Abstract
The current study revisited the question of whether there are brain mechanisms specific to divided attention that differ from those used in selective attention. Increased neuronal activity required to simultaneously process two stimulus dimensions as compared with each separate dimension has often been observed, but rarely has activity induced by a divided attention condition exceeded the sum of activity induced by the component tasks. Healthy participants performed a selective-divided attention paradigm while undergoing functional Magnetic Resonance Imaging (fMRI). The task required participants to make a same-different judgment about either one of two simultaneously presented stimulus dimensions, or about both dimensions. Performance accuracy was equated between tasks by dynamically adjusting the stimulus display time. Blood Oxygenation Level Dependent (BOLD) signal differences between tasks were identified by whole-brain voxel-wise comparisons and by region-specific analyses of all areas modulated by the divided attention task (DIV). No region displayed greater activation or deactivation by DIV than the sum of signal change by the two selective attention tasks. Instead, regional activity followed the tasks’ processing demands as reflected by reaction time. Only a left cerebellar region displayed a correlation between participants’ BOLD signal intensity and reaction time that was selective for DIV. The correlation was positive, reflecting slower responding with greater activation. Overall, the findings do not support the existence of functional brain activity specific to DIV. Increased activity appears to reflect additional processing demands by introducing a secondary task, but those demands do not appear to qualitatively differ from processes of selective attention.
Section: Cognitive and Behavioral Neuroscience
Keywords: attention, selective, divided, dual-task, fMRI
1. Introduction
The concept of attention is traditionally closely linked to the resource theory and its central premise that an organism possesses limited processing capacity and has to select from the multitude of available sensory input (Broadbent, 1958). Attended information is selected and further processed, while unattended input is filtered out. The term selective attention describes the process of focusing resources on specific aspects of all input. Concepts of divided attention are concerned with limitations of performing more than one information-processing task simultaneously. Divided attention thus relates to the optimal allocation of resources between different sets of input by splitting or rapid shifting of the attentional focus, given the inability to process all available information in parallel (Parasuraman, 1998). Attention can be divided between locations in space, between features of a single or of several objects, and between stimuli in one or several sensory modalities (Braun, 1998).
Several neuroimaging studies have investigated whether dividing attention recruits the same brain structures as selective attention, or whether there are mechanisms specific to divided attention. Prefrontal, posterior parietal and premotor cortical areas were more activated by divided attention to two stimulus dimensions than by selective attention to each separate dimension (Corbetta et al., 1991a; Vandenberghe, et al. 1997; Rees et al., 1997; Herath et al., 2001; Loose et al., 2003; Nebel et al., 2005; Weerda et al., 2006; Johnson and Zatorre, 2006). Enhanced activity in the anterior cingulate cortex (ACC) has also been reported, although, where performance accuracy was not equated, this may be explained by differences in error processing and response uncertainty between the selective and divided attention conditions (Carter et al., 1998; 2000; Botvinick et al., 1999). In most studies, activations during divided attention did not exceed the sum of activity induced by the component selective attention tasks. Thus, the increase in neural activity may reflect additional processing demands by introducing a secondary task, but not necessarily qualitatively different processes above and beyond demands on selective attention. Only a few studies provided evidence for activity specific to divided attention:
When participants concurrently discriminated two stimulus features, left superior parietal, left frontal and temporal activations exceeded the summed activity in the single discriminations (Vandenberghe et al., 1997). Analyses were restricted to stimuli presented in the left hemifield. The predominantly right lateralized activations during single discriminations (in accordance with stimulus presentation on the left) may thus explain the mostly left-hemispheric recruitment of additional activity as attentional load increased. Another study presented two successive stimuli in different modalities and identified right inferior frontal activation only when stimuli appeared in close temporal succession and created behavioral interference (Herath et al., 2001). Activation in the single-task conditions was not significant, but activity levels were not reported, thus precluding a direct comparison with the interference condition. Johnson and Zatorre (2006) presented participants with a melody and a shape stimulus “drawn” over the same time frame. Attending to both stimuli activated left dorsolateral prefrontal cortex, with zero activity in the single-task conditions. Subjects were aware that a memory test for the stimuli would follow. The encoding of two simultaneous information streams may have introduced particular control demands to coordinate the continuous update of both streams in working memory.
