Abstract
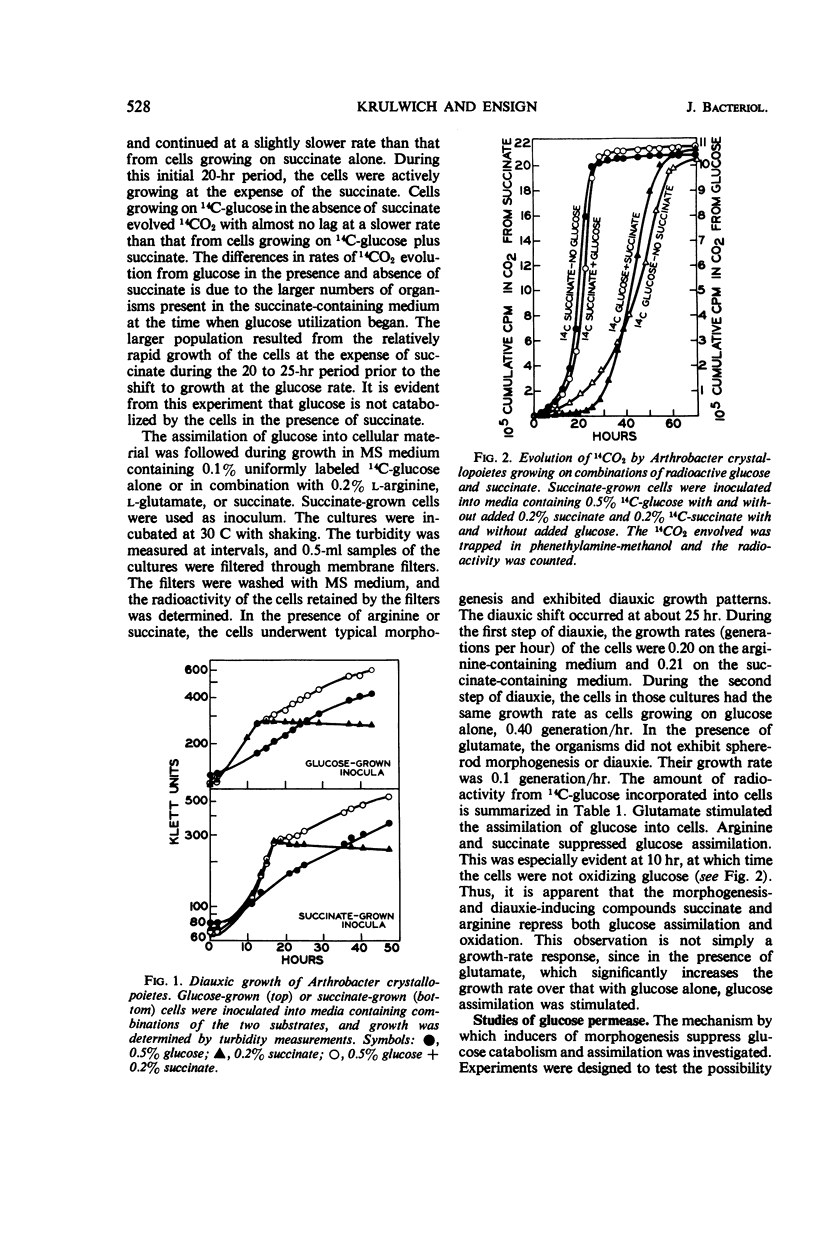
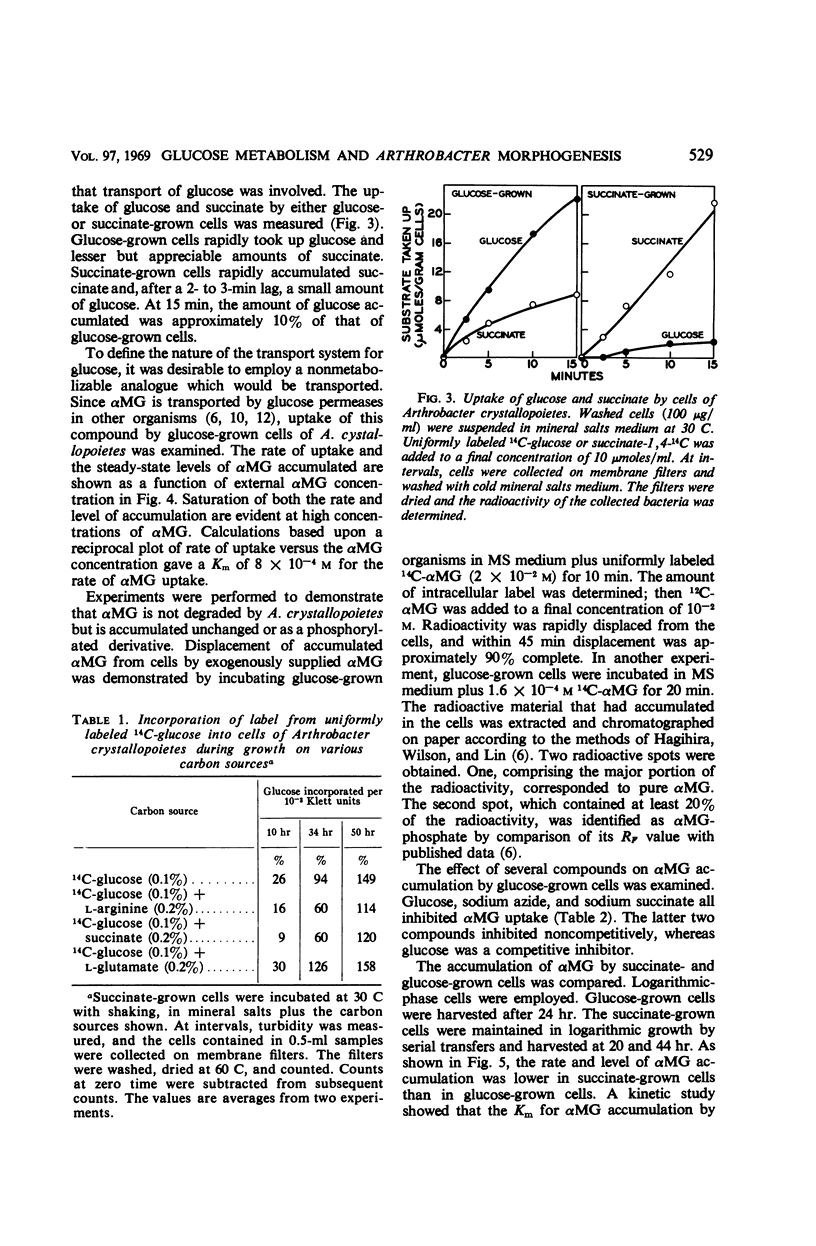
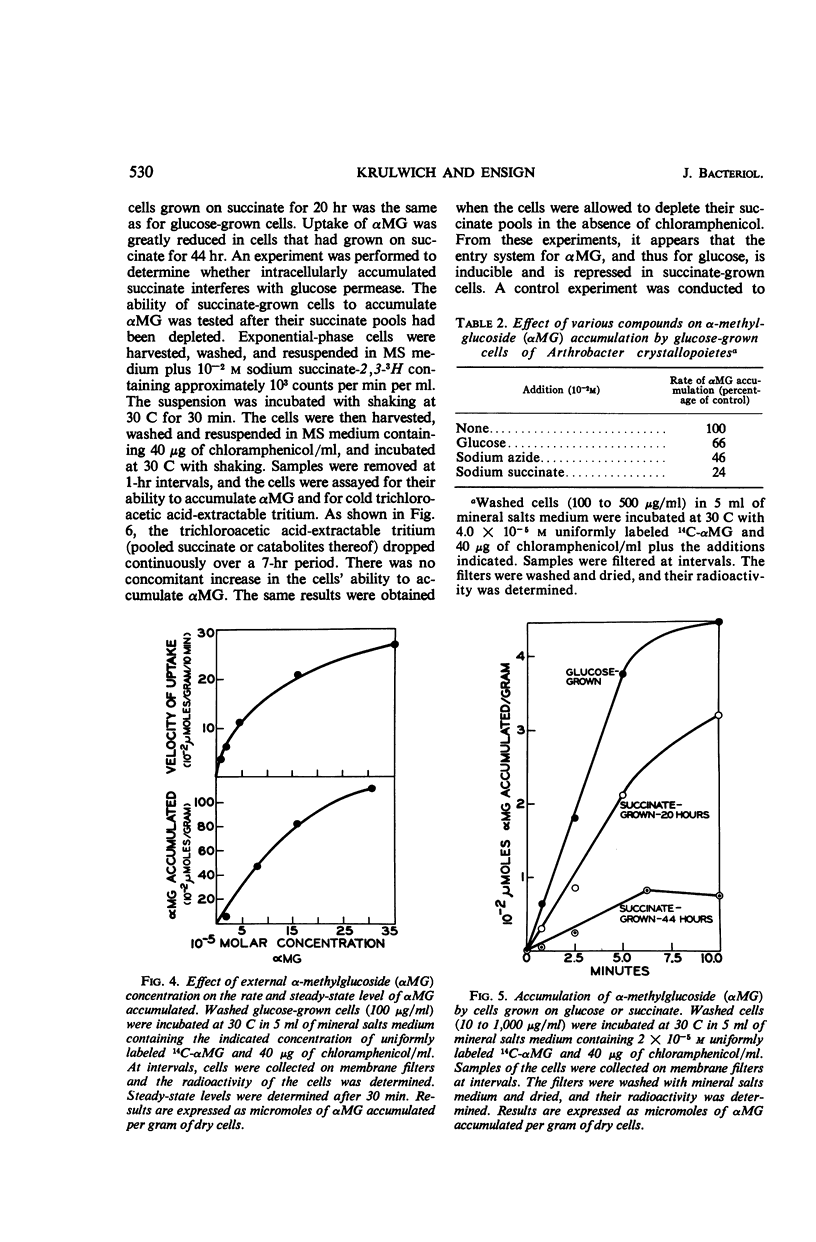
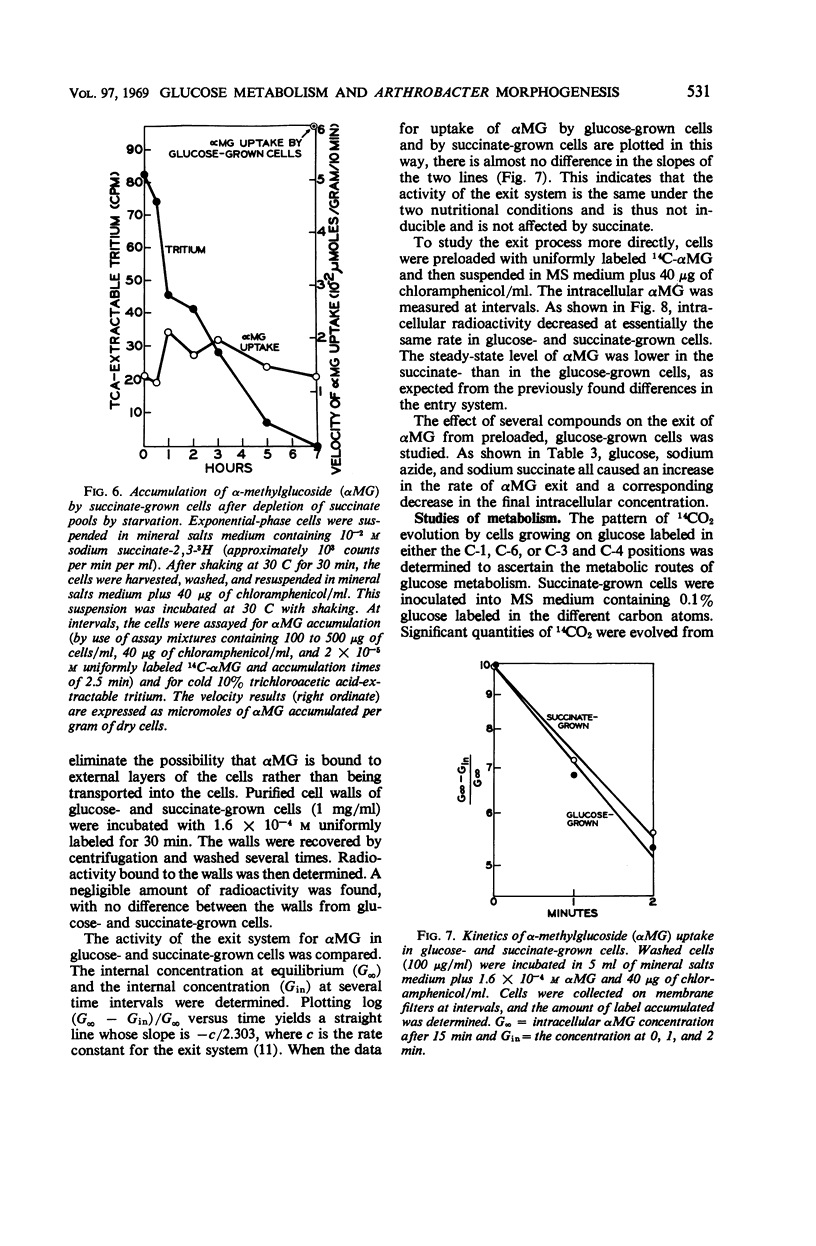
Succinate and several other compounds which induce sphere to rod morphogenesis of A. crystallopoietes were found to suppress both catabolism and assimilation of glucose. Diauxic growth patterns resulted from growth on glucose plus any one of these compounds. Glutamate stimulated growth but was not an inducer of morphogenesis. With this compound, diauxic growth and suppression of glucose catabolism or assimilation did not occur. Glucose permease was studied with α-methylglucoside as substrate. The entry system for glucose was found to involve active transport and to have a Km of 8 × 10−4m. It was inducible, was repressed in succinate-grown cells, and was also inhibited by succinate. The exit system was constitutive and appeared to be less sensitive