Abstract
The Lyme disease spirochete Borrelia burgdorferi must repress expression of outer surface protein C (OspC) to effectively evade specific humoral immunity and to establish persistent infection. This ability largely relies upon a regulatory element, the only operator that has been reported in spirochetal bacteria. Immediately upstream of the ospC promoter, two sets of inverted repeats (IRs) constitute small and large palindromes, in which the right IR of the large palindrome contains the left IR of the small one, and may collectively function as the ospC operator. In the study, the large palindrome with or without the small IR was fused with a flaB promoter, which was used to drive expression of a promoterless ospC copy as a reporter gene, and introduced into OspC-deficient B. burgdorferi. The presence of the large palindrome alone significantly reduced ospC expression driven by the fused flaB promoter in the joint tissue of severe combined immunodeficiency (SCID) mice, and rescued spirochetes from elimination by passively transferred OspC antibody in infected SCID mice and specific immune responses elicited in immunocompetent mice, confirming a function of the IRs as an operator. Inclusion of the small IR further enhanced the ability of the large palindrome to reduce the activity of the fused flaB promoter, indicating that the small IR is a part of the operator. Taken together, the study led to successful verification and dissection of the ospC operator.
Keywords: Bacterial pathogenesis, Gene regulation, Lyme disease
1. Introduction
The Lyme disease spirochete, Borrelia burgdorferi, is one of the most invasive bacterial pathogens, causing persistent infection despite the development of vigorous immune responses [1, 2]. Tight regulation of outer surface protein (Osp) expression is crucial for its pathogenic strategy. The pathogen abundantly expresses OspA/B in the unfed tick [3–6], consistent with an important role of these lipoproteins in spirochetal persistence in the vector [7, 8]. A fresh blood meal down-regulates OspA/B and up-regulates OspC and others, a process that prepares B. burgdorferi for infection of mammals, regardless of whether OspC is required for salivary gland invasion [9–12]. Repression of OspA/B expression in mammals is critical for the maintenance of the enzootic cycle because their expression would ultimately induce a strong humoral response and, as a result, may effectively block acquisition of B. burgdorferi by the vector [13–15], regardless of whether OspA/B can be targeted by borreliacidal antibodies in mammalian tissues [16]. B. burgdorferi abundantly expresses OspC only during early infection when the antigen has an important role and before specific humoral responses have developed [17–19]. OspC is not only a strong immunogen but also an effective target of protective immunity; its expression induces a robust humoral response that imposes tremendous pressure on the pathogen [20, 21]. To cause persistent infection, B. burgdorferi must down-regulate OspC [17, 18, 22, 23]. If B. burgdorferi fails to repress OspC expression or undergo escape mutations on the ospC gene, infection would be cleared [20]. It is also crucial for B. burgdorferi to keep the ospC gene off after it is acquired by the tick vector as OspC antibodies in blood meal may kill spirochetes expressing the antigen in the vector [24], leading to discontinuation of the enzootic cycle.
Only three σ factors can be identified from the entire genome of B. burgdorferi, including the major factor, RpoD (σ70), and two alternative factors, RpoN (σ54) and RpoS (σ38) [25]. Moreover, the two alternative factors compose a regulatory network, in which RpoS expression depends on RpoN [26, 27], greatly limiting their role in contribution to diverse gene regulation. In the unfed tick, the network is silent, so are the RpoS-dependent genes, such as ospC, decorin-binding proteins A/B (dbpA/B), ospF and bbk32 [3, 28–30]. During mammalian infection, B. burgdorferi apparently mobilizes all the three σ factors [31–33]; therefore, selective gene down-regulation must depend on mechanisms other than controlling expression of these factors.
The identification of the ospC operator helps interpret the ability of B. burgdorferi to selectively down-regulate ospC, while actively transcribing other RpoS-dependent genes during mammalian infection [34]. While the regulatory element remains to be verified in a different system, two sets of inverted repeats (IRs) immediately upstream of the ospC promoter constitute small and large palindromes, in which the right IR of the large palindrome contains the left IR of the small one, and may collectively function as an operator but have to be confirmed. Although ospC is probably the most investigated among genes in spirochetal bacteria, the full ospC promoter region remains to be defined even after attempts from two leading groups in the field [35, 36]. In contrast, the flaB promoter is a well defined σ70-dependent promoter, driving constitutive gene expression [37]. In the study, the large palindrome with or without the small right IR was fused with the flaB promoter, which was used to drive expression of a promoterless ospC copy as a reporter gene, and introduced into OspC-deficient B. burgdorferi. The study allowed us to successfully verify and dissect the ospC operator.
2. Results
2.1. Identification of a minimum flaB promoter
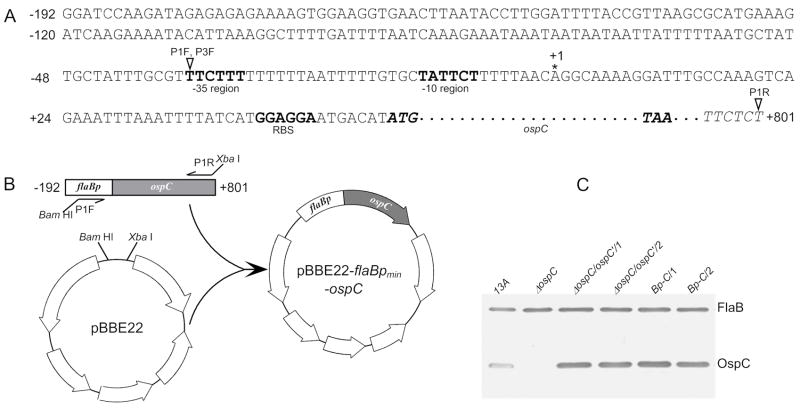
The efficiency for the ospC operator to repress the activity of a downstream promoter is likely position-dependent, so unessential upstream sequences of the flaB promoter should be removed. To identify a minimum flaB promoter, two constructs containing the identical promoterless ospC copy as a reporter gene were used. The construct pBBE22-flaBpmin-ospC, which was created as illustrated in Fig. 1A&B, harbored a flaB promoter sequence extending just to the -35 region, consequently called a minimum promoter; while pBBE22-ospC′ constructed in our previous study [20], contained a flaB promoter extending to −192 from the transcriptional start site and was expected to drive maximum transcriptional activity. The feature of the constructs was summarized in Table 1.
Fig. 1.
Identification of a minimum flaB promoter. (A) Extended flaB promoter sequence and fused promoterless ospC gene. This sequence is already in pBBE22-ospC′, which was constructed from pBBE22 in our previous study [20]. The sequence includes the flaB promoter and upstream sequence, extending to −192 from its transcriptional start site, and a promoterless ospC gene (italic), extending from the start codon ATG (boldface) to +801 from the transcriptional start site of the ospC gene. The stop codon TAA (boldface) of ospC is also present. The −35 and −10 regions, and the putative ribosome-binding site (RBS) (all in boldface type) of the flaB gene are indicated. The asterisk marks the previously identified transcriptional initiation site [44]. The amplification start sites of primers, P1F, P3F and P1R, are pointed with open triangles. P1F and P3F had an identical sequence, but were incorporated with different restriction enzyme sites, and were used for plasmid construction described in this figure and Fig. 3, respectively. (B) Construction of pBBE22-flaBpmin-ospC from pBBE22 and pBBE22-ospC′. An 883-bp fragment was PCR amplified with the use of primers P1F and P1R, and of pBBE22-ospC′ as a template, then cloned into pBBE22. (C) Immunoblot analysis of OspC expression. The parental clone 13A, the ospC mutant (ΔospC), and the clones ΔospC/ospC′/1, ΔospC/ospC′/2, Bp-C/1 and Bp-C/2 were verified for OspC expression by immunoblotting probed with a mixture of FlaB and OspC MAbs.
