with AUC = 0.673. The index alone performed somewhat better than age alone with AUC =0.706. Adjustment for age, sex, and race increased the AUC to 0.726. Additional adjustment for behavioral risk factors and clinical comorbidity increased the AUC only a little further to 0.735. In the Cox proportional hazards models for mortality, the physiologic index could be shown to attenuate the coefficient for age by about 40%. Figure 4 illustrates the much larger attenuation of age by the physiologic index compared to the comorbidity count. Thus, the index itself discriminated mortality risk very well, but did not fully explain the effect of age on mortality risk. As a single factor, the physiologic index predicted mortality slightly better than did age itself.
Figure 4.
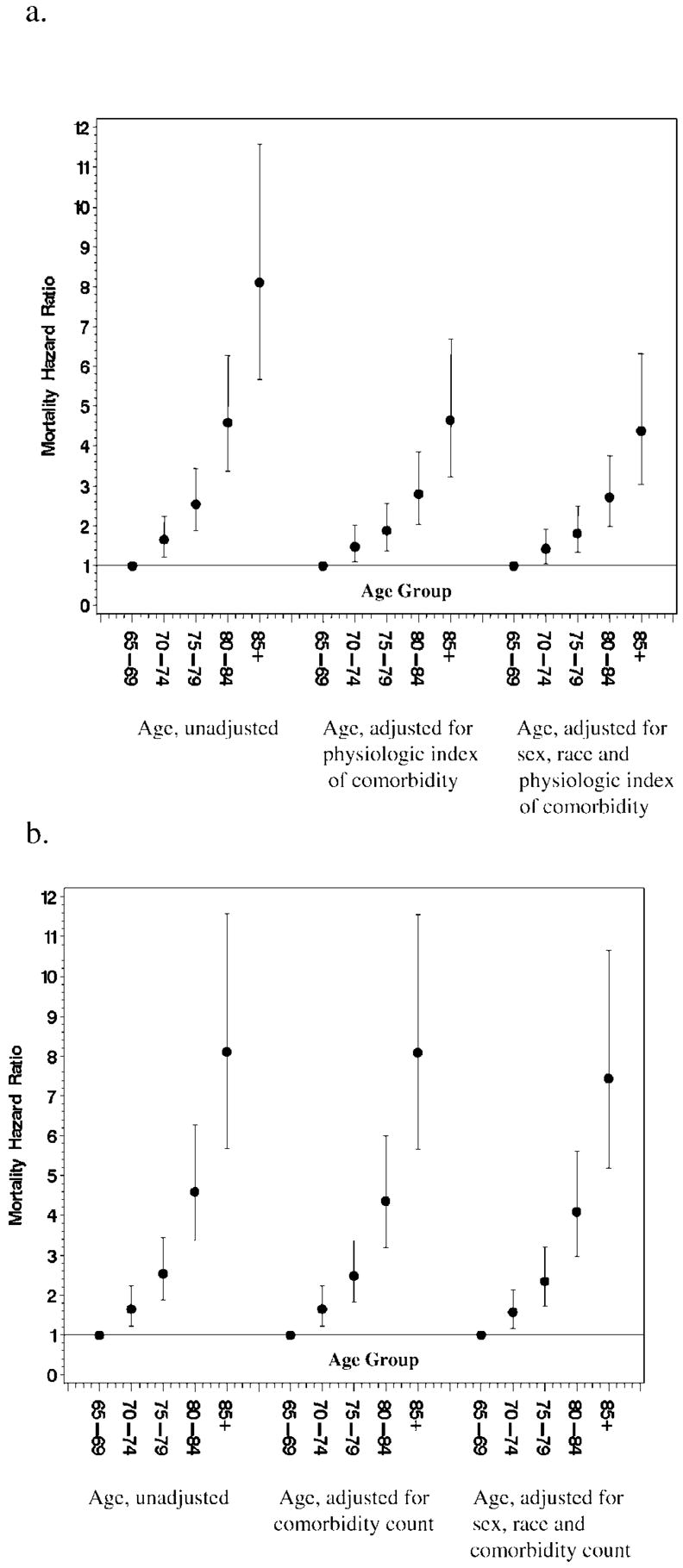
a, Association of age with mortality (i) unadjusted, (ii) adjusted for physiologic index of comorbidity, and (iii) adjusted for age, sex, race, and physiologic index of comorbidity. b, Association of age with mortality (i) unadjusted, (ii) adjusted for comorbidity count, and (iii) adjusted for age, sex, race, and comorbidity count.