This raises the question of which specific cognitive processes differentiate divided from selective attention conditions. Beyond the need to divide attention between stimulus dimensions, such conditions introduce uncertainty regarding the response-relevant stimulus feature, and the need to maintain two sets of behavioral goals and to control attentional resources allocation. There does not appear to be a clear division between divided attention and dual-task interference at an executive level (Braun, 1998). Dividing attention becomes more difficult with increasing executive control demands (Posner and DiGirolamo, 1998), and control demands can be expected to increase with the complexity of the component tasks. Thus, task characteristics appear to influence the processes necessary to divide attention.
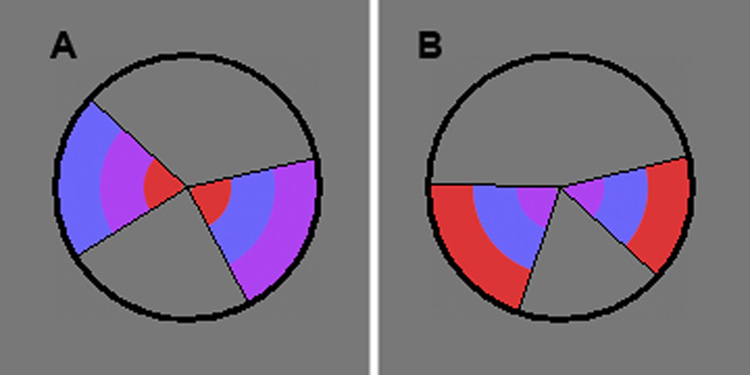
The aim of the present study was to determine if brain activations specific to divided attention could be identified under conditions that induce minimal demands on executive control processes and working memory. A task setting was developed that enabled performance accuracy to be equated between the selective and divided attention conditions in order to minimize differences in error processing and response uncertainty. A single foveally-presented circular stimulus accommodated three tasks related to decisions about each of two stimulus dimensions and about both dimensions combined. The circle contained two wedges, each divided into three rings of color (Fig. 1). Participants had to decide whether either the color order of the rings (selective color, SEL-C) or the angles of the wedges (selective angle, SEL-A) or both of these features (divided attention, DIV) were the same. Participants performed these tasks in two identical fMRI sessions, throughout which performance accuracy was held at 75% for each task by manipulating the time that the stimulus was on the screen (display time, DT).
Figure 1. Examples of the task stimulus.
Participants were instructed to detect a difference in either the angles of the two wedges, in the sequence of color across the three rings, or in either aspect. In (A), there is a difference in the color dimension. In (B), there is a difference in the angle dimension.
2. Results
Behavioral performance
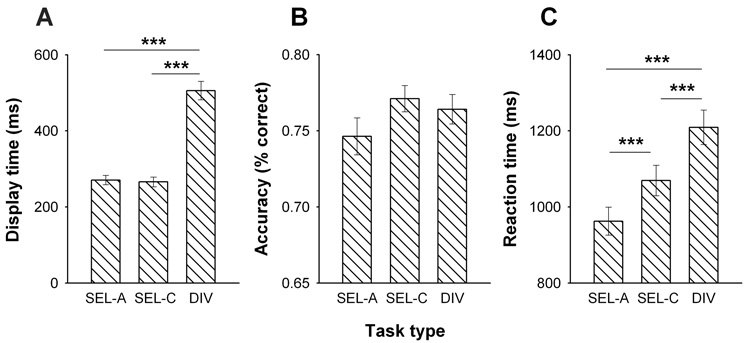
Fig. 2A shows the average DT of the circle stimulus across the three tasks. A main effect of TASK [F(2,48)=262.8, P<0.001] in two-factor ANOVA was due to DIV requiring longer DT than either SEL-A or SEL-C, while SEL-A and SEL-C did not differ. Fig. 2B shows that, for all three tasks, the adjustments in DT successfully manipulated performance accuracy to vary around or just above 75%. For accuracy, ANOVA yielded no main effect of TASK [F(2,48)=2.15, NS], suggesting that it was roughly equated between SEL-A, SEL-C and DIV. RT, however, differed with TASK [F(2,48)=58.4, P<0.001]; it was not only longer for DIV than SEL-C and SEL-A, but also longer for SEL-C than SEL-A (Fig. 2C). There was no main effect of SESSION or SESSION × TASK interaction on any measure.