Table 1.
Constructs and clones used in the study
| Construct or clone | Description | Source |
|---|---|---|
| pBBE22 | pBSV2 carrying a bbe22 copy | [39] |
| pBBE22-ospC′ | pBBE22 carrying promoterless ospC fused with long version of flaB promoter | [20] |
| pBBE22-flaBpmin-ospC | pBBE22 carrying promoterless ospC fused with minimal flaB promoter | This study |
| pBBE22-Co1-flaBp-ospC | pBBE22 carrying promoterless ospC fused with minimal flaB promoter which is fused with long version of ospC operator | This study |
| pBBE22-Co2-flaBp-ospC | pBBE22 carrying promoterless ospC fused with minimal flaB promoter which is fused with short version of ospC operator | This study |
| ΔospC | ospC mutant | [34] |
| ΔospC/FL/1 | Expressing ospC controlled by ospC regulatory elementsa | [34] |
| ΔospC/ospC′/1 | Expressing ospC driven by long version of flaB promoter | This study |
| ΔospC/ospC′/2 | Expressing ospC driven by long version of flaB promoter | This study |
| Bp-C/1 | Expressing ospC driven by minimal flaB promoter | This study |
| Bp-C/2 | Expressing ospC driven by minimal flaB promoter | This study |
| Co1-Bp-C/1 | Expressing ospC driven by minimal flaB promoter which is controlled by long version of ospC operator | This study |
| Co1-Bp-C/2 | Expressing ospC driven by minimal flaB promoter which is controlled by long version of ospC operator | This study |
| Co2-Bp-C/1 | Expressing ospC driven by minimal flaB promoter which is controlled by short version of ospC operator | This study |
| Co2-Bp-C/2 | Expressing ospC driven by minimal flaB promoter which is controlled by short version of ospC operator | This study |
The ospC regulatory elements include both operator and promoter.
The two constructs were electroporated into the ospC mutant, which was generated and characterized in our previous study [34]; 16 and 18 transformants were obtained from the transformation with each construct. Plasmid analyses identified two clones receiving each construct, namely Bp-C/1, Bp-C/2, ΔospC/ospC′/1, and ΔospC/ospC′/2. The clones Bp-C/1and Bp-C/2 received pBBE22-flaBpmin-OspC, while the clones ΔospC/ospC′/1 and ΔospC/ospC′/2 obtained pBBE22-ospC′. These clones shared the same plasmid content as the ospC mutant, which lost lp25, lp5, lp21 and cp9 [34]. OspC expression resulting from introduction of the constructs was confirmed by immunoblot analysis (Fig. 1C), demonstrating that both constructs actively drove in vitro OspC expression.
To more precisely compare the activity of the constructs to drive reporter expression, the Bp-C/1, Bp-C/2, ΔospC/ospC′/1, and ΔospC/ospC′/2 spirochetes were harvested at early log and stationary phase. RNA was prepared and analyzed for ospC and flaB mRNA accumulation by RT-qPCR. At early log phase, introduction of the constructs pBBE22-ospC′ and pBBE22-flaBpmin-ospC led to the accumulation of 2049 and 1926 ospC transcripts, respectively, to match every 1000 flaB mRNA copies (Fig. 2A), indicating that the two promoter versions initiated reporter expression as equally well (P = 0.26). At stationary phase, the minimum flaB promoter drove ospC transcription also as effectively as the long promoter version (P = 0.47); although the two constructs increased ospC mRNA copies to 5281 and 5137, respectively, for every 1000 flaB transcripts produced, representing 2.6- and 2.7-fold increases over early log phase (P values were 5.7 × 10−7 and 6.9 × 10−7, respectively).
Fig. 2.
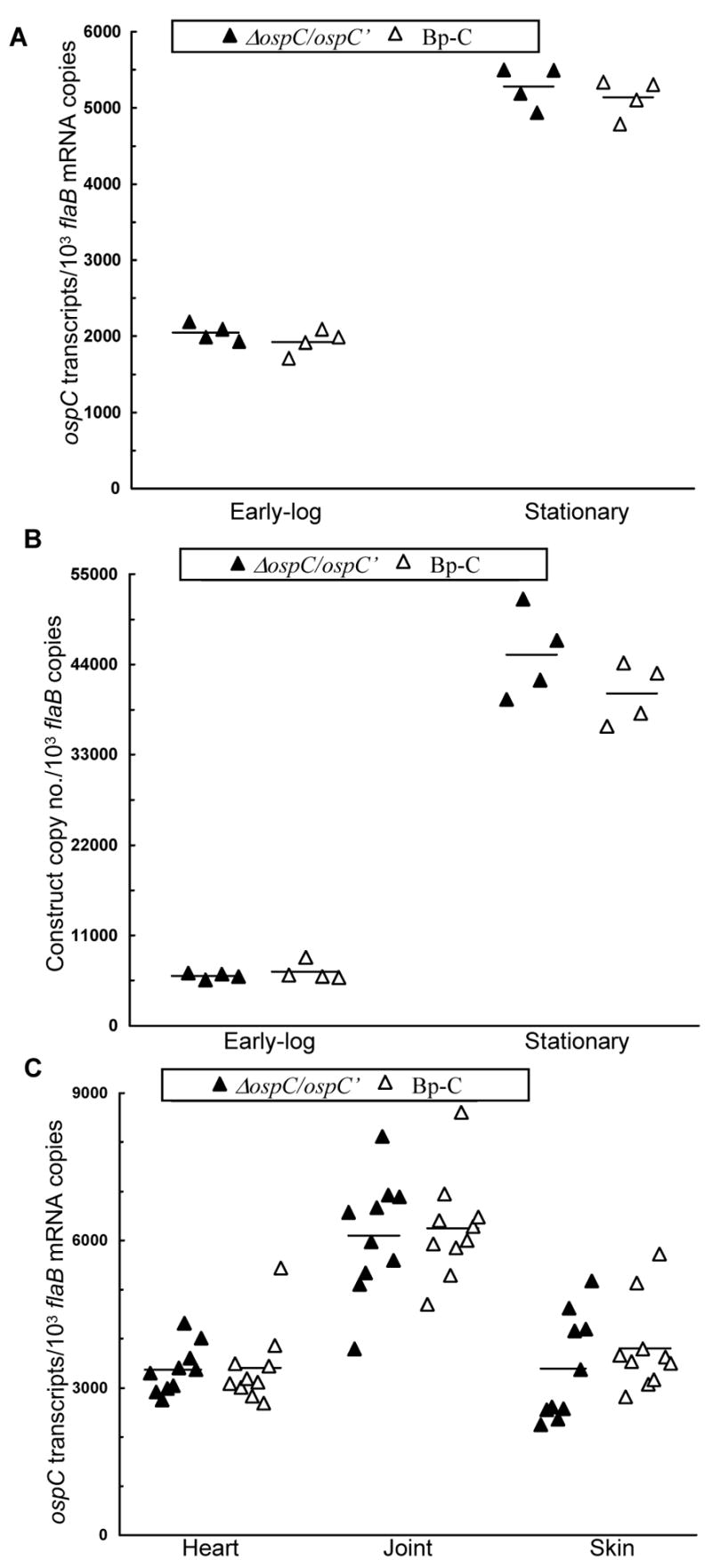
The minimum flaB promoter drives maximum in vitro and in vivo ospC expression. (A) The two constructs drive active in vitro ospC expression. The ΔospC/ospC′/1, ΔospC/ospC′/2, Bp-C/1, and Bp-C/2 spirochetes were grown, in duplicate, to early log and stationary phase at 33°C. RNA was extracted and analyzed by RT-qPCR for flaB and ospC mRNA copy numbers. The expression activity is presented as ospC mRNA copy numbers per 1000 flaB transcripts. Data generated from ΔospC/ospC′/1and ΔospC/ospC′/2 were combined and compared with those obtained from Bp-C/1 and Bp-C/2. (B) B. burgdorferi increases accumulation of constructs from early log to stationary phase. DNA was also extracted from spirochetes grown as described above and quantified for flaB and kan copies. The data are expressed as kan DNA copy numbers per 1000 flaB copies. (C) The two constructs drive similar ospC expression in mice. Subgroups of five SCID mice were inoculated with the clone ΔospC/ospC′/1, ΔospC/ospC′/2, Bp-C/1, or Bp-C/2. All mice were sacrificed one month later; RNA samples were prepared from the heart, joint and skin specimens and quantified for flaB and ospC expression by RT-qPCR. The data are presented as ospC transcripts per 1000 flaB mRNA copies and in two groups by combining the subgroups ΔospC/ospC′/1 and ΔospC/ospC′/2, and Bp-C/1 and Bp-C/2.
