Abstract
Thrombotic microangiopathy, which includes thrombotic thrombocytopenic purpura (TTP), shiga-toxin associated hemolytic uremic syndrome (Stx-HUS) and atypical HUS, is characterized pathologically by the development of hyaline thrombi in the microvasculature and clinically by the manifestations of thrombocytopenia, microangiopathic hemolysis, and organ dysfunction. Renal failure is a predominant complication of both Stx-HUS and atypical HUS, while neurological complications are more prominent in TTP. Other disorders such as lupus or bone marrow transplantations may occasionally present with features of thrombotic microangiopathy. Recent studies have found autoimmune inhibitors or genetic mutations of a von Willebrand factor cleaving metalloprotease ADAMTS13 in patients with TTP. In approximately 30% – 50% of patients with atypical HUS, mutations have been detected complement factor H, membrane cofactor protein (CD46), or factor I. All three proteins are involved in the regulation of complement activation. Additionally autoantibodies of factor H have been described in patients without genetic mutations. These advances illustrate that dysregulation of VWF homeostasis or complement activation due to genetic or autoimmune mechanisms may lead to syndrome of thrombotic microangiopathy.
Keywords: ADAMTS13, Hemolytic uremic syndrome, Shear stress, Thrombotic microangiopathy, Thrombotic thrombocytopenic purpura, Regulators of complement activation, von Willebrand factor
Thrombotic microangiopathy is a clinico-pathological syndrome consisting of thrombotic thrombocytopenia, the hemolytic uremic syndrome, and other disorders characterized by hyaline thrombi in the microvasculature of various organs and the presence of fragmented red blood cells and thrombocytopenia in the peripheral blood. Widespread thrombosis in the microcirculation causes consumptive thrombocytopenia and creates abnormally high levels of shear stress that fragment the red blood cells. The thrombosis often affects the brain or kidney, causing mental changes, seizures, hematuria, hypertension, and/or renal function impairment. The heart and other vital organs may also be affected.
Thrombotic thrombocytopenic purpura (TTP) is a relatively uncommon yet intriguing disorder that frequently presents mysteriously and abruptly with the development of VWF- and plateletrich thrombi in the arterioles and capillaries of brain, heart, and other organs. Neurological and renal abnormalities such as confusion, focal neurological deficits, seizures, hematuria and proteinuria are common. Nevertheless, hypertension or advanced renal failure requiring dialysis is rare in TTP. Without treatment, the disease is associated with a very high mortality rate (> 90%). When treated with plasma infusion or plasma exchange, 70%–90% of the patients survive the acute episodes. However, relapse occurs in more than one third of the patients who achieve remission, contributing to further morbidity and mortality. A subset of patients develops chronic TTP, requiring long-term plasma exchange.
Initially described as a distinct uremic disorder following a prodrome of hemorrhagic diarrhea, the hemolytic uremic syndrome (HUS) has been closely intertwined with TTP, as evidenced by frequent use of the hybrid term TTP/HUS in the literature. HUS has also been extended to include atypical cases that are not preceded by a diarrheal prodrome. Other conditions such as systemic lupus erythematosus, bone marrow or solid organ transplants, chemotherapeutic or other medications, disseminated intravascular coagulopathy, and metastasizing malignancies may occasionally be complicated with the syndrome of thrombotic microangiopathy with variable renal and neurological involvement.
Recent studies have delineated the molecular defects of TTP and some of the atypical HUS cases, demonstrating that the syndrome of thrombotic microangiopathy may result from distinct pathogenetic processes. This article reviews the critical advances in the studies of TTP and HUS.
THROMBOTIC THROMBOCYTOPENIC PURPURA
VWF and hemostasis in the microcirculation
von Willebrand factor (VWF), a glycoprotein synthesized in vascular endothelial cells and megakaryocytes, supports platelet adhesion and aggregation at sites of vessel injury under high shear stresses. This unique property of VWF is believed to result from its large molecular structure that is conformationally flexible in response to shear stress. When VWF is attached to exposed blood vessel matrix components at a site of injury, it is exposed to high levels of shear stress at the boundary between the flowing blood and the vessel wall, which unfold the conformation of VWF from a globular to an elongated form, providing the substrate to support platelet adhesion and aggregation. Because large multimers are conformationally more responsive to shear stress than small multimers, the size of VWF multimers is a major determinant of VWF activity. Deficiency of VWF, particularly the large multimers, causes hemorrhagic diathesis characterized by mucocutaneous capillary bleeding.