Discussion
This simple method of summarizing five noninvasive tests of chronic disease into a more continuous physiologic index of comorbidity provided very powerful discrimination of risk for mortality and disability from very, very low risk to extremely high risk in this community-dwelling cohort of older adults. A simple comorbidity count was also very effective for identifying individuals at high absolute risk, but did not discriminate among individuals at low risk.
Noninvasive tests have several advantages over clinical diagnosis in defining disease burden. The extent of disease can be assessed at the middle to low range of disease, thus providing further discrimination within the pre- or subclinical range of disease. Testing can reduce variability that may occur with clinical diagnosis threshold. For example, there are known variations in diagnostic threshold related to age, sex, race, and socioeconomic status (29–31). Furthermore, such methods can provide more continuous rather than dichotomous or ordinal scales of severity. Disadvantages include the cost of testing and interpretation of the results. Increasingly, however, such testing is becoming more automated and miniaturized.
This index was developed to summarize comorbidity at the organ level, parallel to the World Health Organization’s International Classification of Functioning Health and Disease (32). In our framework, we did not include nonspecific age-related abnormalities or the function at the person level as part of comorbidity. We would hypothesize that age-related changes in hormones and inflammation may well be strongly related to subclinical disease burden and that this in turn will also be linked to physical function and frailty (33–35).
A similar, but broader nosology was recently proposed as a framework for developing comorbidity indices and called for research that would develop and refine such an index. Such indices have many potential uses in clinical care and clinical research in older adults, including risk assessment, prognosis, evaluation of treatment effects, communication about disease burden, and evaluation of the role of comorbidity in observational studies and in clinical trials. Additional research is needed to determine whether other aspects of physiologic assessment improve prediction of outcomes or are more efficient in certain settings (36,37).
It is noteworthy that the physiologic index predicted mortality and disability better than age itself and substantially attenuated the effect of age on mortality risk. The ability of a factor or factors to explain the strong age effect on risk has been held forth as one potential criterion for defining a biomarker of aging (38). In that sense, the burden of subclinical chronic disease could be considered to be a biomarker of aging.
Strengths of this study include the unique data on multiple systems that were obtained in the CHS along with the detailed outcomes ascertainment. However, it is important to note that there are limitations to be considered. First, there was attrition of participants so that those with all five measurements would represent a healthier subset of participants in the study. Additionally, because the examinations were developed primarily to assess cardiovascular disease, the choice of measures was limited to those already available. Finally, the tests were examined at only one point in time and do not incorporate rate of change and treatment effects, which may also be important to risk.
These findings illustrate the large burden of disease that exists in older populations. The spectrum of disease by testing was quite broad and extremely heterogeneous. Older adults who have very minimal disease by this combination of tests were rather rare. Their low mortality rates validate this group as an important exceptional survival cohort. Such individuals may have unique life histories or perhaps genetic characteristics that predispose them to longevity and protection from age-related chronic disease, providing an opportunity to better understand optimal health in old age.
Acknowledgments
The research reported in this article was supported by grants R01-AG-023629 and 5-P30-AG-024827 and by contracts N01-HC-35129, N01-HC-45133, N01-HC-75150, N01-HC-85079 through N01-HC-85086, N01 HC-15103, N01 HC-55222, and U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contributions from the National Institute of Neurological Disorders and Stroke.
A full list of participating CHS investigators and institutions can be found at http://www.chs-nhlbi.org.