To explore how the constructs more effectively increased ospC mRNA accumulation at stationary rather than early log phase, the copy numbers of the kan gene on the constructs and the chromosomal flaB gene were determined by qPCR. As shown in Fig. 2B, B. burgdorferi produced, on average, six copies of the constructs to match each copy of the linear chromosome at early log phase and increased to over 40 copies at stationary phase, representing a nearly 7-fold increase (P = 7.1 × 10−6). Compared to a less than 3-fold increase in ospC mRNA (Fig. 2A), the fused flaB promoter did not more efficiently initiate reporter expression at stationary phase. Instead, increased ospC mRNA accumulation during stationary phase apparently resulted from a dramatic rise in copy numbers of the ospC gene associated with the shuttle vector per cell.
Next, the activity of the two constructs to initiate ospC expression was compared in the murine host. Subgroups of five SCID mice were inoculated with the clones Bp-C/1, Bp-C/2, ΔospC/ospC′/1, or ΔospC/ospC′/2. In all 20 animals, joint swelling evolved around 10 days post-inoculation and developed into severe arthritis within a week (data not shown), indicating that all four clones were infectious. Mice were euthanized one month post-inoculation; RNA was extracted and quantified for ospC and flaB transcripts. As shown in Fig. 2C, the two constructs led to similar ospC mRNA accumulation in the heart (P = 0.89), joint (P = 0.77), and skin tissue (P = 0.36), indicating that the minimum flaB promoter initiated reporter expression as efficiently as the full-length promoter.
2.2. The presence of the large palindrome alone is able to effectively reduce the activity of a fused flaB promoter in joints of SCID mice but inclusion of the small IR further enhances the ability
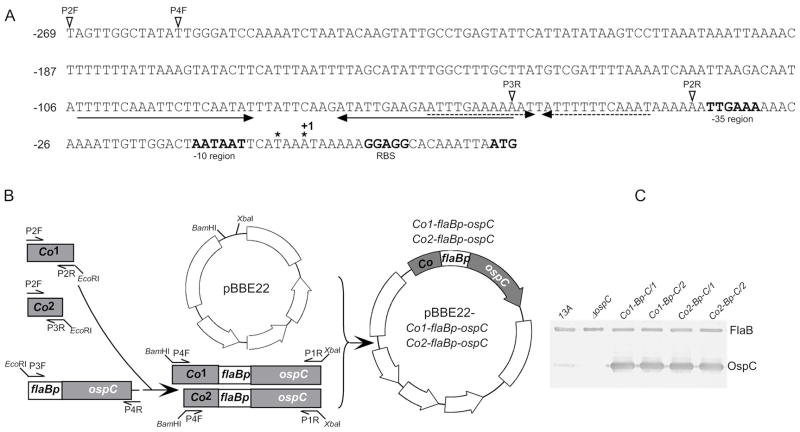
Immediately upstream of the ospC promoter, two sets of IRs constitute small and large palindromes, in which the right IR of the large palindrome essentially contains the left IR of the small one (Fig. 3A), and may collectively function as the operator. To dissect the regulatory sequence, pBBE22-Co1-flaBp-ospC, containing the large palindrome and the small IR, and pBBE22-Co2-flaBp-ospC, harboring only the large one, were generated as diagramed in Fig. 3A&B before being electroporated into the ospC mutant. Nine and 16 transformants were obtained from the transformation with each construct. Plasmid analyses identified two clones receiving each construct, namely Co1-Bp-C/1, Co1-Bp-C/2, Co2-Bp-C/1 and Co2-Bp-C/2. These clones shared the same plasmid content as the ospC mutant [34]. Active OspC expression resulting from the introduction of the constructs was confirmed by immunoblot analysis (Fig. 3C).
Fig. 3.
Generation of B. burgdorferi expressing ospC driven by a fused flaB promoter under control of the ospC operator. (A) The regulatory elements including extended ospC promoter and operator. Upstream regions of the ospC gene including two large inverted repeats (indicated by a pair of divergent arrows) and two small inverted repeats (indicated by a pair of dashed divergent arrows), the extended −35 and −10 regions, the putative ribosome-binding site (RBS), and the start codon ATG (all in boldface type) are indicated. The asterisks mark two previously identified transcriptional initiation sites [45, 46]; the downstream one is designated as +1 because of a larger distance to the −10 region. The amplification starting sites of the four primers, P2F, P4F, P2R and P3R used for vector construction are pointed with open triangles. (B) Construction of pBBE22-Co1-flaBp-ospC and pBBE22-Co2-flaBp-ospC. Two fragments, Co1 and Co2, were PCR amplified with the use of reverse primers P2R and P3R, respectively, and a common forward primer P2F, and of borrelial DNA as a template. The fragment flaBp-OspC was generated by PCR using the primers P3F (see Fig. 1 for the binding site of P3F) and P4R (the binding site of P4R within the construct pBBE222-flaBpmin-ospC is not shown) and pBBE22-flaBpmin-ospC as a template. The fragments Co1 and Co2 were fused with flaBp-ospC, forming Co1-flaBp-ospC and Co2-flaBp-ospC, respectively, amplified by nested PCR with use of the primers P4F and P1R (see Fig. 1 for the binding site of P1R), and cloned into pBBE22. (C) Immunoblot analysis of OspC expression. The parental clone 13A, the ospC mutant, and the clones Co1-Bp-C/1, Co1-Bp-C/2, Co2-Bp-C/1, and Co2-Bp-C/2 were verified for OspC expression by immunoblot probed with a mixture of FlaB and OspC MAbs.
To examine whether the presence of the operator versions influences the in vitro activity of the fused promoter, the Co1-Bp-C/1, Co1-Bp-C/2, Co2-Bp-C/1 and Co2-Bp-C/2 spirochetes were harvested at early log and stationary phases. RNA was prepared and analyzed for ospC expression by RT-qPCR. The clones Bp-C/1 and Bp-C/2 were used as a control. No significant differences were noted in ospC mRNA accumulation by the three genotypes either at early log or stationary phase (data not shown), indicating that the presence of the ospC operator sequences did not affect the activity of the fused flaB promoter when spirochetes were grown in vitro.