Figure 2. Performance across task conditions.
Average (± SEM) display time (A), response accuracy (B) and reaction time (C) of all subjects (n=25) performing angle discriminations (Sel-A), color discriminations (Sel-C) or combined angle and color discriminations (divided attention, DIV). Each subject was tested twice, and results are averaged over both test sessions.
fMRI
Average task activation maps
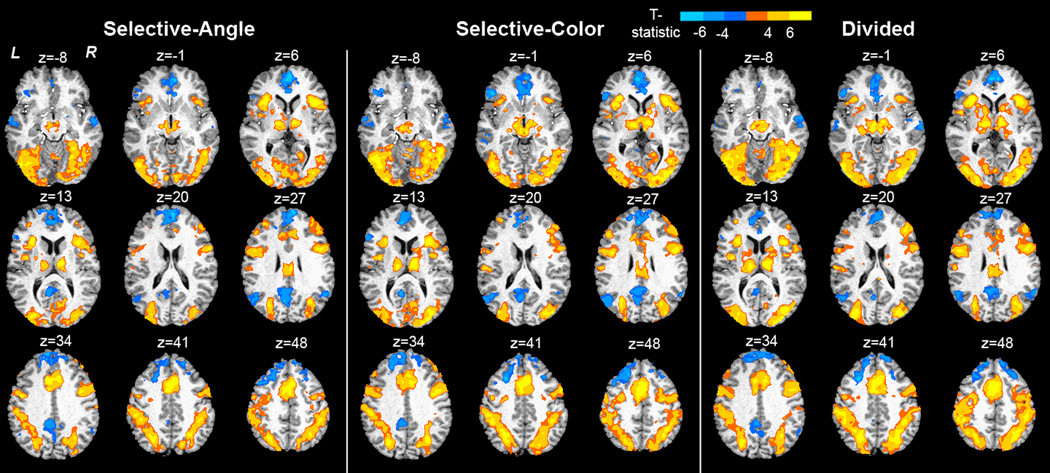
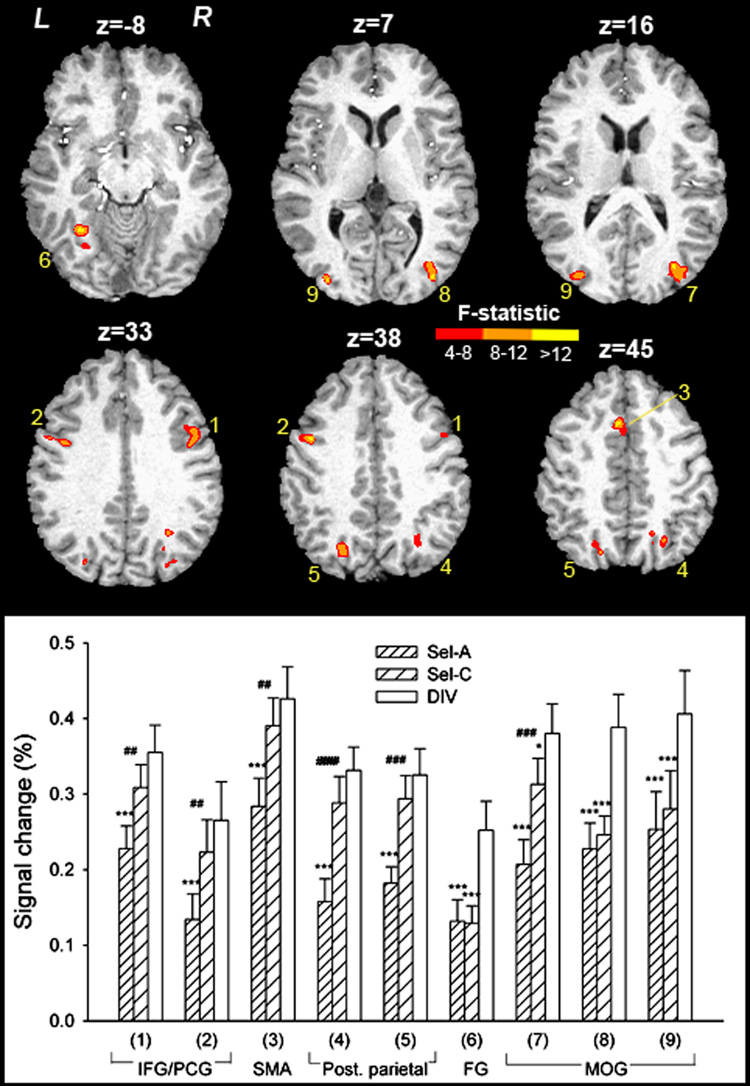
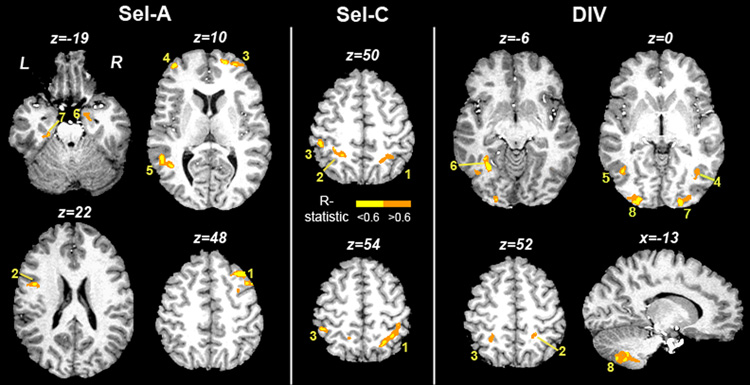
Fig. 3 displays task maps for Sel-A, Sel-C and DIV, averaged across sessions, obtained by voxel-wise t-tests against zero. Anatomical coordinates are listed in Supplementary Table 1–Supplementary Table 3. Activations and deactivations bear close similarity across tasks and include activation within typical frontoparietal attention regions and deactivation within so-called default resting areas. To explore task-specific BOLD effects, voxel-wise two-factor ANOVA for repeated measures was performed with TASK (Sel-A, Sel-C, DIV) and SESSION (1, 2) as within-subject factors. Nine regions displayed a main effect of TASK (Table 1, Fig. 4): left and right inferior frontal gyri (IFG) / precentral gyri, medial frontal cortex in the area of the Supplementary Motor Area (SMA) reaching into anterior cingulate sulcus, left and right posterior parietal cortex, left fusiform gyrus, and left and right extrastriate visual cortex. The inset graph in Fig. 4 shows activity in these regions across tasks. Activations were significantly different from zero in each task, but always highest during DIV. In all visual areas including fusiform gyrus, activation was significantly higher in DIV than in