References
- 1.Hoffman C, Rice D, Sung HY. Persons with chronic conditions. Their prevalence and costs. JAMA. 1996;276:1473–1479. [PubMed] [Google Scholar]
- 2.Sachdev M, Sun JL, Tsiatis AA, Nelson CL, Mark DB, Jollis JG. The prognostic importance of comorbidity for mortality in patients with stable coronary artery disease. J Am Coll Cardiol. 2004;43:576–582. doi: 10.1016/j.jacc.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 3.Guralnik JM, LaCroix AZ, Everett DF, Kovar MG. Aging in the eighties: the prevalence of comorbidity and its association with disability. Adv Data. 1989;170:1–8. [Google Scholar]
- 4.Charlson ME, Sax FL, MacKenzie R, Fields SD, Braham RL, Douglass RG., Jr Assessing illness severity: does clinical judgement work? J Chronic Dis. 1986;39:439–452. doi: 10.1016/0021-9681(86)90111-6. [DOI] [PubMed] [Google Scholar]
- 5.Ash AS, Ellis RP, Pope GC, et al. Using diagnosis to describe populations and predict costs. Health Care Financ Rev. 2000;21:7–28. [PMC free article] [PubMed] [Google Scholar]
- 6.Waterer GW, Wan JY, Kritchevsky SB, et al. Airflow limitation is underrecognized in well-functioning older people. J Am Geriatr Soc. 2001;49:1032–1038. doi: 10.1046/j.1532-5415.2001.49205.x. [DOI] [PubMed] [Google Scholar]
- 7.Newman AB, Siscovick DS, Manolio TA, et al. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation. 1993;88:837–845. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 8.Longstreth WT, Jr, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 9.Newman AB, Haggerty CL, Kritchevsky SB, Nevitt MC, Simonsick EM for the Health ABC Collaborative Research Group. Walking performance and cardiovascular response: associations with age and morbidity – The Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2003;58A:715–720. doi: 10.1093/gerona/58.8.m715. [DOI] [PubMed] [Google Scholar]
- 10.Kuller LH, Shemanski L, Psaty BM, et al. Subclinical disease as an independent risk factor for cardiovascular disease. Circulation. 1995;92:720–726. doi: 10.1161/01.cir.92.4.720. [DOI] [PubMed] [Google Scholar]
- 11.Enright PL, McBurnie MA, Bittner V, et al. The 6-min walk test: a quick measure of functional status in elderly adults. Chest. 2003;123:387–398. doi: 10.1378/chest.123.2.387. [DOI] [PubMed] [Google Scholar]
- 12.Shlipak MG, Fried LF, Cushman M, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293:1737–1745. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 13.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 14.O’Leary DH, Polak JF, Wolfson SK, Jr, et al. Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Health Study. CHS Collaborative Research Group. Stroke. 1991;22:1155–1163. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 15.Barzilay JI, Kronmal RA, Gottdiener JS, et al. The association of fasting glucose levels with congestive heart failure in diabetic adults ≥65 years. J Am Coll Cardiol. 2004;43:2236–2241. doi: 10.1016/j.jacc.2003.10.074. [DOI] [PubMed] [Google Scholar]
- 16.Fried LF, Katz R, Sarnak MJ, et al. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol. 2005;16:3728–3735. doi: 10.1681/ASN.2005040384. [DOI] [PubMed] [Google Scholar]
- 17.Longstreth WT, Jr, Dulberg C, Manolio TA, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2002;33:2376–2382. doi: 10.1161/01.str.0000032241.58727.49. [DOI] [PubMed] [Google Scholar]
- 18.Kuller LH, Arnold AM, Longstreth WT, Jr, et al. White matter grade and ventricular volume on brain MRI as markers of longevity in the cardiovascular health study. Neurobiol Aging. 2007;28:1307–1315. doi: 10.1016/j.neurobiolaging.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 20.Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108:161–175. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 21.Higgins MW, Enright PL, Kronmal RA, et al. Smoking and lung function in elderly men and women: the Cardiovascular Health Study. JAMA. 1993;269:2741–2748. [PubMed] [Google Scholar]