The ability of the two operator versions to influence the activity of a fused promoter was examined in mice. Subgroups of five SCID mice were inoculated with the clone Co1-Bp-C/1, Co1-Bp-C/2, Co2-Bp-C/1 or Co2-Bp-C/2. An additional 10 mice were inoculated with the clone Bp-C/1 or Bp-C/2 as a control. In all 30 mice, joint swelling evolved around 10 days post-inoculation and developed into severe arthritis within a week (data not shown), indicating that all mice were infected. Animals were euthanized one month post-inoculation for the assessment of ospC transcriptional activity. As shown in Fig. 4, incorporation of either ospC operator version did not change reporter gene expression in heart (P > 0.05) or skin tissue (P > 0.05). In the joint, however, the presence of the long operator version reduced ospC mRNA accumulation by 44% (P = 1.9 × 10−7), compared with a 22% decline caused by the presence of the short version (P = 0.05), indicating that the ospC operator is able to effectively reduce the activity of the fused flaB promoter in joint tissues and, thus, confirming the function of the ospC operator. Moreover, the longer operator reduced ospC expression 28% more effectively than the shorter version (P = 0.02), indicating that the small IR also contributes to the function of the operator to reduce the activity of the downstream promoter.
Fig. 4.
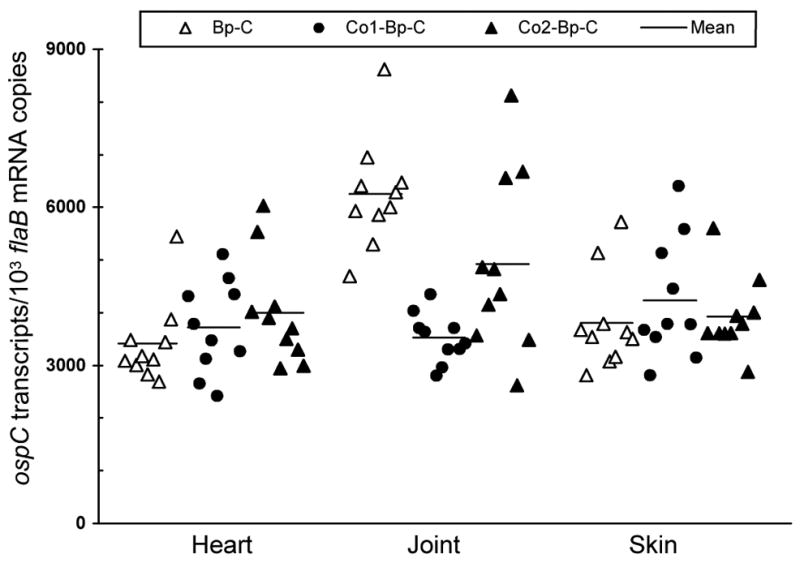
The long ospC operator version more effectively reduces the activity of a fused flaB promoter in joints of SCID mice. Subgroups of five SCID mice were inoculated with the clone Bp-C/1, Bp-C/2, Co1-Bp-C/1, Co1-Bp-C/2, Co2-Bp-C/1 or Co2-Bp-C/2. One month later, mice were euthanized; heart, joint and skin specimens were used for RNA extraction. RNA samples were quantified for flaB and ospC expression by RT-qPCR. Data are presented as ospC transcripts per 1000 flaB mRNA copy numbers in three groups by combining the subgroups Bp-C/1 and Bp-C/2, Co1-Bp-C/1 and Co1-Bp-C/2, and Co2-Bp-C/1 and Co2-Bp-C/2.
2.3. The presence of the large palindrome alone confers the responsiveness of a fused flaB promoter to treatment with OspC MAb but inclusion of the small IR further enhances the responsiveness
To further examine the influence of the ospC operator on the activity of a fused promoter, subgroups of 10 SCID mice were challenged with the clone Bp-C/1, Bp-C/2, Co1-Bp-C/1, Co1-Bp-C/2, Co2-Bp-C/1 or Co2-Bp-C/2. An additional group of 10 mice were inoculated with the clone ΔospC/FL/1 as a control. This clone was generated by introducing a full-length ospC gene carried by pBBE22 into the ospC mutant in our previous study [34]. In all 70 mice, joint swelling evolved around 10 days post-inoculation and developed into severe arthritis within a week (data not shown). At 3 weeks, five mice from each group received 100 μg of either OspC MAb or purified murine IgG as a control. One week later, mice were euthanized; heart, joint, and skin samples were used for bacterial culture. As expected, spirochetes were recovered from each specimen harvested from all of the 35 IgG-treated mice (Table 2). As a positive control, the ΔospC/FL/1 spirochetes were recovered from all of the joints and most of the heart and skin specimens of the five MAb-treated mice. Transferred MAb cleared infections in each tissue from all 10 mice that had been inoculated with either the clone Bp-C/1 or Bp-C/2. In contrast, the Co1-Bp-C/1, Co1-Bp-C/2, Co2-Bp-C/1 or Co2-Bp-C/2 spirochetes were grown from each of the joints, some of the skin specimens, but none of the heart samples, which were taken from the 20 MAb-treated mice (Table 2). These data indicated that the presence of either operator version provides B. burgdorferi with the ability to avoid elimination by the OspC antibody, again, confirming the function of the ospC operator.
Table 2.
The ospC operator helps B. burgdorferi evade clearance by OspC MAba
| No. of cultures positive/Total no. of specimens examined
|
|||||
|---|---|---|---|---|---|
| Clone | Treatment received | Heart | Joint | Skin | All sites |
| ΔospC/FL/1 | mouse IgG | 5/5 | 5/5 | 5/5 | 15/15 |
| OspC MAb | 3/5 | 5/5 | 4/5 | 12/15 | |
| Bp-C/1 | mouse IgG | 5/5 | 5/5 | 5/5 | 15/15 |
| OspC MAb | 0/5 | 0/5 | 0/5 | 0/15 | |
| Bp-C/2 | mouse IgG | 5/5 | 5/5 | 5/5 | 15/15 |
| OspC MAb | 0/5 | 0/5 | 0/5 | 0/15 | |
| Co1-Bp-C/1 | mouse IgG | 5/5 | 5/5 | 5/5 | 15/15 |
| OspC MAb | 0/5 | 5/5 | 3/5 | 8/15 | |
| Co1-Bp-C/2 | mouse IgG | 5/5 | 5/5 | 5/5 | 15/15 |
| OspC MAb | 0/5 | 5/5 | 3/5 | 8/15 | |
| Co2-Bp-C/1 | mouse IgG | 5/5 | 5/5 | 5/5 | 15/15 |
| OspC MAb | 0/5 | 5/5 | 2/5 | 7/15 | |
| Co2-Bp-C/2 | mouse IgG | 5/5 | 5/5 | 5/5 | 15/15 |
| OspC MAb | 0/5 | 5/5 | 3/5 | 8/15 | |
Groups of 10 BALB/c SCID mice were inoculated with the clone ΔospC/FL/1, Bp-C/1, Bp-C/2, Co1-Bp-C/1, Co1-Bp-C/2, Co2-Bp-C/1 or Co2-Bp-C/2. Three weeks later, five animals from each group received a single dose of either OspC MAb or murine IgG as a control and were sacrificed within 1 week. Heart, tibiotarsal joint and skin specimens were harvested and cultured for spirochetes in BSK-H complete medium.
RNA was extracted from joint specimens harvested from all 35 IgG-treated mice and 25 of the 35 MAb-treated mice, then analyzed for ospC and flaB mRNA transcripts by RT-qPCR. The clearance of infection excluded 10 mice initially infected with either the clone Bp-C/1 or Bp-C/2 and treated with MAb. As previously reported [34], in response to MAb treatment, the ΔospC/FL/1 spirochetes reduced ospC expression by 87-fold (P = 3.2 × 10−7) (data not shown). Consistent with the data presented in Fig. 4, addition of the long and short operator versions led to 41% (P = 6.2 × 10−6) and 21% (P = 0.03) reductions, respectively, in ospC mRNA accumulation (Fig. 5). MAb treatment further reduced ospC expression by 4.5- and 2.5-fold in spirochetes harboring the long (P = 1.7 × 10−12) and the short operator versions (P = 3.3 × 10−6), respectively, indicating that either version reduces the activity of a fused flaB promoter in response to treatment with OspC MAb. Furthermore, the long operator version showed a 59% more effectiveness in reducing ospC expression than the short one in response to treatment with OspC MAb (P = 7.1 × 10−6), again, indicating that the small IR is also crucial for the function of the operator.