either of the selective attention tasks. In all other regions, only Sel-A differed from DIV, and Sel-C differed from Sel-A. Activation by DIV never exceeded the summed activation by Sel-A and Sel-C. No regions were identified as displaying a main effect of SESSION or a TASK × SESSION interaction.
Figure 3. Activation maps for Sel-A, Sel-C and DIV.
Maps were obtained by voxel-wise t-tests against zero. Group activation maps are overlaid onto an anatomical scan in Talairach space. Anatomical activation coordinates are listed in Suppl. Table 1–Suppl. Table 3. z = mm from AC-PC.
Table 1.
Regions displaying an effect of task
| Brain Region | Side | Center of Mass (mm) X, Y, Z | Brodmann Area(s) | Volume (µl) | |||
|---|---|---|---|---|---|---|---|
| 1 | Precentral gyrus, inferior frontal gyrus | R | 43.2 | 5.1 | 31.5 | 4, 44 | 938 |
| 2 | Precentral gyrus, inferior frontal gyrus | L | −43.3 | 2.7 | 34.8 | 4, 44 | 574 |
| 3 | Medial frontal gyrus / supplementary motor area | B | −3 | 11.7 | 46.7 | 6 | 426 |
| 4 | Superior parietal lobule, intraparietal sulcus, precuneus | R | 22.9 | −64.4 | 45 | 7 | 849 |
| 5 | Superior parietal lobule, intraparietal sulcus, precuneus | L | −20.9 | −68.8 | 40.9 | 7 | 862 |
| 6 | Fusiform gyrus | L | −27.7 | −55 | −7.5 | 19, 37 | 442 |
| 7 | Middle/superior occipital gyrus, precuneus | R | 31.2 | −77.3 | 21.9 | 18, 19, 39 | 1431 |
| 8 | Middle occipital gyrus | R | 35.6 | −79.4 | 6.4 | 18, 19 | 425 |
| 9 | Middle occipital gyrus | L | −32.4 | −83.6 | 10.3 | 18, 19 | 537 |
Regions in Talairach space where BOLD signal intensity displayed a main effect of task in voxel-wise ANOVA. Numbers of brain regions correspond to ROIs in Fig. 4. L = left, R = right, B = bilateral.