than the entry system to inhibition by azide. The properties of the glucose permease system may account for the slow growth of the organism on glucose and the preferred use of other substrates for growth. Studies of metabolic pathways for glucose metabolism indicated the operation of the Embden-Meyerhof-Parnas (EMP) and pentose phosphate pathways and of the tricarboxylic acid cycle. Cells grown on glucose plus limiting amounts of succinate or other inducers of morphogenesis metabolized the glucose only after exhaustion of the inducers. Under these circumstances, the organisms employed the EMP pathway to a greater extent than when growing on glucose as sole carbon source.
Full text
PDF

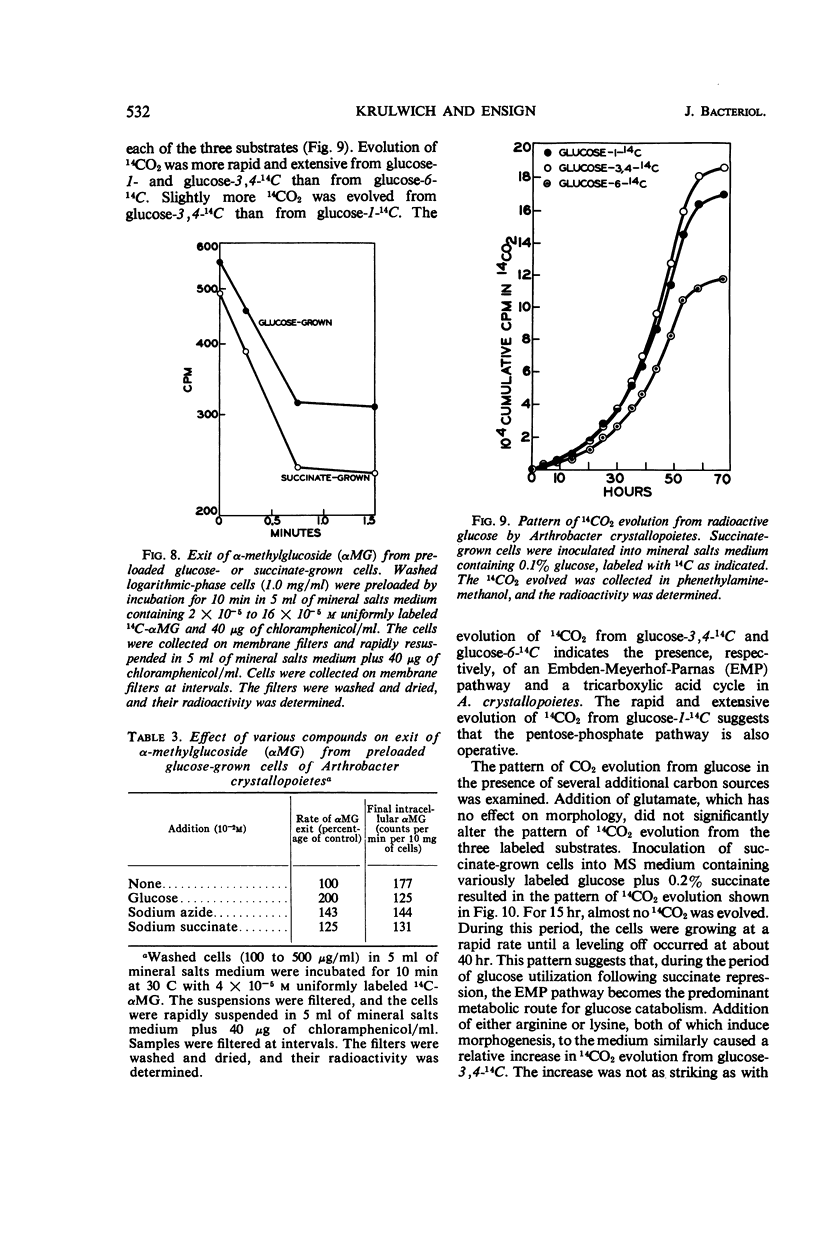
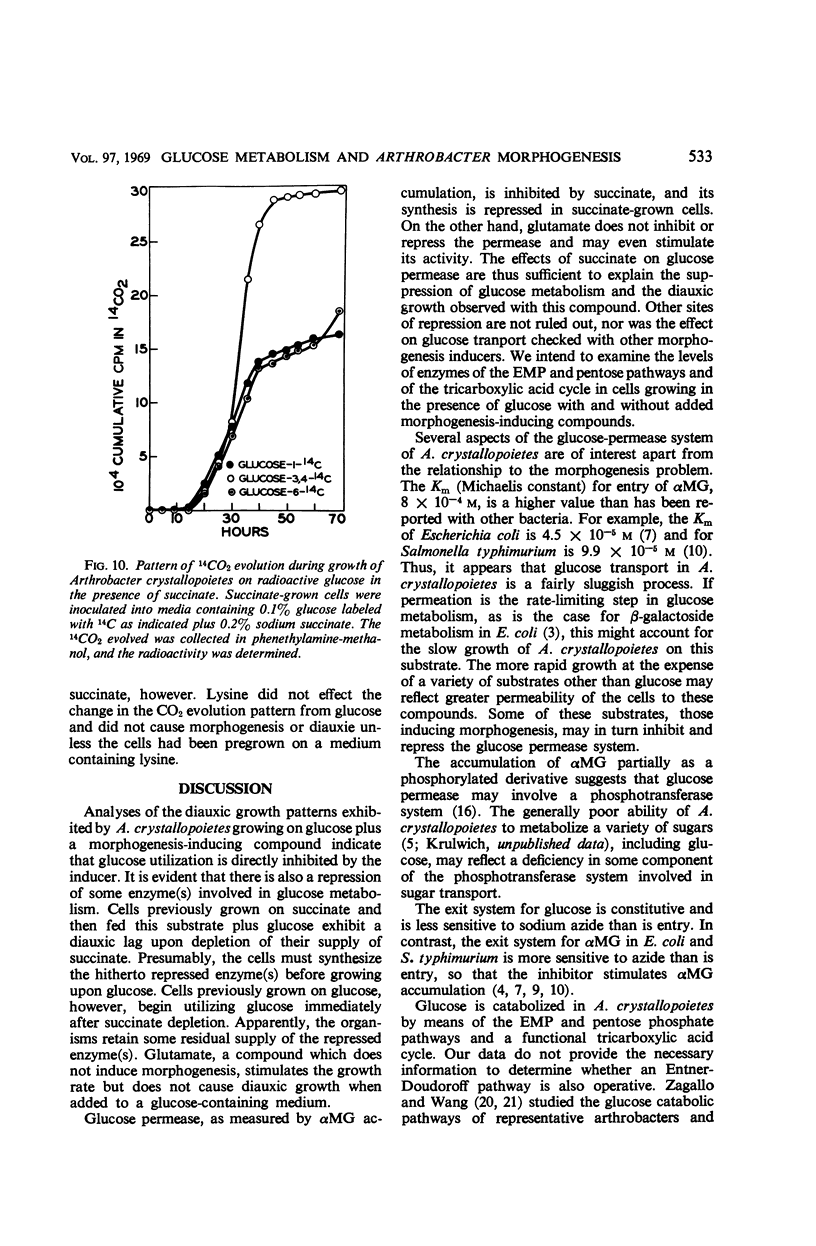

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brammar W. J., Clarke P. H., Skinner A. J. Biochemical and genetic studies with regulator mutants of the Pseudomonas aeruginosa 8602 amidase system. J Gen Microbiol. 