Fig. 5.
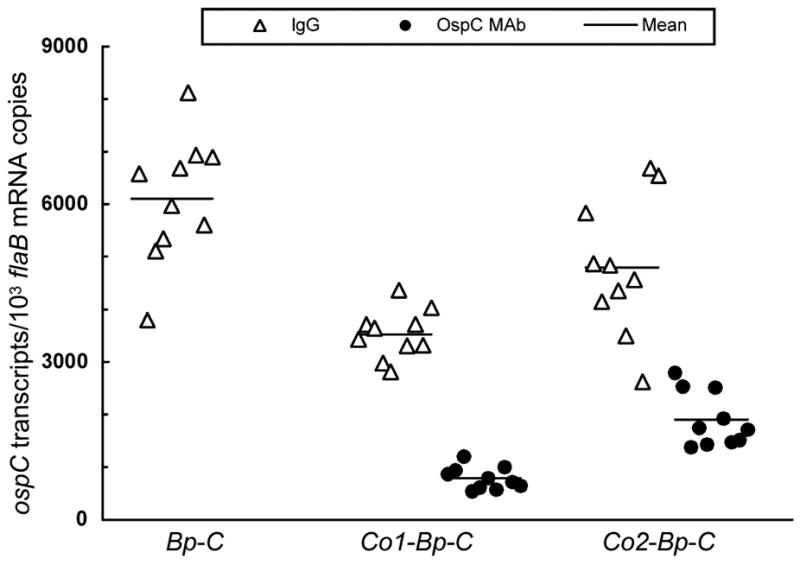
The long ospC operator version more effectively reduces the activity of a fused flaB promoter in response to treatment with OspC MAb. Subgroups of 10 SCID mice were inoculated with the clone Bp-C/1, Bp-C/2, Co1-Bp-C/1, Co1-Bp-C/2, Co2-Bp-C/1 or Co2-Bp-C/2. Three weeks later, five mice from each subgroup received a single dose of 100 μg of either OspC MAb or purified murine IgG as a control. One week later, mice were euthanized; RNA was extracted from joint specimens harvested from the 30 IgG-treated mice and from the 20 mice infected with the Co1-Bp-/1, Co1-Bp-C/2, Co2-Bp-C/1, or Co2-Bp-C/2 bacteria and treated with OspC MAb. RNA samples were quantified for flaB and ospC expression by RT-qPCR. Data are presented as ospC transcripts per 1000 flaB mRNA copies in three groups by combining the subgroups Bp-C/1 and Bp-C/2, Co1-Bp-C/1 and Co1-Bp-C/2, and Co2-Bp-C/1 and Co2-Bp-C/2. The Bp-C/1 and Bp-C/2 bacteria were cleared by OspC MAb; therefore no expression data were obtained from this treatment.
2.4. Verification of the function of the ospC operator during chronic infection of immunocompetent mice
Next, the function of the ospC operator was further verified in immunocompetent mice. Groups of five BALB/c mice were challenged with the clone Bp-C/1, Bp-C/2, Co1-Bp-C/1, Co1-Bp-C/2, Co2-Bp-C/1 or Co2-Bp-C/2. An additional group of 10 mice were challenged with the clone ΔospC/FL/1 as a positive control. Ear biopsies taken for bacterial culture at 3 weeks post-inoculation showed that all of the 40 mice were infected (data not shown). Animals were sacrificed 4 months post-inoculation; heart, joint and skin samples were harvested for bacterial isolation. The ΔospC/FL/1 spirochetes were grown from each of the heart, joint and skin specimens of 10 inoculated mice (Table 3). Although the Bp-C/1 and Bp-C/2 spirochetes were recovered from most of the skin specimens, they were cleared from the heart and joint tissues of 10 inoculated mice. In contrast, B. burgdorferi was grown from each of the skin specimens and most of the heart and joint tissues of 20 mice that had been challenged with the clone Co1-Bp-C/1, Co1-Bp-C/2, Co2-Bp-C/1 or Co2-Bp-C/2, indicating that either ospC operator version helps B. burgdorferi evade the immune system. Therefore, the study successfully verified the function of the ospC operator in immunocompetent mice.
Table 3.
The ospC operator facilitates immune evasion of B. burgdorferia
| No. of cultures positive/Total no. of specimens examined
|
||||
|---|---|---|---|---|
| Clone | Heart | Joint | Skin | All sites |
| ΔospC/FL/1 | 10/10 | 10/10 | 10/10 | 30/30 |
| Bp-C/1 | 0/5 | 0/5 | 3/5 | 3/15 |
| Bp-C/2 | 0/5 | 0/5 | 4/5 | 4/15 |
| Co1-Bp-C/1 | 3/5 | 5/5 | 5/5 | 13/15 |
| Co1-Bp-C/2 | 3/5 | 4/5 | 5/5 | 12/15 |
| Co2-Bp-C/1 | 4/5 | 2/5 | 5/5 | 11/15 |
| Co2-Bp-C/2 | 4/5 | 3/5 | 5/5 | 12/15 |
Groups of five or 10 BALB/c mice were inoculated with the clone ΔospC/FL/1, Bp-C/1, Bp-C/2, Co1-Bp-C/1, Co1-Bp-C/2, Co2-Bp-C/1 or Co2-Bp-C/2. Mice were sacrificed 4 months post-inoculation. Heart, tibiotarsal joint and skin specimens were harvested and cultured for spirochetes in BSK-H complete medium. Fisher’s exact test P values were 0.01 and 1.2 × 10−4 between the genotypes Bp-C and Co1-Bp-C in heart and joint tissues, 7.1 × 10−4 and 0.03 between Bp-C and Co2-Bp-C in heart and joint tissues, and 0.63 and 0.14 between Co1-Bp-C and Co2-Bp-C in heart and joint tissues, respectively.
3. Discussion
As a σ70-dependent promoter, the flaB promoter is one of the most active promoters, which drives constitutive gene expression during the entire enzootic cycle of B. burgdorferi. To verify and dissect the newly identified operator of the RpoS-dependent ospC gene, the flaB promoter was used as a reporter promoter and fused with a promoterless ospC as a reporter gene. The study not only verified the function of the ospC operator but also demonstrated that all the IRs collectively act as a cis-element to influence the activity of a downstream promoter.
Identification of the ospC operator predicts the existence of a repressor [34]. However, nothing is known about the regulatory protein or how it is induced during the enzootic cycle of B. burgdorferi. In vitro ospC expression is always associated with the upregulation of RpoS [26, 35, 36, 38], suggesting that the repressor is not induced in vitro. Our previous study showed that deletion of the operator does not influence in vitro ospC expression, thus, ruling out the involvement of the operator-repressor system in ospC regulation [34]. In the current study, the presence of either ospC operator version did not affect the activity of the fused flaB promoter to initiate a reporter gene expression in cultivated B. burgdorferi, further confirming that the potential repressor is not expressed under in vitro conditions.
The current study showed that the copy number of shuttle vectors dramatically increased as spirochetes grew from early log to stationary phase. B. burgdorferi produced approximately six copies of the introduced construct to match each copy of the linear chromosome at early log phase and increased to more than 40 copies at stationary phase. This increase in ospC gene copy number resulted in a dramatic increase in mRNA accumulation, clearly indicating that manipulating the copy number of a plasmid can achieve selective regulation of specific genes, a potential strategy for gene regulation that has been ignored in B. burgdorferi. However, it should be noted that the constructs were modified from pBBE22 [39], which was derived from cp9 [40]. It remains to be confirmed whether the constructs are replicated and maintained as the endogenous plasmid. It is also interesting to know whether the copy numbers of plasmids change during in vitro cultivation and the enzootic cycle of B. burgdorferi.