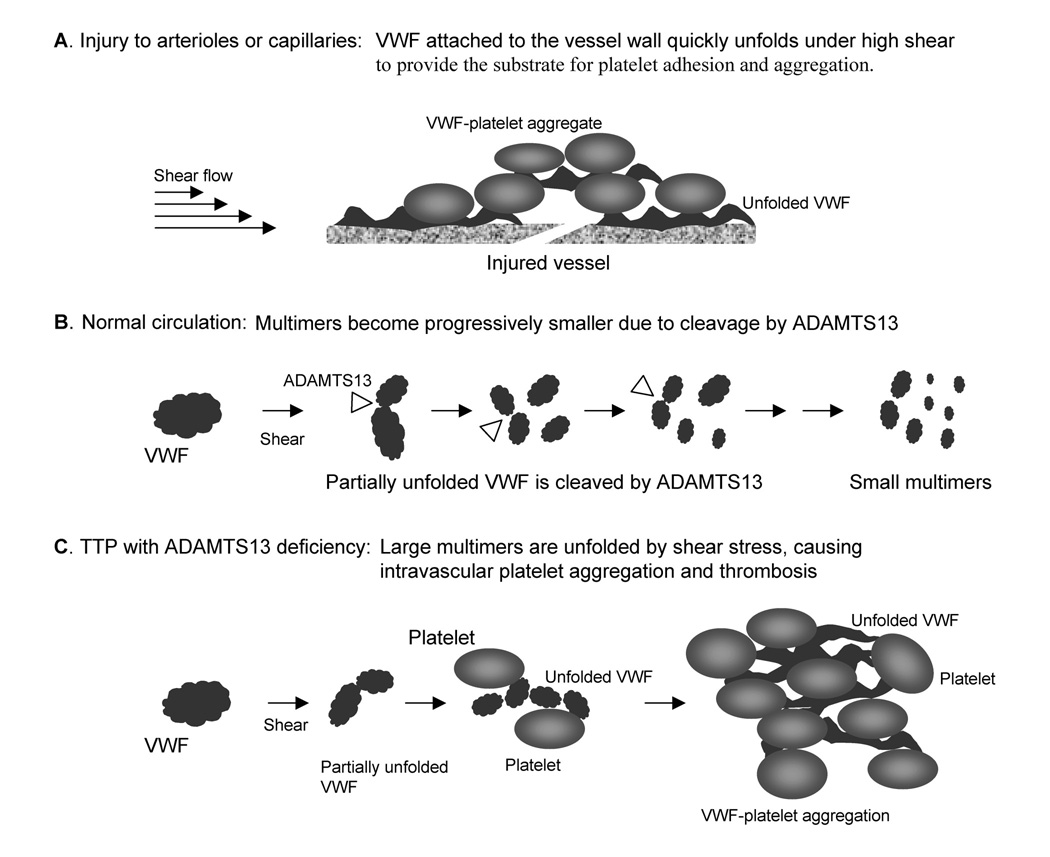
The process of conformational unfolding also occurs after a VWF molecule enters the circulation. However, since VWF is exposed to high shear stresses only very briefly during each cycle of passage through the microcirculation, the conformational change is much less efficient once a VWF molecule enters the circulation. ADAMTS13, a circulating zinc metalloprotease, cleaves VWF whenever one or more of its cleavage sites are exposed by shear stress. This proteolysis converts endothelial derived high molecular weight VWF polymer to a series of disulfide-bonded multimers as observed in the plasma samples. Deficiency of ADAMTS13 leads to a shift of plasma VWF multimers to larger sizes. Most importantly, in the absence of ADAMTS13, endothelial secreted VWF polymer and the large multimers in the circulation will eventually become fully unfolded by shear stress to become elongated forms, creating an environment favoring VWF-platelet binding, platelet aggregation, and microvascular thrombosis. The complex interaction among VWF, platelet, ADAMTS13, and shear stress is depicted in Figure 1[1].
Figure 1.
Schematic depiction of TTP due to ADAMTS13 deficiency. A. When a large VWF multimer is attached to the extracellular matrix at a site of injury, it is conformationally unfolded by high levels of wall shear stress to an elongated form, providing the substrate for platelets adhesion and hemostasis. B. In the normal circulation, VWF and platelets do not interact to form aggregates because ADAMTS13 cleaves the VWF multimer whenever one or more of its cleavage sites are exposed by shear stress. This process keeps VWF in inactive, globular forms as it become progressively smaller in size. C. In the absence of ADAMTS13, VWF multimers eventually become fully unfolded by shear stress, causing intravascular platelet thrombosis characteristic of TTP.
The process of VWF-platelet binding promoted by ADAMTS13 deficiency is likely to be affected by multiple factors such as the shear stress profile in the microvasculature, the reactivity of platelets, and the rate of VWF release from microvascular endothelial cells. Despite these compounding factors, serial investigation of individual TTP cases often detects a clear inverse correlation between the ADAMTS13 activity level and the VWF-platelet binding, as represented by the severity of thrombocytopenia and depletion of ultra large and large VWF multimers. In our experience, when ADAMTS levels decrease below 15% – 20% of normal level, a shift of the VWFmultimers to larger sizes is detectable by SDS agarose gel electrophoresis. Further decrease of ADAMTS13 activity below 10% of normal begins to result in clinically apparent thrombosis, thrombocytopenia, and progressive depletion of the large multimers. The threshold values cited here may differ when other versions of ADAMTS13 assays are used.
Recently several studies have demonstrated that when endothelial cells are exposed to cytoactive molecules such as histamine or calcium ionophore A23187, the secreted VWF may remain anchored to endothelial surface, providing a substrate to support platelet adhesion[2–4]. ADAMTS13 causes detachment of the platelets, presumably by cleaving the anchored VWF. In the absence of ADAMTS13, these platelet strands tend to persist. It remains to be determined whether this process may contribute to the exacerbation of TTP in association with the stresses of infection, surgery, or pregnancy.
Structure and function of ADAMTS13
ADAMTS13 belongs to a family of extra-cellular, multi-domain zinc metalloproteases in which the catalytic metalloprotease domain with a zinc-binding active-site motif is similar to that of ADAM proteases and is followed by a disintegrin-like domain, a central thrombospondin type 1 repeat (TSR), a cysteine-rich domain, a cysteine-free spacer domain and usually one or more TSRs (Table 1 and Figure 2). ADAMTS13 is constitutively active in the circulation with a narrow range of activity (79% – 127%) in normal individuals. Recombinant expression studies reveal that ADAMTS13 constructs truncated at the central TSR domain retain a small fraction of VWF-cleaving activity[5]. The addition of the cysteine-rich and the spacer domains markedly increases the proteolytic potency to approximately 50% of the full-length enzyme. Since the spacer domain is essential for interaction with the anti-ADAMTS13 antibodies of TTP, ADAMTS13 variants devoid of the spacer domain are not recognized or suppressed by TTP IgG antibodies. Such non-suppressible ADAMTS13 variants may be exploited to circumvent the therapeutic difficulties of TTP posed by anti-ADAMTS13 antibodies[5].
Table 1.