Figure 4. Regions displaying a main effect of TASK in voxel-wise ANOVA.
Group activation maps are overlaid onto an anatomical scan in Talairach space. The numbers correspond to ROIs in Table 1 and in the bottom graph showing average activity. IFG/PCG = inferior frontal and precentral gyri, SMA = Supplementary Motor Area, Post. parietal = posterior parietal cortex, FG = fusiform gyrus, MOG = middle occipital gyri. z = mm from AC-PC. *** P<0.001, paired t-test, comparing each selective attention task with the divided attention task. ## P<0.01, ### P<0.001, paired t-test, comparing the two selective attention tasks.
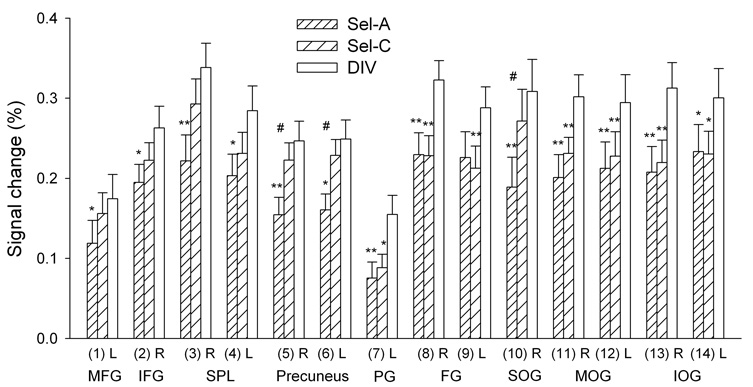
To further probe for differences between the selective and divided attention tasks, the DIV activation map (right panel of Fig. 3, Supplementary Table 3) was used as a template to extract BOLD signal during all three task conditions. Thus, activity during Sel-A, Sel-C or DIV was averaged within regions activated by DIV and underwent two-factor ANOVA (TASK × SESSION). Only the largest ROI, comprising frontal, parietal, temporal, occipital, cerebellar and subcortical regions, displayed a main effect of TASK [F(2,48)=3.59, P<0.05]. This region was subdivided according to anatomical boundaries defined by the AFNI Talairach Demon, and the resulting ROIs again underwent two-factor ANOVA. Table 2 lists coordinates and Fig. 5 shows activity levels for regions that displayed a main effect of TASK [F(2,48)>3.28, P<0.05 in each case]. Again, most regions associated with visual processing [middle and inferior occipital gyri (MOG, IOG), fusiform and parahippocampal gyri] displayed higher activation during DIV than during Sel-A or Sel-C, while Sel-A and Sel-C did not differ. In all other regions [middle frontal gyri (MFG), IFG, superior parietal lobule (SPL), precuneus, and right superior occipital gyrus (SOG) as the only visual region], activation was higher during DIV than during Sel-A but not Sel-C, and sometimes significantly higher during Sel-C than Sel-A. Activation by DIV never exceeded the sum of activation by Sel-A and Sel-C. No region displayed a main effect of SESSION or TASK × SESSION interaction.
Table 2.