1967 Apr;47(1):87–102. doi: 10.1099/00221287-47-1-87. [DOI] [PubMed] [Google Scholar]
- Brown O. R., Reda S. Enzyme and permeability changes during morphogenesis of Nocardia corallina. J Gen Microbiol. 1967 May;47(2):199–205. doi: 10.1099/00221287-47-2-199. [DOI] [PubMed] [Google Scholar]
- COHEN G. N., MONOD J. Bacterial permeases. Bacteriol Rev. 1957 Sep;21(3):169–194. doi: 10.1128/br.21.3.169-194.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLESBERG E., WATSON J. A., HOFFEE P. A. The glucose effect and the relationship between glucose permease, acid phosphatase, and glucose resistance. Cold Spring Harb Symp Quant Biol. 1961;26:261–276. doi: 10.1101/sqb.1961.026.01.033. [DOI] [PubMed] [Google Scholar]
- ENSIGN J. C., WOLFE R. S. NUTRITIONAL CONTROL OF MORPHOGENESIS IN ARTHROBACTER CRYSTALLOPIETES. J Bacteriol. 1964 Apr;87:924–932. doi: 10.1128/jb.87.4.924-932.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIHIRA H., WILSON T. H., LIN E. C. STUDIES ON THE GLUCOSE-TRANSPORT SYSTEM IN ESCHERICHIA COLI WITH ALPHA-METHYLGLUCOSIDE AS SUBSTRATE. Biochim Biophys Acta. 1963 Nov 15;78:505–515. doi: 10.1016/0006-3002(63)90912-0. [DOI] [PubMed] [Google Scholar]
- HOFFEE P., ENGLESBERG E. Effect of metabolic activity on the glucose permease of bacterial cells. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1759–1765. doi: 10.1073/pnas.48.10.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFEE P., ENGLESBERG E., LAMY F. THE GLUCOSE PERMEASE SYSTEM IN BACTERIA. Biochim Biophys Acta. 1964 Mar 30;79:337–350. [PubMed] [Google Scholar]
- HORECKER B. L., THOMAS J., MONOD J. Galactose transport in Escherichia coli. II. Characteristics of the exit process. J Biol Chem. 1960 Jun;235:1586–1590. [PubMed] [Google Scholar]