Characteristics of proteins involved in the pathogenesis of TTP and atypical HUS
| Protein | ADAMTS13 | CFH | IF | MCP |
|---|---|---|---|---|
| Gene, RefSeq | NM 139026 | NM 000186 | NM 000204 | NM 002389 |
| Genomic size | 37 kb | 95 kb | 63 kb | 43 kb |
| Location | 9q34 | 1q32 | 4q25 | 1q32 |
| No. of exons | 29 | 23 | 13 | 14 |
| cDNA size | 4.7 kb | 4.0 kb | 2.0 kb | 3.4 kb |
| Amino acid residues (precursor) | 1427 | 1231 | 583 | 392 |
| Mol wt (kD) | ||||
| Predicted | 154 | 139 | 65.7 | 43.7 |
| Observed | 190 | 155 | 88 | 59–68 (BC1, BC2) |
| 43 | (50 & 38) | 51–58 (C1, C2) | ||
| Tissue expression | Liver (stellate cells) | Liver | Liver, EC, MNC, etc. | Kidney, fetal heart, brain, MNC, spermatozoa |
| Subcellular location | Plasma | Plasma | Plasma | Transmembrane protein |
| Plasma concentration | 1 µg/mLa | 3.5 mg/mL | 35 µg/mL | - |
| Function | Cleavage of sheared VWF | Cofactor of IF ↓ alternative C3 convertase | Cleavage of C3b, C4b | Cofactor of IF Activation of Treg |
| Causes of deficiency | Inhibitory antibodies | Genetic mutations | Genetic Mutations | Genetic Mutations |
| Genetic mutations | Autoantibody | |||
| Disorders of deficiency | TTP | MPGN* | Pyogenic infection | Atypical HUS |
| Atypical HUS | Vasculitis | |||
| Atypical HUS | ||||
HUS: hemolytic uremic syndrome; EC: endothelial cell; MNC: mononuclear cells; MPGN: membranoproliferative glomerulonephropathy; TTP: thrombotic thrombocytopenic purpura; Treg: regulatory T cells.
Estimated
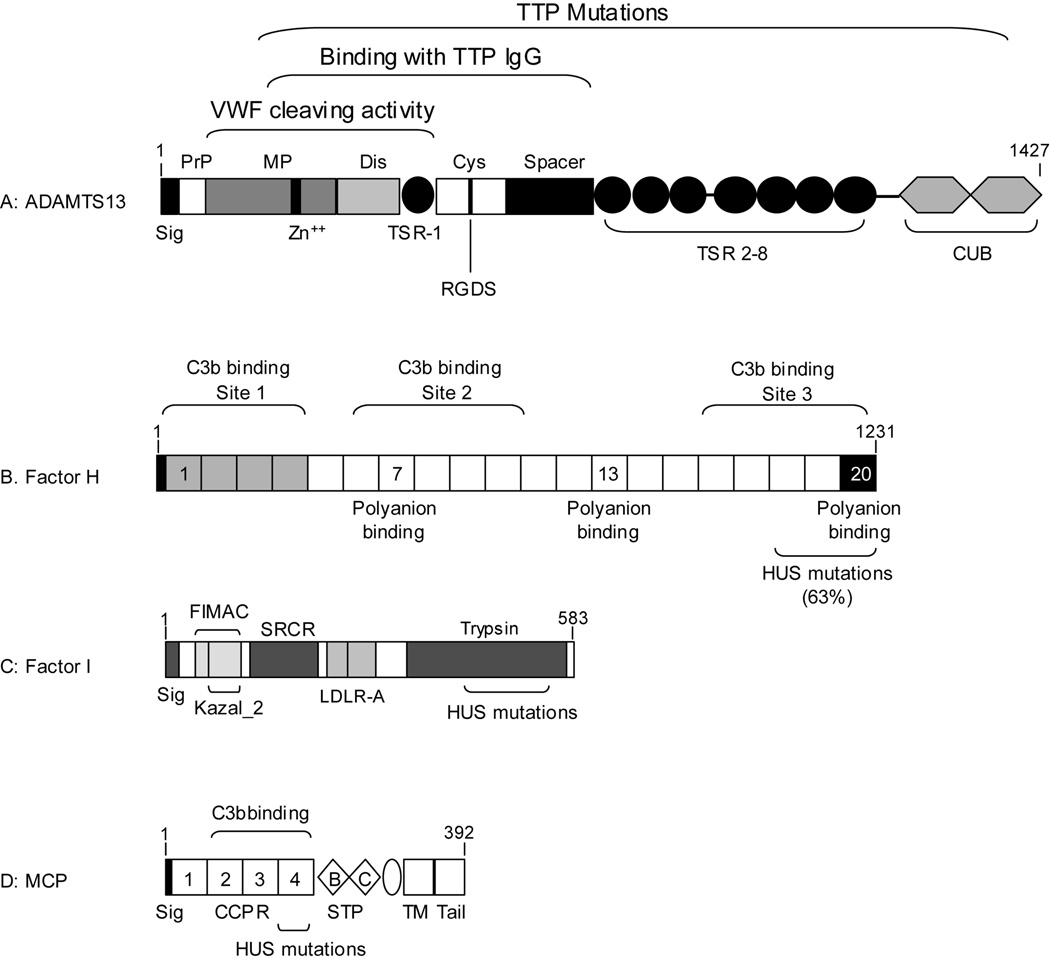
Figure 2.
Schematic depiction of the domain structures of proteins involved in the pathogenesis of TTP and atypical HUS. A. ADAMTS13. Sig: signal peptide; PrP: propeptide; MP: metalloprotease domain; Cys: cysteine-rich region. TSR: thrombospondin type 1 repeat. The amino acid sequence of the zinc-binding motif in the MP domain and the RGDS sequence in the cysteine-rich region are shown. Brackets indicate the segments exhibiting VWF cleaving activity or binding with the IgG of TTP. Mutations of ADAMTS13 have been detected throughout the entire span of the protein. B. Factor H. The protein consists of twenty complement control protein repeats (CCPR). The three C3b binding sites and three polyanion binding domains are indicated. The first C3b binding site (gray) has cofactor activity for complement factor I. The major polyanion-binding site at CCPR20 is highlighted in black. The majority of the mutations associated with HUS cluster at the C-terminal end, particularly CCPR20, which is critical for host binding. C. Complement factor I. The single-chain precursor form is depicted. FIMAC: factor I major attack complex; Kazal_2: Kazal-type serine protease inhibitor domain; SRCR: Scavenger receptor cysteine-rich domain; LDLR-A: LDL receptor domain class A; Trypsin: trypsin type protease domain. Four mutations have been detected in the protease domain of the protein. D. MCP (membrane cofactor protein). CCPR: complement control protein repeat. STP: serine/threonine/proline-rich domain; TM: transmembranous domain; Tail: cytoplasmic anchor. The region of the protein critical for C3b binding and cofactor activity is highlighted in the bracket. HUS mutations have been detected in CCPR4.