Previous studies showed B. burgdorferi exhibiting tissue-differential ospC expression, with significantly higher expression in both heart and skin tissues than the joints of SCID mice [17, 41]. Recent identification of the ospC operator led to a successful interpretation of this differential expression pattern [34]. Deletion of the operator results in a dramatic increase in ospC expression in joints, but does not affect expression in either heart or skin tissues, suggesting that the potential repressor is induced only in joints of SCID mice [34]. In the current study, the fused ospC operator versions did not affect the activity of the reporter flaB promoter either in heart or skin tissues, instead reducing ospC expression driven by the fused promoter in the joints of SCID mice, indicating the repressor is expressed only in this specific type of tissue in the absence of specific humoral pressure and allowing successful verification of the function of the operator. Because the long version showed more effectiveness in reducing ospC expression, the study also led to dissection of the operator. It remains to be determined whether the copy number of the constructs is tissue-dependent or changes during the course of infection. However, these factors should not affect our data interpretations because all our constructs should behave in similar manners under each specific condition.
The biological significance for the existence of the ospC operator in B. burgdorferi is to confer the ability to evade specific humoral immunity. Previous studies showed that OspC antibodies administered to immunodeficient mice or the specific humoral response induced during infection of immunocompetent mice constitute an overwhelming force to reduce ospC expression to a baseline level in all tissues [17, 18, 22, 23]. A recent study also showed that lack of the operator allows the ospC promoter to drive constitutive gene expression in all tissues and, as a result, completely diminishes the ability of B. burgdorferi to evade specific humoral immunity [34]. In the current study, lack of the operator allowed the fused flaB promoter to drive constitutive ospC expression in all tissues of SCID mice and, as a consequence, diminished the ability to evade specific OspC antibody. Addition of the operator to the upstream of the flaB promoter allowed B. burgdorferi to effectively evade clearance by OspC MAb in the joint tissue, an ability that was gained apparently through the down-regulation of ospC expression. Because the long operator version conferred B. burgdorferi a higher responsiveness to treatment with OspC antibody, the study, again, not only verified the function of the operator but also led to a successful dissection of the operator. The involvement of the fused ospC operator was unable to rescue B. burgdorferi from elimination by the administration of OspC MAb in the heart tissue, speculating that the sudden presence of specific antibody in a large quantity overwhelmed spirochetes before the down-regulation of ospC expression driven by the fused promoter could occur. This explanation is supported by our chronic infectivity study conducted in immunocompetent mice, showing B. burgdorferi that was given the operator was grown from most heart specimens collected 4 months after initial inoculation.
Although B. burgdorferi with constitutive ospC expression driven by a fused flaB promoter was eradicated by OspC MAb from all tissues of infected SCID mice, this genotype was able to persist in the skin of immunocompetent mice. Our previous study showed that spirochetes with increased OspC expression via the introduction of an extra ospC copy fused with a flaB promoter are cleared from all tissues of infected immunocompetent mice, unless escape mutations occur on the constitutively expressed copy [20]. Different ospC expression levels might cause this discrepancy. In the previous study, two ospC copies, the native gene and the introduced copy, might lead to higher OspC expression, making the pathogen more vulnerable to specific humoral immunity. This interpretation is supported by our other study [34], in which robust ospC expression driven by the ospC promoter without control of its operator completely abrogates the ability of B. burgdorferi to persist in immunocompetent mice.
During a transcriptional initiation, the σ factor of a RNA polymerase holoenzyme mediates the initial interaction of the polymerase with a specific promoter. Binding of a repressor to its operator may block the interaction, thus preventing a transcriptional initiation. In a bacterium, the same RNA polymerase can use all σ factors the cell expresses, albeit it may exhibit various utilization efficiencies for different σ factors. In addition, the concentration of a specific σ factor in the cell and its ability to mediate the interaction with a target promoter may affect the efficiency of an operator to influence transcriptional initiation. All of these might collectively contribute to the reduced effectiveness of the ospC operator to influence the activity of the fused σ70-dependent flaB promoter observed in the current study.
A palindromic sequence may form a cruciform structure that interacts with a regulatory protein with high affinity. There are two sets of IRs, potentially forming small and large cruciform structures immediately upstream of the ospC promoter (Fig. 3A). However, because the right IR of the large palindrome contains the left IR of the small palindrome, it is unclear how these four IRs are arranged to form secondary and tertiary structures. Our previous study clearly showed that the large palindrome is required for the operator’s activity; in the native promoter-operator system, however, we have not been able to address whether the small one contributes to the repressing activity [34]. In the current study, the ospC promoter was replaced, allowing us to dissect the operator without the influence of the native promoter. The study showed that although the large palindrome alone was able to effectively reduce the activity of the fused flaB promoter, the involvement of all the four IRs significantly improved the repressing activity. In our previous study, deletion of the sequence immediately upstream of the large palindrome has little effect on the activity of the operator, but removal of the left IR of the palindrome completely diminishes the operator’s function [34]. Taken together, these studies demonstrate that the operator includes all the four IRs.
4. Materials and methods
4.1. Construction of pBBE22-flaBpmin-ospC
As illustrated in Fig. 1, an 883-bp fragment was PCR (polymerase chain reaction) amplified with the use of primers P1F and P1R (Table 4), and of pBBE22-ospC′ as a template; digested with BamHI and XbaI; purified; and cloned into pBBE22 (a gift from S. Norris) [39] after the vector was digested with the same restriction enzymes. The recombinant plasmid pBBE22-ospC′ was constructed from pBBE22 in our previous study [20]. The insert within pBBE22-flaBpmin-ospC was sequenced to ensure that the insert and its flanking sequences were arranged as designed.
Table 4.
Primers used in this study
| Primer name | Primer sequence (5′ to 3′)a |
|---|---|
| P1F | TAGGATCCTTCTTTTTTTTTAATTTTTGTGCTA |
| P1R | TTCCTCTAGAGAAGAGCTTAAAGTTAA |
| P2F | TAGTTGGCTATATTGGGATCCAA |
| P2R | CAGAGAATTCTTTATTTGAAAAAATAATTTTTTC |
| P3F | ACAGGAATTCTTTTTTTTTAATTTTTGTGCTATTCT |
| P3R | TTGAATTCTTTTTCAAATTCTTCAATATCTTGA |
| P4F | TTGGGATCCAAAATCTAATACAAGT |
| P4R | GGAAATCTTCCTTGAAGCT |
| P5F | GCAGCTAATGTTGCAAATCTTTTC |
| P5R | GCAGGTGCTGGCTGTTGA |
| P6F | ATGCCTCTTCCGACCATCAAGCA |
| P6R | GATCGCAGTGGTGAGTAACCATGCA |
The underlined sequences are restriction enzyme sites: BamHI sites (P1F and P4F), a XbaI site (P1R), and EcoRI sites (P2R, P3F and P3R).
4.2. Construction of pBBE22-Co1-flaBp-ospC and pBBE22-Co2-flaBp-ospC
As illustrated in Fig. 3, 238-bp and 219-bp fragments, namely Co1 and Co2, were PCR amplified with the use of two reverse primers, P2R and P3R (Table 4), respectively, and a common forward primer, P2F, and of borrelial DNA as a template. A third fragment, designated flaBp-ospC, was generated by PCR using the primers P3F and P4R, and the construct pBBE22-flaBpmin-OspC as a template. After digestion with EcoRI and subsequent purification, the fragments Co1 and Co2, respectively, were fused with flaBp-ospC. Resulting fragments were used as templates and PCR amplified with the use of primers P4F and P1R, generating Co1-flaBp-ospC and Co2-flaBp-ospC, respectively. After digestion with BamHI and XbaI plus subsequent purification, the two fragments were cloned into pBBE22 after the vector was digested with the same enzymes, completing the construction of pBBE22-Co1-flaBp-ospC and pBBE22-Co2-flaBp-ospC, which were then sequenced to ensure that the inserts and their flanking sequences were arranged as designed.