- Halpern Y. S., Lupo M. Effect of glucose and other carbon compounds on the transport of alpha-methylglucoside in Escherichia coli K12. Biochim Biophys Acta. 1966 Sep 5;126(1):163–167. doi: 10.1016/0926-6585(66)90046-x. [DOI] [PubMed] [Google Scholar]
- KESSLER D. P., RICKENBERG H. V. The competitive inhibition of alpha-methylglucoside uptake in Escherichia coli. Biochem Biophys Res Commun. 1963 Mar 25;10:482–487. doi: 10.1016/0006-291x(63)90383-8. [DOI] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C. Activity of an autolytic N-acetylmuramidase during sphere-rod morphogenesis in Arthrobacter crystallopoietes. J Bacteriol. 1968 Sep;96(3):857–859. doi: 10.1128/jb.96.3.857-859.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C., Tipper D. J., Strominger J. L. Sphere-rod morphogenesis in Arthrobacter crystallopoietes. I. Cell wall composition and polysaccharides of the peptidoglycan. J Bacteriol. 1967 Sep;94(3):734–740. doi: 10.1128/jb.94.3.734-740.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C., Tipper D. J., Strominger J. L. Sphere-rod morphogenesis in Arthrobacter crystallopoietes. II. Peptides of the cell wall peptidoglycan. J Bacteriol. 1967 Sep;94(3):741–750. doi: 10.1128/jb.94.3.741-750.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessie T. G., Neidhardt F. C. Formation and operation of the histidine-degrading pathway in Pseudomonas aeruginosa. J Bacteriol. 1967 Jun;93(6):1800–1810. doi: 10.1128/jb.93.6.1800-1810.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- ZAGALLO A. C., WANG C. H. Comparative carbohydrate catabolism in Arthrobacter. J Gen Microbiol. 1962 Nov;29:389–401. doi: 10.1099/00221287-29-3-389. [DOI] [PubMed] [Google Scholar]
- Zagallo A. C., Wang C. H. Comparative carbohydrate catabolism in corynebacteria. J Gen Microbiol. 1967 Jun;47(3):347–357. doi: 10.1099/00221287-47-3-347. [DOI] [PubMed] [Google Scholar]