ADAMTS13 deficiency and TTP
Several large series of cases have confirmed the prevalence of ADAMTS13 deficiency among patients with the acquired form of TTP, as reviewed elsewhere[1]. The percentage of patients with ADAMTS13 deficiency ranges from 13% to 100%. This variation in prevalence is attributable to at least two reasons: the stringency of the criteria for defining the study cases and the assay used to measure ADAMTS13 activity. In some studies, the cases were classified based on the diagnosis provided by the referring physicians or the findings at the time of presentation.
Such series invariably included patients without TTP. When a patient appears to have “TTP” without ADAMTS13 deficiency, it is imperative to investigate the presence of an underlying cause that is not apparent at the time of presentation. In our experience, such unapparent causes may include systemic lupus erythematosus or related disorders, occult metastatic cancers, paroxysmal nocturnal hemoglobinuria with widespread mesenteric microvascular thrombosis, and atypical HUS. These conditions require different therapeutic approaches targeted to the specific causes.
Different ADAMTS13 assays have yielded opposite results in some cases, one of which has been described[6]. This and other similar incidences should serve as a precaution that each assay result needs to be carefully scrutinized and corroborated with clinical and laboratory data. VWF multimer analysis by agarose gel electrophoresis or VWF proteolytic profiling by polyacrylamide gel analysis may be particularly helpful in identifying inconsistent assay results.
Two types of ADAMTS13 deficiency have been recognized: autoimmune IgG inhibitors of ADAMTS13 and mutations of the ADAMTS13 gene. Autoimmune inhibitors account for > 90% cases of ADAMTS13 deficiency.
Hereditary deficiency of ADAMTS13
More than 55 mutation of the ADAMTS13 gene have been described from > 60 patients with the inherited forms of TTP[1]. One mutation, 4143insA, has been detected in both homozygous and heterozygous forms in multiple pedigrees from Europe, suggesting that they may have common ancestry. In addition to mutations, at least 26 polymorphisms have been described in the ADAMTS13 gene. The polymorphisms may not be entirely benign, as some have been shown to affect ADAMTS13 expression depending on the sequence context[7;8].
The patients usually present during the neonatal period with periodic episodes of thrombocytopenia and microangiopathic hemolysis that improve quickly upon infusion of plasma infusion. In patients with less severe disease, TTP may first presents later in life and relapse sporadically. In our series of 25 cases of hereditary TTP, 30% had chronic neurological deficits due to ischemic strokes or kernicterus. Acute renal failure occurred at least once in 5 cases. However, it was reversible in each case after the patient was promptly treated with plasma therapy. Chronic renal failure has been described in patients with inherited TTP that did not receive periodic plasma infusion. Furthermore, one case of inherited TTP has been described that developed chronic renal failure due to a factor H mutation[9]. Long-term plasma infusion is an effective but challenging therapy for hereditary TTP. The risk of serious complications and the possible co-existence of other disorders should be taken into consideration when individual cases are evaluated for therapeutic options.
Acquired deficiency of ADAMTS13
Plasma mixing studies have detected the presence of inhibitory antibodies of ADAMTS13 in 50% – 90% of the acquired TTP cases[1]. In patients whose inhibitory antibodies are too low to be detectable by plasma mixing studies, IgG isolated from the plasma samples may yield positive inhibition. Anti-ADAMTS13 antibody is detectable by ELISA in 97% – 100% of the patients with acquired ADAMTS13 deficiency[10;11]. Nevertheless, the ELISA may yield false positive results in 5% – 15% of the general population without TTP. These false positive cases can be identified by adding ADAMTS13 protein, which blocks the binding of anti-ADAMTS13 IgG but not of the non-specific IgG[11].
The etiologies of ADAMTS13 inhibitory antibodies are unknown in most cases. The widespread use of ticlopidine in the 1990’s was accompanied by a surge of TTP in approximately 1 per 2000 to 4000 patients treated, which represents an increase by at least 65 to 130 folds over the background incidence. No other etiologies of ADAMTS13 inhibitors have been identified.
Plasma exchange is believed to control thrombosis by replenishing the missing enzyme until the endogenous ADAMTS13 recovers over the waning inhibitors. Many patients continue to have decreased ADAMTS13 activity levels during remission, indicating that the immune response does not subside completely and the patients are at risk of relapse. In chronic TTP, the ADAMTS13 inhibitors persist at higher levels, preventing the recovery of endogenous ADAMTS13 to detectable levels. In such patients, rituximab, a chimeric anti-CD20 monoclonal antibody, has been used to deplete the B cells and induce remission of TTP. The efficacy of rituximab therapy in patients with chronic refractory TTP has prompted investigators to study its use in patients with acute TTP or a history of TTP with multiple relapses. Because the course of TTP is unpredictable, the role of rituximab requires rigorous investigation in such cases.