Regions activated by DIV that displayed an effect of task
| Brain Region | Side | Center of Mass (mm) X, Y, Z | Brodmann Area(s) | Volume (µl) | |||
|---|---|---|---|---|---|---|---|
| 1 | Middle frontal gyrus | L | −32.2 | −3.5 | 46.9 | 9, 46 | 2162 |
| 2 | Inferior frontal gyrus | R | 46.7 | 10 | 21 | 44, 45 | 3613 |
| 3 | Superior parietal lobule | R | 28.1 | −60.8 | 50.3 | 7 | 3267 |
| 4 | Superior parietal lobule | L | −26.6 | −60.4 | 50.2 | 7 | 3105 |
| 5 | Precuneus | R | 20.8 | −66.5 | 41.8 | 7 | 8400 |
| 6 | Precuneus | L | −20.5 | −66.8 | 41 | 7 | 6510 |
| 7 | Parahippocampal gyrus | L | −26.9 | −41.1 | −10.6 | 19, 28, 34 | 1550 |
| 8 | Fusiform gyrus | R | 35.6 | −54.3 | −12.2 | 19, 37 | 5165 |
| 9 | Fusiform gyrus | L | −35 | −56.8 | −12.5 | 19, 37 | 5899 |
| 10 | Superior occipital gyrus | R | 34.5 | −79.5 | 27.3 | 19 | 551 |
| 11 | Middle occipital gyrus | R | 32.7 | −80.5 | 7.7 | 18, 19 | 8410 |
| 12 | Middle occipital gyrus | L | −33.3 | −81.5 | 7.1 | 18, 19 | 10003 |
| 13 | Inferior occipital gyrus | R | 32.4 | −81.8 | −5.5 | 18, 19 | 1300 |
| 14 | Inferior occipital gyrus | L | −31.7 | −84.3 | −6.3 | 18, 19 | 2322 |
Regions in Talairach space identified as displaying significant BOLD signal increases during the divided attention condition that displayed a main effect of TASK in ANOVA performed on regional averages. L = left, R = right.
Figure 5. Activation levels for regions activated by DIV that displayed a main effect of TASK.
The numbers correspond to ROIs in Table 2. l = left, r = right. MFG = middle frontal gyrus, IFG = inferior frontal gyrus, SPL = superior parietal lobule, PG = parahippocampal gyrus, FG = fusiform gyrus, SOG = superior occipital gyrus, MOG = middle occipital gyrus, IOG = inferior occipital gyrus. * P<0.05, ** P<0.01, paired t-test, comparing each selective attention task with the divided attention task. # P<0.05, paired t-test, comparing the two selective attention tasks.
In general, the signal in frontal and parietal regions mirrored the processing demands of the tasks as reflected by RT performance (Fig. 2C). In contrast, activation in occipital and inferotemporal regions reflected the attentional function (selective vs. divided), or, alternatively, the length of the visual stimulation as indexed by DT (Fig. 2A), which was longer for DIV but equal for Sel-A and Sel-C. To test this latter possibility, Pearson correlations were established between individual subjects’ average DT and BOLD signal during DIV of regions displaying a main effect of TASK (Table 2, Fig. 5). Only session 1 data were used because an association between the two variables would more likely be detected within the more constrained time period of a single session. Significant correlations were identified only for visual regions: left MOG (r=0.50), right MOG (r=0.45), left IOG (r=0.50) and left fusiform gyrus (r=0.48). Furthermore, individual difference values were calculated between DIV and the average of both selective attention tasks for DT and regional BOLD signal. Correlations were identified in left IOG (r=0.44) and fusiform gyrus (r=0.47), indicating that greater differences in DT were associated with greater activation differences between the selective and divided attention conditions. No non-sensory processing regions displayed such association. Overall, activation differences in visual processing regions may thus mirror visual stimulus length.
To further probe for activation specific to DIV, voxel-wise paired t-tests compared activation by DIV with the sum of activation by Sel-A and Sel-C. T-tests were one-tailed, testing for more activation during DIV than during the added selective attention tasks in task-positive voxels, and for more deactivation in task-negative voxels. No region was identified as being more activated or more deactivated by DIV than by Sel-A and Sel-C combined.
