Abstract
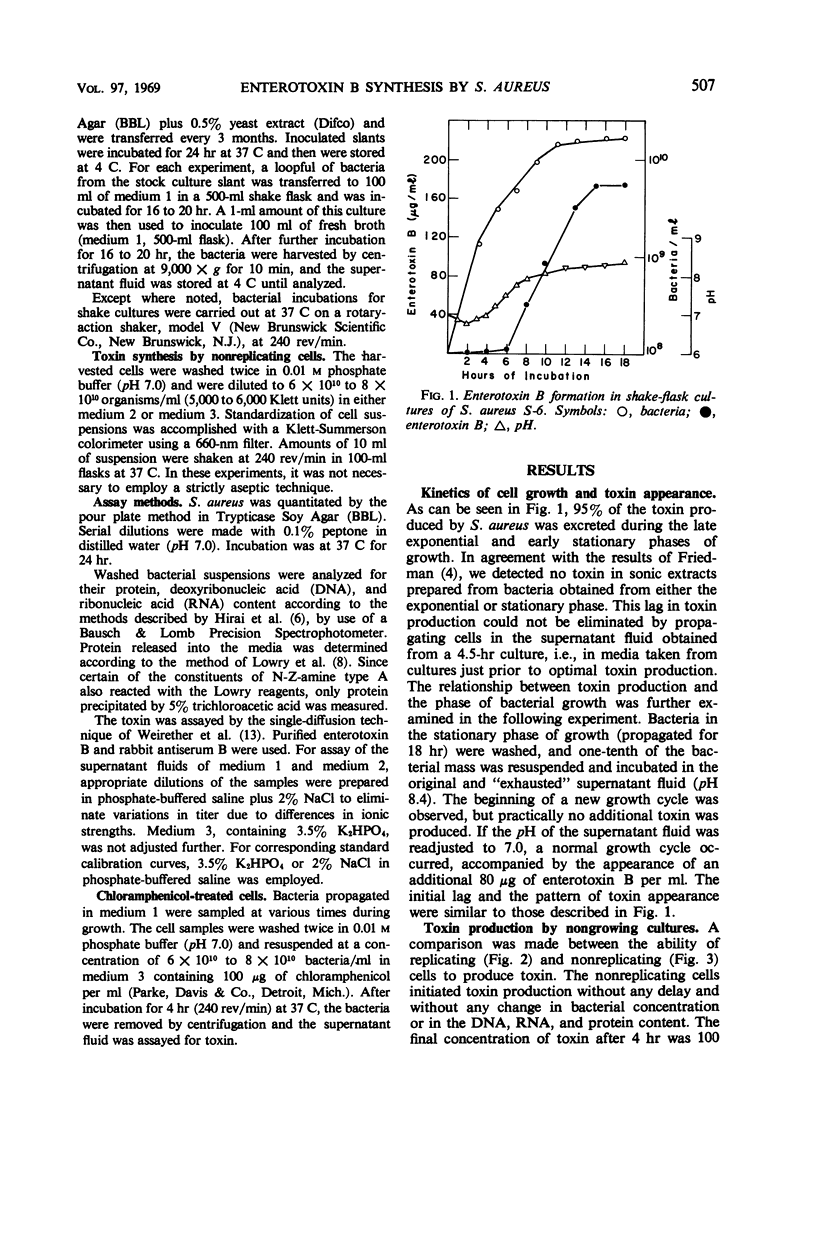
Although 95% of the enterotoxin B produced by Staphylococcus aureus appears during the latter part of the exponential phase of growth, growth per se is not necessary for toxin synthesis. A procedure is described whereby a concentrated suspension (at least 6 × 1010 cells per ml) of a 16-hr culture of S. aureus was found to be capable of producing toxin, without replication, when air and glucose were present. This technique allows the growth requirement to be separated from toxin formation. Although higher (100 μg/ml) concentrations of toxin appeared in the medium when nitrogen was present, lower levels (30 μg/ml) were produced in the absence of N-Z-amine A. Toxin production proceeded without any net increase in deoxyribonucleic acid, ribonucleic acid, or protein. Chloramphenicol did not inhibit toxin formation in a nitrogen-free medium. The optimal pH for toxin production in a nitrogen-free medium was 8.0 to 8.5; for synthesis in a medium where nitrogen was available, the optimal pH was 7.0 to 7.5. Increasing the rate of aeration increased toxin release during growth, but decreased the amount of toxin subsequently produced when the bacteria were resuspended. These results suggest the presence of a precursor pool in the cells collected after 16 hr of growth.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergdoll M. S., Chu F. S., Borga C. R., Huang I. Y., Weiss K. F. The staphylococcal enterotoxins. Jpn J Microbiol. 1967 Dec;11(4):358–368. doi: 10.1111/j.1348-0421.1967.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Friedman M. E. Inhibition of staphylococcal enterotoxin B formation by cell wall blocking agents and other compounds. J Bacteriol. 1968 Mar;95(3):1051–1055. doi: 10.21236/ad0833372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M. E. Inhibition of staphylococcal enterotoxin B formation in broth cultures. J Bacteriol. 1966 Jul;92(1):277–278. doi: 10.1128/jb.92.1.277-278.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato E., Khan M., Kujovich L., Bergdoll M. S. Production of enterotoxin a. Appl Microbiol. 1966 Nov;14(6):966–972. doi: 10.1128/am.14.6.966-972.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Markus Z., Silverman G. J. Enterotoxin B production by nongrowing cells of Staphylococcus aureus. J Bacteriol. 1968 Oct;96(4):1446–1447. doi: 10.1128/jb.96.4.1446-1447.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean R. A., Lilly H. D., Alford J. A. Effects of meat-curing salts and temperature on production of staphylococcal enterotoxin B. J Bacteriol. 1968 Apr;95(4):1207–1211. doi: 10.1128/jb.95.4.1207-1211.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwald A. J., Lincoln R. E. Streptomycin inhibition of elaboration of staphylococcal enterotoxic protein. J Bacteriol. 1966 Jul;92(1):279–280. doi: 10.21236/ad0481024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weirether F. J., Lewis E. E., Rosenwald A. J., Lincoln R. E. Rapid quantitative serological assay of staphylococcal enterotoxin B. Appl Microbiol. 1966 Mar;14(2):284–291. doi: 10.1128/am.14.2.284-291.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]