4.3. Transformation of B. burgdorferi and selection of transformants
Constructs were electroporated into the ospC mutant, which was generated and characterized in our previous study [34]; resulting transformants were screened as described previously [42]. Selected transformants were first surveyed for the presence of lp28-1 as this plasmid is essential for infection of an immunocompetent host [42, 43]. Only clones that contained lp28-1 were further analyzed for plasmid content as described previously [42]. Selected clones were grown to late-log phase in Barbour-Stoenner-Kelly H (BSK-H) complete medium at 33°C (Sigma Chemical Co., St. Louis, MO), and subjected to immunoblot analysis probed with a mixture of FlaB and OspC monoclonal antibodies (MAbs), as described previously [20].
4.4. In vitro characterization of transformants
Transformants were grown in BSK-H complete medium to middle log and stationary phase at 33°C, and harvested by centrifugation. RNA samples were prepared and quantified for the cDNA copy numbers of flaB and ospC by reverse transcription-quantitative PCR (RT-qPCR) as previously described [17].
4.5. SYBR green qPCR analysis of kan and flaB gene copy numbers
Spirochetes were grown and harvested as described above; DNA was extracted using tissue DNA columns per the manufacturer’s instruction (Sigma). The copy numbers of the borrelial linear chromosome and the constructs were quantified by using the primers P5F and P5R or P6F and P6R (Table 4) specific for the genes flaB and kan, respectively. The recombinant plasmids pNCO1T-actin-flaB, which was constructed previously [42], and pBBE22 were used as DNA concentration standards for flaB and kan, respectively. Purified plasmids were determined for DNA concentrations by measuring the optical density at 260 nm wavelength, converted to copy numbers and 10-fold serially diluted within a range of 102 – 107 DNA copies/μl. The iTaq SYBR Green Supermix with ROX (Bio-Rad Laboratories, Hercules, CA) and the ABI PRISM™ 7900 sequence detection system (Applied Biosystems, Foster City, CA) were used for qPCR assays. Amplification was performed in a final volume of 10-μl on the ABI PRISM™ 384-well clear optical reaction plate (Applied Biosystems). Two sets of 12 wells on each plate were run for either flaB or kan DNA concentration standards. Both standards and samples were amplified in duplicate wells. A PCR program with the following parameters was used: 50°C for 2 min; 95°C for 5 min; 40 cycles of 95°C for 20 sec, 60°C for 1 min. The mean DNA copy numbers of flaB and kan in each sample were calculated from duplicate wells. Data were expressed as the copy number of kan per 1000 flaB copies.
4.6. Mouse infection study
SCID mice (BALB/c background; ages, 4 -- 8 weeks; provided by the Division of LSU Laboratory Animal Medicine) were given one single intradermal/subcutaneous injection of 104 spirochetes. Animals were examined for the development of arthritis at 2-day intervals, starting at day 7, and sacrificed one month post-inoculation. Joint, heart, and skin specimens were collected for RNA preparation. RNA was converted to cDNA and quantified for the mRNA copy numbers of flaB and ospC by qPCR as described previously [20].
4.7. Passive immunization of infected SCID mice
BALB/c SCID mice were inoculated and monitored for the development of arthritis as described above. Infected mice received a single subcutaneous injection of 100 μg of OspC MAb or purified mouse IgG as a control 3 weeks post-inoculation, as described previously [34]. Mice were sacrificed 1 week after passive immunization; heart, tibiotarsal joint, and skin specimens were aseptically collected for spirochete culture as previously described [42]. RNA was extracted from joint samples, and used for ospC expression analyses as described above.
4.8. Chronic infectivity study
Groups of five to 10 BALB/c mice (ages, 4 -- 8 weeks; provided by the Division of LSU Laboratory Animal Medicine) were given one single intradermal/subcutaneous injection of 104 spirochetes. Ear biopsies were conducted for bacterial isolation 3 weeks after inoculation. Mice were euthanized 4 months post-inoculation; heart, tibiotarsal joint and skin specimens were aseptically collected for spirochete culture as previously described [42].
4.9. Statistical analysis
qPCR Data were analyzed by using a one-way analysis of variance (ANOVA), followed by a two-tailed Student t test to compare two treatments and calculate P values. Data obtained from infection studies were analyzed by using Fisher’s exact test. Calculated P values of ≤0.05 were considered significant.
Acknowledgments
We thank S. Norris for providing pBBE22, P. Rosa for critically reading the manuscript, and M. Kearney for assistance with statistical analysis.
This work was supported in part by a career development award and a grant from NIH/NIAMS, an Arthritis Foundation Investigators award, and P20RR020159 (PI, Kousoulas) from NIH/NCRR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Seiler KP, Weis JJ. Immunity to Lyme disease: protection, pathology and persistence. Curr Opin Immunol. 1996;8:503–9. doi: 10.1016/s0952-7915(96)80038-0. [DOI] [PubMed] [Google Scholar]
- 2.Steere AC. Lyme disease. N Engl J Med. 2001;345:115–25. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- 3.Schwan TG, Piesman J, Golde WT, Dolan MC, Rosa PA. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci U S A. 1995;92:2909–13. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwan TG, Piesman J. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J Clin Microbiol. 2000;38:382–8. doi: 10.1128/jcm.38.1.382-388.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohnishi J, Piesman J, de Silva AM. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc Natl Acad Sci U S A. 2001;98:670–5. doi: 10.1073/pnas.98.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Silva AM, Telford SR, 3rd, Brunet LR, Barthold SW, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–5. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang XF, Pal U, Alani SM, Fikrig E, Norgard MV. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J Exp Med. 2004;199:641–8. doi: 10.1084/jem.20031960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neelakanta G, et al. Outer surface protein B is critical for Borrelia burgdorferi adherence and survival within Ixodes ticks. PLoS Pathog. 2007;3:e33. doi: 10.1371/journal.ppat.0030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fingerle V, Goettner G, Gern L, Wilske B, Schulte-Spechtel U. Complementation of a Borrelia afzelii OspC mutant highlights the crucial role of OspC for dissemination of Borrelia afzelii in Ixodes ricinus. Int J Med Microbiol. 2007;297:97–107. doi: 10.1016/j.ijmm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Grimm D, et al. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc Natl Acad Sci U S A. 2004;101:3142–7. doi: 10.1073/pnas.0306845101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart PE, et al. Delineating the requirement for the Borrelia burgdorferi virulence factor OspC in the mammalian host. Infect Immun. 2006;74:3547–53. doi: 10.1128/IAI.00158-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pal U, et al. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J Clin Invest. 2004;113:220–30. doi: 10.1172/JCI19894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsao J, Barbour AG, Luke CJ, Fikrig E, Fish D. OspA immunization decreases transmission of Borrelia burgdorferi spirochetes from infected Peromyscus leucopus mice to larval Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 2001;1:65–74. doi: 10.1089/153036601750137705. [DOI] [PubMed] [Google Scholar]