Correlation maps
To explore regional activity associated with performance of Sel-A, Sel-C and DIV, correlations were established, for each task averaged across sessions, between subjects’ voxel-wise BOLD values and their average RT. Fig. 6 and Table 3, Table 4 and Table 5 detail maps for significant correlations. All correlations were positive, i.e. more activity was associated with longer RT. Thus, regions were less activated or more deactivated in participants who responded faster, possibly reflecting a more automatic response mode. Average activity in most clusters identified in Sel-C and DIV was positive (one-sample t-test); only activity in the cerebellar region identified in DIV did not differ from zero. Average activity in most clusters identified in Sel-A did not differ from zero (a frontal region was positive and right parahippocampal gyrus negative). Supplementary Fig. 1–Supplementary Fig. 3 show scatter plots for all correlations.
Figure 6. Anatomical maps for correlations between subjects’ voxel-wise BOLD values and average RT.
Group activation maps are overlaid onto an anatomical scan in Talairach space. The numbers correspond to ROIs in Table 3, Table 4 and Table 5. z = mm from AC-PC.
Table 3.
Regions displaying correlations during Sel-A
| Brain Region | Side | Center of Mass (mm) X, Y, Z | Brodmann Area(s) | Volume (µl) | r | Average BOLD * | |||
|---|---|---|---|---|---|---|---|---|---|
| 1 | Middle frontal/precentral gyrus | R | 34.3 | 16.7 | 47.7 | 6, 8 | 1209 | 0.75 | NS |
| 2 | Inferior frontal/precentral gyrus | L | −44.2 | 0.9 | 21.2 | 6, 9 | 390 | 0.68 | positive |
| 3 | Middle frontal gyrus, anterior | R | 23.8 | 54.7 | 12.8 | 10 | 684 | 0.73 | NS |
| 4 | Middle frontal gyrus, anterior | L | −36.8 | 50.3 | 6.2 | 10 | 488 | 0.69 | NS |
| 5 | Middle/superior temporal gyrus | L | −44.4 | −51.4 | 12 | 21, 22, 39 | 932 | 0.70 | NS |
| 6 | Parahippocampal gyrus, amygdale | R | 12.3 | −5.1 | −14 | 28, 34 | 381 | 0.75 | negative |
| 7 | Parahippocampal gyrus | L | −28.2 | −29.4 | −17.6 | 35, 36 | 678 | 0.76 | NS |
Regions in Talairach space where BOLD signal intensity and reaction time were correlated during the angle discrimination. L = left, R = right.
This column indicates whether average activation in each cluster was positive, negative or not significantly different from zero (NS) in one-sample t-tests. Numbers of brain regions correspond to those in the left segment of Fig. 6.
Table 4.
Regions displaying correlations during Sel-C
| Brain Region | Side | Center of Mass (mm) X, Y, Z | Brodmann Area(s) | Volume (µl) | r | Average BOLD * | |||
|---|---|---|---|---|---|---|---|---|---|
| 1 | Inferior parietal lobule, precuneus | R | 29.8 | −46.1 | 54.5 | 7, 40 | 1160 | 0.69 | positive |
| 2 | Inferior parietal lobule, precuneus | L | −23.8 | −46.3 | 47.1 | 7, 40 | 595 | 0.75 | positive |
| 3 | Inferior parietal lobule | L | −42.7 | −37.4 | 52 | 40 | 404 | 0.65 | positive |
Regions in Talairach space where BOLD signal intensity and reaction time were correlated during the color discrimination. L = left, R = right.
This column indicates whether average activation in each cluster was positive, negative or not different from zero in one-sample t-test. Numbers of brain regions correspond to those in the middle segment of Fig. 6.
Table 5.