- 22.Fitti JE, Kovar MG. National Center for Health Statistics. Vital and Health Statistics, Series 1 No. 21. DHHS Pub. No. (PHS) 87–1323. Public Health Service, Washington DC: US Government Printing Office; October 1987, The supplement on aging to the 1984 National Health Interview Survey. [PubMed] [Google Scholar]
- 23.Orme J, Reis J, Herz E. Factorial and indiscriminate validity of the center for epidemiological studies depression (CES-D) scale. J Clin Psychol. 1986;42:28–33. doi: 10.1002/1097-4679(198601)42:1<28::aid-jclp2270420104>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 24.Schulz R, Beach SR, Ives DG, Martire LM, Ariyo AA, Kop WJ. Association between depression and mortality in older adults: the Cardiovascular Health Study. Arch Intern Med. 2000;160:1761–1768. doi: 10.1001/archinte.160.12.1761. [DOI] [PubMed] [Google Scholar]
- 25.Ettinger WH, Fried LP, Harris T, et al. Self-reported causes of physical disability in older people: the Cardiovascular Health Study. J Am Geriatr Soc. 1994;42:1035–1044. doi: 10.1111/j.1532-5415.1994.tb06206.x. [DOI] [PubMed] [Google Scholar]
- 26.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–464. [Google Scholar]
- 27.Heagerty PJ, Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics. 2005;61:92–105. doi: 10.1111/j.0006-341X.2005.030814.x. [DOI] [PubMed] [Google Scholar]
- 28.Heagerty PJ, Lumley T, Pepe MS. Time dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337–344. doi: 10.1111/j.0006-341x.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 29.Franse LV, Di Bari M, Shorr RI, et al. Type 2 diabetes in older well-functioning people: who is undiagnosed? Data from the Health, Aging, and Body Composition Study. Diabetes Care. 2001;24:2065–2070. doi: 10.2337/diacare.24.12.2065. [DOI] [PubMed] [Google Scholar]
- 30.Ayanian JZ, Zaslavsky AM, Weissman JS, Schneider EC, Ginsburg JA. Undiagnosed hypertension and hypercholesterolemia among uninsured and insured adults in the Third National Health and Nutrition Examination Survey. Am J Public Health. 2003;93:2051–2054. doi: 10.2105/ajph.93.12.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whittle J, Conigliaro J, Good CB, et al. Racial differences in the use of invasive cardiovascular procedures in the Department of Veterans Affairs Medical System. N Engl J Med. 1993;329:621–627. doi: 10.1056/NEJM199308263290907. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. The international classification of functioning, disability, and health (ICF) Geneva, Switzerland: World Health Organization; 2002. Toward a common language of functioning, disability, and health. [Google Scholar]
- 33.Fried LP, Tangen CM, Walston J, Newman AB, et al. for the Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56A:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 34.Newman AB, Gottdiener JS, McBurnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56A:M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 35.Walston J, McBurnie MA, Newman A, et al. for the Cardiovascular Health Study Collaborative Research Group. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 36.Karlamangla A, Tinetti M, Guralnik J, Studenski S, Wetle T, Reuben D. Comorbidity in older adults: nosology of impairment, diseases, and conditions. J Gerontol A Biol Sci Med Sci. 2007;62A:296–300. doi: 10.1093/gerona/62.3.296. [DOI] [PubMed] [Google Scholar]
- 37.Lash TL, Mor V, Wieland D, Ferrucci L, Satariano W, Silliman RA. Methodology, design and analytic technique to address measurement of comorbid disease. J Gerontol A Biol Sci Med Sci. 2007;62A:281–285. doi: 10.1093/gerona/62.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masoro EJ. Are age-associated diseases an integral part of aging? In: Masoro EJ, Austad SN, editors. Handbook of the Biology of Aging. 6. San Diego, CA: Academic Press; 2006. pp. 43–62. [Google Scholar]