Animal model of ADAMTS13 deficiency
ADAMTS13 deficient mice generated by gene targeting are viable without disease manifestations (Table 2)[3]. Crossing of the mice to the genetic background of the CASA/Rk strain results in the appearance of spontaneous TTP-like hyaline thrombi and decreased survival in a subset of the ADAMTS13-deficient mice. The C57BL/6J strain of mice used in generating the ADAMTS13-null founder allele primarily express a form of ADAMTS13 truncated after the sixth TSR motif, while the CASA/Rk strain expresses the full-length form. It is speculated that the wild-type C57BL/6J mice may harbor mutations that compensate for the relative ADAMTS13 deficiency. Genetic cross onto the CASA/Rk strain might lose the protective mutations, leading to the emergence of the TTP phenotype in the ADAMTS13 deficient mice. Further investigation of the protective genes in C57BL/6J strain of mice against TTP may provide clues for understanding the variation of disease severity in TTP.
Table 2.
Animal models of TTP and HUS
| Authors | Host | Findings |
|---|---|---|
| ADAMTS13 | ||
| Motto et al, 2005 | Mouse | No phenotype in the founder strain with mixed C57BL/6J and 129X1/SvJ genetic background. |
| Spontaneous TTP in mice with CASA/Rk genetic background. | ||
| Stx-2 precipitates TTP in ADAMTS13-null mice with CASA/Rk background. | ||
| STEC* or Shiga toxins (Stx-1 or Stx-2) | ||
| Tesh et al, 1993 | Mouse | Stx causes proteinuria, necrosis of renal tubular cells, and death. |
| Stx-2 is more potent than Stx-1. | ||
| No thrombotic microangiopathy | ||
| Shibolet et al, 1997 | Rat (kidney) | Perfusion of kidney with Shiga toxin causes direct renal tubular necrosis |
| Yamamoto et al, 2005 | Rat (kidney) | Renal tubular necrosis, glomerular platelet aggregates, apoptosis and TNF-a expression in the medulla |
| Woods et al, 2002 | Ferret | Renal thrombotic microangiopathy |
| Gunzer et al, 2002 | Gnotobiotic piglet | Colitis, renal thrombotic microangiopathy, and myelin sheath degeneration |
| Siegler et al, 2003 | Baboon | Stx-2 more potent than Stx-1 in causing HUS |
| Raife et al, 2004 | Greyhound dog | Thrombotic microangiopathy of the kidney and skin in natural cases |
| Lepirudin may prevent the lethal effect of Stx-2 in experimental animals. | ||
| Factor H | ||
| Hogasen et al, 1995 | Pig | Homozygous deficiency causes MPGN** |
| Pickering et al , 2002 | Mouse | Homozygous deficiency causes MPGN** |
Shiga toxin producing E. coli.
Membranoproliferative glomerulonephropathy
SHIGA-TOXIN ASSOCIATED HEMOLYTIC UREMIC SYNDROME (Stx-HUS)
Stx-HUS is characterized by microvascular thrombi and tubular cell damage in the kidney. Other organs may also be affected with thrombosis in severe cases. Most cases of post-diarrhea HUS are caused by shiga toxin-producing E. coli O157:H7 or S dysenteriae serotype 1. The history, epidemiology, molecular microbiology, clinical presentation, and management of shiga toxin-associated HUS have been expertly reviewed recently[12]. A typical case develops thrombocytopenia, microangiopathic hemolysis and azotemia one week after having bloody diarrhea due to infection with E. coli O157:H7. Occasionally the diarrheal prodrome may not be evident.
Many strains of shiga toxin producing E. coli have not been associated with the development of hemorrhagic diarrhea or HUS, indicating that other features of the organisms are necessary for virulence. Most cases of colitis or HUS are infected with E. coli of the shiga toxin 2 (Stx-2) genotype, which is 56% homologous to the genes of shiga toxin 1 (Stx-1). Although Stx-2 binds less avidly than Stx-1 to glycolipid cell surface receptor globotriaocylceramide (Gb3), Stx-2 is more stable in the acidic environment of endosomes, and therefore is more likely to inhibit the eEF-1-dependent binding of aminoacyl tRNA to the 60-S subunit of ribosomes. The resulting inhibition of protein synthesis is cytotoxic to the target cells such as glomerular endothelial cells. Endothelial cell injury and detachment expose the underlying thrombogenic glomerular basement, causing activation of platelets and the coagulation cascade[13]. This sequence of events is further supported by the findings of endothelial swelling and detachment, platelet-base membrane attachment, and fibrin clots observed in the lesions of human and animal HUS. Shiga toxins may also be cytotoxic to glomerular mesangial cells and renal tubular cells, contributing to the severity of renal dysfunction. Other studies have demonstrated that shiga toxins may increase the endothelial expression of adhesion molecules such as E-selectin, ICAM-1, VCAM-1, P-selectin, glycoprotein αvβ3, and PCAM-1, promoting the adhesion of leukocytes and platelets. Shiga toxins may also cause renal mesangial and tubular cell apoptosis and increase the expression of IL-6 and monocyte chemoattractant protein 1 in the kidney and of TNFα and IL-1β in monocytes/macrophages. The latter cytokines may up-regulate the expression of Gb3 receptors on endothelial cells, thereby rendering the cells more susceptible to the cytotoxic effects of shiga toxins. Lipopolysaccharide from the microorganisms may also augment the pathogenesis of shiga toxins, perhaps via its effects on the expression of inflammatory cytokines and/or the secretion of von Willebrand factor.
With perhaps a few exceptions, patients with E. coli O157-H7 or diarrhea associated HUS have normal ADAMTS13 activity levels and no inhibitory antibodies. In contrast to the lesions of TTP, the thrombi in the renal glomeruli of Stx-HUS contain no or little VWF[14]. In the presence of ADAMTS13, abnormal shear stress created by microvascular thrombosis enhances the cleavage of VWF, shifting its size to smaller multimers. The difference in ADAMTS13, VWF and pathological features clearly indicates that Stx-HUS and TTP are distinct in pathogenesis.