- 14.Tsao JI, Wootton JT, Bunikis J, Luna MG, Fish D, Barbour AG. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc Natl Acad Sci U S A. 2004;101:18159–64. doi: 10.1073/pnas.0405763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Silva AM, Fish D, Burkot TR, Zhang Y, Fikrig E. OspA antibodies inhibit the acquisition of Borrelia burgdorferi by Ixodes ticks. Infect Immun. 1997;65:3146–50. doi: 10.1128/iai.65.8.3146-3150.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strother KO, Hodzic E, Barthold SW, de Silva AM. Infection of mice with Lyme disease spirochetes constitutively producing outer surface proteins A and B. Infect Immun. 2007;75:2786–94. doi: 10.1128/IAI.01307-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang FT, et al. Borrelia burgdorferi changes its surface antigenic expression in response to host immune responses. Infect Immun. 2004;72:5759–67. doi: 10.1128/IAI.72.10.5759-5767.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang FT, Jacobs MB, Bowers LC, Philipp MT. An immune evasion mechanism for spirochetal persistence in Lyme borreliosis. J Exp Med. 2002;195:415–22. doi: 10.1084/jem.20011870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tilly K, Bestor A, Jewett MW, Rosa P. Rapid clearance of Lyme disease spirochetes lacking OspC from skin. Infect Immun. 2007;75:1517–9. doi: 10.1128/IAI.01725-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Q, Seemanapalli SV, McShan K, Liang FT. Constitutive expression of outer surface protein C diminishes the ability of Borrelia burgdorferi to evade specific humoral immunity. Infect Immun. 2006;74:5177–84. doi: 10.1128/IAI.00713-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fung BP, McHugh GL, Leong JM, Steere AC. Humoral immune response to outer surface protein C of Borrelia burgdorferi in Lyme disease: role of the immunoglobulin M response in the serodiagnosis of early infection. Infect Immun. 1994;62:3213–21. doi: 10.1128/iai.62.8.3213-3221.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang FT, Nelson FK, Fikrig E. Molecular adaptation of Borrelia burgdorferi in the murine host. J Exp Med. 2002;196:275–80. doi: 10.1084/jem.20020770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crother TR, et al. Temporal analysis of the antigenic composition of Borrelia burgdorferi during infection in rabbit skin. Infect Immun. 2004;72:5063–72. doi: 10.1128/IAI.72.9.5063-5072.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilmore RD, Jr, Piesman J. Inhibition of Borrelia burgdorferi migration from the midgut to the salivary glands following feeding by ticks on OspC-immunized mice. Infect Immun. 2000;68:411–4. doi: 10.1128/iai.68.1.411-414.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fraser CM, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–6. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 26.Hubner A, Yang X, Nolen DM, Popova TG, Cabello FC, Norgard MV. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc Natl Acad Sci U S A. 2001;98:12724–9. doi: 10.1073/pnas.231442498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang XF, Alani SM, Norgard MV. The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc Natl Acad Sci U S A. 2003;100:11001–6. doi: 10.1073/pnas.1834315100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagman KE, et al. Decorin-binding protein A (DbpA) of Borrelia burgdorferi is not protective when immunized mice are challenged via tick infestation and correlates with the lack of DbpA expression by B. burgdorferi in ticks. Infect Immun. 2000;68:4759–64. doi: 10.1128/iai.68.8.4759-4764.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He M, Boardman BK, Yan D, Yang XF. Regulation of expression of the fibronectin-binding protein BBK32 in Borrelia burgdorferi. J Bacteriol. 2007;189:8377–80. doi: 10.1128/JB.01199-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller JC, von Lackum K, Babb K, McAlister JD, Stevenson B. Temporal analysis of Borrelia burgdorferi Erp protein expression throughout the mammal-tick infectious cycle. Infect Immun. 2003;71:6943–52. doi: 10.1128/IAI.71.12.6943-6952.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caimano MJ, et al. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol Microbiol. 2007;65:1193–217. doi: 10.1111/j.1365-2958.2007.05860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fisher MA, et al. Borrelia burgdorferi σ54 is required for mammalian infection and vector transmission but not for tick colonization. Proc Natl Acad Sci U S A. 2005;102:5162–7. doi: 10.1073/pnas.0408536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caimano MJ, Eggers CH, Gonzalez CA, Radolf JD. Alternate sigma factor RpoS is required for the in vivo-specific repression of Borrelia burgdorferi plasmid lp54-borne ospA and lp6.6 genes. J Bacteriol. 2005;187:7845–52. doi: 10.1128/JB.187.22.7845-7852.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Q, McShan K, Liang FT. Identification of an ospC operator critical for immune evasion of Borrelia burgdorferi. Mol Microbiol. 2007;64:220–31. doi: 10.1111/j.1365-2958.2007.05636.x. [DOI] [PubMed] [Google Scholar]
- 35.Eggers CH, Caimano MJ, Radolf JD. Analysis of promoter elements involved in the transcriptional initiation of RpoS-dependent Borrelia burgdorferi genes. J Bacteriol. 2004;186:7390–402. doi: 10.1128/JB.186.21.7390-7402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang XF, et al. Analysis of the ospC regulatory element controlled by the RpoN-RpoS regulatory pathway in Borrelia burgdorferi. J Bacteriol. 2005;187:4822–9. doi: 10.1128/JB.187.14.4822-4829.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ge Y, Old IG, Saint Girons I, Charon NW. Molecular characterization of a large Borrelia burgdorferi motility operon which is initiated by a consensus σ70 promoter. J Bacteriol. 1997;179:2289–99. doi: 10.1128/jb.179.7.2289-2299.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilbert MA, Morton EA, Bundle SF, Samuels DS. Artificial regulation of ospC expression in Borrelia burgdorferi. Mol Microbiol. 2007;63:1259–73. doi: 10.1111/j.1365-2958.2007.05593.x. [DOI] [PubMed] [Google Scholar]
- 39.Purser JE, Lawrenz MB, Caimano MJ, Howell JK, Radolf JD, Norris SJ. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol Microbiol. 2003;48:753–64. doi: 10.1046/j.1365-2958.2003.03452.x. [DOI] [PubMed] [Google Scholar]
- 40.Stewart PE, Thalken R, Bono JL, Rosa P. Isolation of a circular plasmid region sufficient for autonomous replication and transformation of infectious Borrelia burgdorferi. Mol Microbiol. 2001;39:714–21. doi: 10.1046/j.1365-2958.2001.02256.x. [DOI] [PubMed] [Google Scholar]
- 41.Crother TR, Champion CI, Wu XY, Blanco DR, Miller JN, Lovett MA. Antigenic composition of Borrelia burgdorferi during infection of SCID mice. Infect Immun. 2003;71:3419–28. doi: 10.1128/IAI.71.6.3419-3428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu Q, et al. Association of linear plasmid 28-1 with an arthritic phenotype of Borrelia burgdorferi. Infect Immun. 2005;73:7208–15. doi: 10.1128/IAI.73.11.7208-7215.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grimm D, et al. Experimental assessment of the roles of linear plasmids lp25 and lp28-1 of Borrelia burgdorferi throughout the infectious cycle. Infect Immun. 2004;72:5938–46. doi: 10.1128/IAI.72.10.5938-5946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ge Y, Old IG, Girons IS, Charon NW. The flgK motility operon of Borrelia burgdorferi is initiated by a σ70-like promoter. Microbiology. 1997;143(Pt 5):1681–90. doi: 10.1099/00221287-143-5-1681. [DOI] [PubMed] [Google Scholar]
- 45.Marconi RT, Samuels DS, Garon CF. Transcriptional analyses and mapping of the ospC gene in Lyme disease spirochetes. J Bacteriol. 1993;175:926–32. doi: 10.1128/jb.175.4.926-932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Margolis N, Hogan D, Cieplak W, Jr, Schwan TG, Rosa PA. Homology between Borrelia burgdorferi OspC and members of the family of Borrelia hermsii variable major proteins. Gene. 1994;143:105–10. doi: 10.1016/0378-1119(94)90613-0. [DOI] [PubMed] [Google Scholar]