Animal model of Stx-HUS
Both Stx-producing E. coli and purified shiga toxins have been administered to various animal models. Thrombotic microangiopathy is detected in ferrets, gnotobiotic piglets, baboons and greyhound dogs, but not in mice or rats, although Stx may cause direct renal tubular necrosis and acute renal failure (Table 2). Interestingly, intravenous Stx-2 injection caused a TTP-like disease in ADAMTS13-deficient mice of the CASA/Rk strain, with VWF-rich thrombi detected in multiple organs[3]. In one study, high concentrations of Stx-1 induce rapid release of VWF from cultured vascular endothelial cells and slightly delay the ADAMTS13-induced detachment of adherent platelets[15]. Together, these observations suggest that shiga toxins may precipitate the development of TTP in ADAMTS13 deficient mice by promoting VWF secretion and VWF-platelet thrombus formation on endothelial surface. Further studies are necessary to delineate the relevance of these observations to the pathogenesis of TTP or HUS.
ATYPICAL HEMOLYTIC UREMIC SYNDROME
The disorder of atypical HUS occurs in individuals without TTP, prodromal infection with Stx-producing microorganisms, or other obvious etiologies. Occasionally a diarrheal illness may trigger the onset of HUS. Atypical HUS may be genetically transmitted and is recurrent in most cases. The thrombocytopenia in HUS is not as closely linked to the severity of disease as in TTP. Mental change or seizures may occur. The course of renal function is quite unpredictable. Some of the cases have advanced, irreversible renal failure at the first presentation. Others fully recover the renal function. In still other cases, renal function recovers, but subsequently deteriorates without relapsing hematological complications. Occasionally, a patient presents with HUS only after receiving a renal graft for end-stage renal failure. Hypertension and fluid overload are common and may be severe during the acute phase of atypical HUS. Hypertension often persists after the hematological abnormalities have remitted. Because TTP overlaps with HUS in the severity of renal dysfunctions or neurological abnormalities, ADAMTS13 analysis may be needed to exclude the diagnosis of TTP in suspected cases. Before ADAMTS13 analysis became available, some patients with TTP received the diagnosis of atypical HUS because they had prominent renal failure. Table 3 compares the features of hereditary HUS with that of hereditary TTP.
Table 3.
Clinical and laboratory features of hereditary TTP and hemolytic uremic syndrome.
| Disorder | Hereditary TTP | Hereditary atypical HUS | ||
|---|---|---|---|---|
| Gene affected | ADAMTS13 | CFH | IF | MCP |
| No. of cases | > 60 | > 50 | 4 | 13 |
| Location of mutations | Entire ADAMTS13 gene | CCPR18-20: 63% | Trypsin domain | CCPR4 |
| Inheritance | Recessive | Dominant with variable penetrance | ||
| Age affected, yrs | Newborn – adult | Newborn – adult | Newborn – adult | Child – adult |
| Neurological complications | Common | Uncommon | Uncommon | Uncommon |
| Hypertension | Rare | Common | Common | Common |
| Acute renal failure | Occasional | Yes | Yes | Yes |
| Chronic renal failure | Uncommon | Common | Common | Common |
| Diagnosis | ADAMTS13 < 10% Parents: partial deficiency Genetic study | Factor H assay Genetic study | Factor I assay Genetic study | MCP assay Genetic study |
| Plasma infusion | Effective (every 2–3 weeks) | Probably effective, ?optimal regimen | Probably ineffective | |
| Relapse after renal transplant | Renal transplant not needed | Yes | Yes | No (0 of 3 cases) |
| Animal model | Yes | Yes (MPGN) | No | No |
CCPR: complement control protein repeat; CFH: complement factor H; HUS: hemolytic uremic syndrome; IF: complement factor I; MCP: membrane co-factor protein; MPGN: membranoproliferative glomerulonephropathy; TTPL thrombotic thrombocytopenic purpura.
Molecular basis of atypical HUS
Mutations in one of three proteins involved in regulation of complement activation (Table 1 and Figure 2) have been identified in approximately 30% – 50% of both familial and sporadic atypical HUS patients[16;17]. Two of these proteins, complement factor H (CFH) and membrane cofactor protein (MCP; or CD46) are members of the RCA (regulator of complement activation) cluster located on chromosome 1q32. Both proteins are cofactors of complement factor I (IF), which is a circulating serine protease that cleaves and inactivates surface bound C3b and C4b in the presence of cofactors. Furthermore, CFH antibodies have been detected in 3 patients without mutations in CFH, MCP, or IF[18]. Together, defects in these proteins provide direct evidence that in a subset of patients, uncontrolled complement activation resulting from dysregulation of C3 cleavage plays an important role in the pathogenesis of HUS (Figure 3).
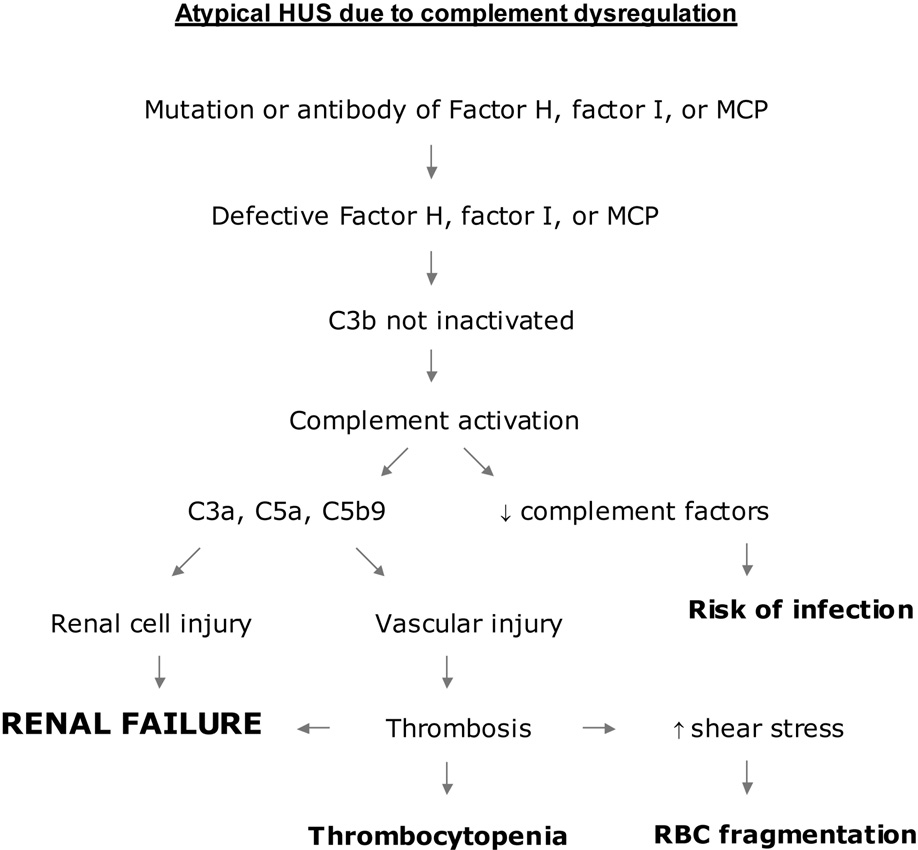
Figure 3.
A pathogenetic scheme of atypical HUS due to complement dysregulation. Mutation or autoantibodies of factor H (CFH), factor I (IF), or membrane cofactor protein (MCP, or CD46) cause defective regulation of the alternative pathway. Excessive complement activation generates cytoactive molecules such as C3a, C5a and C5b9, which injure both renal parenchymal and endothelial cells. Endothelial injury may create a prothrombotic state, leading to local accumulation of platelet-fibrin thrombi. Thrombocytopenia and hemolysis are apparent only when microvascular thrombosis is widespread. In the absence of HUS, renal parenchymal injury may continue to occur, leading to the development of renal failure. Incessant complement activation may increase the risk of pyogenic infection by depleting essential complement components.
HUS due a mutation in the CFH, IF, or MCP is transmitted as an autosomally dominant trait with considerable variability in penetrance. Further complicating the association of CFH or IF deficiency with HUS, patients with severe CFH may develop membranoglomerulonephropathy without HUS[19;20]. Separately, more than 20 cases of IF deficiency have been described from 19 pedigrees that are characterized by increased risk of pyogenic infection but no apparent risk of HUS or renal failure[21]. The reasons for the phenotypic variation in these patients remain unknown.
Haplotype analysis suggests that the risk of atypical HUS may be linked to CFH and MCP even in those without a detectable mutation in either gene[17]. It is speculated that genetic variability in CFH, MCP or the other regulators in the C1q32 cluster could modify the risk of developing HUS.
Diagnosis and treatment of atypical HUS
Diagnosis of deficiencies in the complement regulatory proteins often requires extensive investigative studies. Measurement of protein levels in blood samples may yield suggestive or informative results: a defective regulation of C3 cleavage may decrease the C3 level as a consequence of alternative pathway complement consumption; the levels of the mutant proteins may be decreased in the serum or blood cells; and some of the factor H mutants display unusual mobility in the electrophoresis. However, complement activation is not always present, and some of the mutations do not affect the expression of the dysfunctional proteins. Therefore, detailed genetic and functional analyses are often necessary to demonstrate the involvement of a complement regulator in the pathogenesis of HUS.
The outcome of atypical HUS remains dismal for many patients that require dialysis or renal transplantation. Anecdotally, some of the patients with atypical HUS improve in response to plasma infusion or exchange. It is conceivable that plasma therapy may benefit patients with mutations or antibodies of CFH or IF by replenishing the missing protein or removing the antibodies. Nevertheless, even after initial improvement with plasma therapy, some of the responding patients go on to develop advanced renal failure. Furthermore, except in patients with MCP defects, relapse of HUS is common after renal transplantation. Experimental and clinical evidence suggests that unregulated activation of the alternative complement pathway causes both renal parenchymal and vascular injuries (Figure 3). The florid consequences of the vascular aspect of tissue injury may have hampered a full appreciation of the role of renal parenchymal cell injury. In fact, it is conceivable that the syndrome of HUS only develops when vascular injury causes extensive microthrombi. Renal parenchymal injury may continue to occur in the absence of thrombocytopenia or microangiopathic hemolysis. This scheme may explain why some patients develop advanced renal failure without episodes of HUS, and suggests that the practice of instituting plasma therapy only during episodes of HUS is inadequate for such patients. Thus, it will be important in future studies to explore whether long-term plasma or factor replacement therapy during the periods of hematological remission improves the prognosis of patients with defects in CFH or IF.
Animal models of atypical HUS
A porcine and a murine model of factor H deficiency have been described (Table 2). In these animals, factor H deficiency causes membranoglomerulonephropathy (MPGN) without evidence of thrombotic microangiopathy.
PROSPECTIVE
Until recently, physicians instituted plasma therapy for patients of TTP or HUS without knowing its mechanisms of action. The identification of the molecular defects underlying the pathogenesis of TTP and atypical HUS is beginning to impact on the diagnosis and treatment of the diseases. As specific tests are more reliable and widely available, the nature and course of the diseases are becoming better delineated. Already, therapeutic options are being tailored based on individual molecular defects. It should be feasible in the future to replace plasma therapy or renal transplantation with bioengineered proteins or gene/cell therapies. There is interesting experimental evidence that bioengineered ADAMTS13 variants may help overcome the therapeutic barriers imposed by ADAMTS13 inhibitors[5].
Both the hereditary and acquired forms of TTP and HUS shows considerable variability in severity, suggesting that genetic and environmental factors play important roles in modifying the expression of the diseases. It will be critical in future studies to elucidate the nature of these modifiers. The animal models will be invaluable in providing information that is difficult or impossible to obtain from human subjects.
ACKNOWLEDGEMENT
The author thanks many investigators whose important works contribute to the advances in the studies of TTP and HUS but regrettably are not included in the reference list for limitation of space.
Grant support: The works cited in this article were supported in part by grants R01 HL62136 and R01 HL72876 from the National Heart, Lung and Blood Institute of the NIH.
REFERENCES
- 1.Tsai HM. Current concepts in thrombotic thrombocytopenic purpura. Annu Rev Med. 2006;57:419–436. doi: 10.1146/annurev.med.57.061804.084505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong JF, Moake JL, Nolasco L, et al. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100:4033–4039. doi: 10.1182/blood-2002-05-1401. [DOI] [PubMed] [Google Scholar]
- 3.Motto DG, Chauhan AK, Zhu G, et al. Shigatoxin triggers thrombotic thrombocytopenic purpura in genetically susceptible ADAMTS13-deficient mice. J Clin Invest. 2005;115:2752–2761. doi: 10.1172/JCI26007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonnefoy A, Daenens K, Feys HB, et al. Thrombospondin-1 controls vascular platelet recruitment and thrombus adherence in mice by protecting (sub)endothelial VWF from cleavage by ADAMTS-13. Blood. 2006;107:955–964. doi: 10.1182/blood-2004-12-4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou W, Dong L, Ginsburg D, et al. Enzymatically-active ADAMTS13 variants are not inhibited by anti-ADAMTS13 autoantibodies-A novel therapeutic strategy? J Biol Chem. 2005;280:39934–39941. doi: 10.1074/jbc.M504919200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savasan S, Lee SK, Ginsburg D, Tsai HM. ADAMTS13 gene mutation in congenital thrombotic thrombocytopenic purpura with previously reported normal VWF cleaving protease activity. Blood. 2003;101:4449–4451. doi: 10.1182/blood-2002-12-3796. [DOI] [PubMed] [Google Scholar]
- 7.Bestetti G, Stellari A, Lattuada A, et al. ADAMTS 13 genotype and vWF protease activity in an Italian family with TTP. Thromb Haemost. 2003;90:955–956. doi: 10.1160/TH03-03-0150. [DOI] [PubMed] [Google Scholar]
- 8.Plaimauer B, Fuhrmann J, Mohr G, et al. Modulation of ADAMTS13 secretion and specific activity by a combination of common amino-acid polymorphisms and a missense mutation. Blood. 2006;107:118–125. doi: 10.1182/blood-2005-06-2482. [DOI] [PubMed] [Google Scholar]
- 9.Noris M, Bucchioni S, Galbusera M, et al. Complement factor H mutation in familial thrombotic thrombocytopenic purpura with ADAMTS13 deficiency and renal involvement. J Am Soc Nephrol. 2005;16:1177–1183. doi: 10.1681/ASN.2005010086. [DOI] [PubMed] [Google Scholar]
- 10.Rieger M, Mannucci PM, Kremer Hovinga JA, et al. ADAMTS13 autoantibodies in patients with thrombotic microangiopathies and other immunomediated diseases. Blood. 2005;106:1262–1267. doi: 10.1182/blood-2004-11-4490. [DOI] [PubMed] [Google Scholar]
- 11.Tsai HM, Raoufi M, Zhou W, et al. ADAMTS13-Binding IgG are present in patients with thrombotic thrombocytopenic purpura. (manuscript submitted) [PMC free article] [PubMed] [Google Scholar]
- 12.Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet. 2005;365:1073–1086. doi: 10.1016/S0140-6736(05)71144-2. [DOI] [PubMed] [Google Scholar]
- 13.Obrig TG, Del Vecchio PJ, Brown JE, et al. Direct cytotoxic action of Shiga toxin on human vascular endothelial cells. Infect Immun. 1988;56:2373–2378. doi: 10.1128/iai.56.9.2373-2378.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai HM, Chandler WL, Sarode R, et al. von Willebrand factor and von Willebrand factor-cleaving metalloprotease activity in Escherichia coli O157:H7-associated hemolytic uremic syndrome. Pediatr Res. 2001;49:653–659. doi: 10.1203/00006450-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Nolasco LH, Turner NA, Bernardo A, et al. Hemolytic uremic syndrome-associated Shiga toxins promote endothelial-cell secretion and impair ADAMTS13 cleavage of unusually large von Willebrand factor multimers. Blood. 2005;106:4199–4209. doi: 10.1182/blood-2005-05-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dragon-Durey MA, Fremeaux-Bacchi V. Atypical haemolytic uraemic syndrome and mutations in complement regulator genes. Springer Semin Immunopathol. 2005;27:359–374. doi: 10.1007/s00281-005-0003-2. [DOI] [PubMed] [Google Scholar]
- 17.Fremeaux-Bacchi V, Kemp EJ, Goodship JA, et al. The development of atypical haemolytic-uraemic syndrome is influenced by susceptibility factors in factor H and membrane cofactor protein: evidence from two independent cohorts. J Med Genet. 2005;42:852–856. doi: 10.1136/jmg.2005.030783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dragon-Durey MA, Loirat C, Cloarec S, et al. Anti-Factor H autoantibodies associated with atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2005;16:555–563. doi: 10.1681/ASN.2004050380. [DOI] [PubMed] [Google Scholar]
- 19.Levy M, Halbwachs-Mecarelli L, Gubler MC, et al. H deficiency in two brothers with atypical dense intramembranous deposit disease. Kidney Int. 1986;30:949–956. doi: 10.1038/ki.1986.278. [DOI] [PubMed] [Google Scholar]
- 20.Dragon-Durey MA, Fremeaux-Bacchi V, Loirat C, et al. Heterozygous and homozygous factor h deficiencies associated with hemolytic uremic syndrome or membranoproliferative glomerulonephritis: report and genetic analysis of 16 cases. J Am Soc Nephrol. 2004;15:787–795. doi: 10.1097/01.asn.0000115702.28859.a7. [DOI] [PubMed] [Google Scholar]
- 21.Vyse TJ, Spath PJ, Davies KA, et al. Hereditary complement factor I deficiency. QJM. 1994;87:385–401. [PubMed] [Google Scholar]