Regions displaying correlations during DIV
| Brain Region | Side | Center of Mass (mm) X, Y, Z | Brodmann Area(s) | Volume (µl) | r | Average BOLD * | |||
|---|---|---|---|---|---|---|---|---|---|
| 1 | Inferior frontal gyrus | R | 43.2 | 4.7 | 20.2 | 9, 44 | 481 | 0.67 | positive |
| 2 | Inferior parietal lobule, postcentral gyrus | R | 24.3 | −41.2 | 47 | 5, 40 | 568 | 0.74 | positive |
| 3 | Inferior parietal lobule, intraparietal sulcus | L | −25.9 | −49.8 | 43.6 | 40 | 639 | 0.79 | positive |
| 4 | Middle temporal gyrus (posterior) | R | 37 | −63.5 | 2.4 | 37 | 613 | 0.69 | positive |
| 5 | Middle temporal gyrus (posterior) | L | −40.5 | −61 | −1.3 | 37 | 511 | 0.63 | positive |
| 6 | Fusiform / parahippocampal gyrus | L | −29 | −54.5 | −6.7 | 19 | 646 | 0.69 | positive |
| 7 | Middle occipital gyrus | R | 23.5 | −89.2 | 7.2 | 18 | 1795 | 0.79 | positive |
| 8 | Middle / inferior occipital gyrus | L | −26.3 | −90.1 | 3.9 | 18, 19 | 1814 | 0.74 | positive |
| 9 | Cerebellum (inferior) | L | −12.3 | −56.9 | −40.6 | 1319 | 0.64 | NS | |
Regions in Talairach space where BOLD signal intensity and reaction time were correlated during the combined angle and color discrimination. L = left, R = right.
This column indicates whether average activation in each cluster was positive, negative or not significantly different from zero (NS) in one-sample t-test. Numbers of brain regions correspond to those in the right segment of Fig. 6.
We next tested task-specificity of the correlations statistically. Within each cluster identified as correlating with RT in one task, correlations between average BOLD signal and RT were now established for each of the other two tasks. In four out of seven clusters that displayed BOLD-RT correlations in Sel-A, no significant correlation was found in Sel-C, and correlation coefficients differed significantly between Sel-A and Sel-C. These were left and right anterior MFG and parahippocampal gyri (regions 3, 4, 6 and 7 in Table 3). In contrast, all three clusters that displayed correlations in Sel-C displayed significant correlations also in Sel-A, and coefficients did not differ between tasks. The only cluster that displayed a BOLD-RT correlation in DIV but not in Sel-A or Sel-C, and where correlations differed significantly between DIV and each of the component tasks, was the left cerebellum (region 9 in Table 5).
3. Discussion
The selective and divided attention tasks employed in the present study induced widespread activations that included frontoparietal areas typically engaged by visual attentional processes (Kanwisher and Wojciulik, 2000; Corbetta and Shulman, 2002). Deactivation was seen in areas that commonly deactivate with external task demands and are active at rest, forming a “default” network of resting brain function (Gusnard and Raichle, 2001). Our aim was to test the hypothesis that a dual-task condition that requires dividing attention between two stimulus dimensions will induce brain activation that is unique for DIV or exceeds the sum of activation by the two component tasks. Evidence in support of this hypothesis would suggest the existence of brain functions specific to the simultaneous processing of more than one stimulus dimension.
Our findings do not suggest the existence of such specialized brain functions. No region displayed activity in DIV that exceeded the sum of activation by Sel-A and Sel-C. This was the case even when ROI analyses were performed on all regions activated by DIV. Activity differed between tasks in several frontoparietal and visual areas where DIV always induced the greatest activation, but this may be explained by the added processing load of the additional task, rather than processes specific to divided attention. In support of this notion, activation in all fronto-parietal regions followed the tasks’ processing demands as reflected by RT. Activity in occipital and inferotemporal visual regions differed between the selective and divided attention conditions and not between Sel-A and Sel-C, but this appeared to reflect the different-length stimulus DT rather than the different attentional functions. Direct comparisons also did not identify any activation or deactivation by DIV that exceeded the added activity in Sel-A and Sel-C.
Correlations between participants’ RT performance and BOLD activity that were task-selective are interpreted to reflect task-specific processing functions. Notably, activity in left and right anterior MFG and parahippocampal gyri correlated with performance only during Sel-A. As greater activation was always associated with slower responding, engaging these regions may hinder performance. Higher cognitive control functions associated with prefrontal activations (Duncan and Owen, 2000; Miller and D’Esposito, 2005) may preclude a faster, more automatic performance mode. Interindividual differences in MFG activation may thus reflect different performance strategies, such that participants with lower activity had progressed to a more automatic performance mode. Parahippocampal gyrus activity increases with attention to shapes (Corbetta et al., 1991a; b); thus, lower activity with faster responding during angle-discriminations may, again, reflect performance benefits of an increasing automaticity. But left parahippocampal gyrus also activates with reading color words (Pulvermueller and Hauk, 2006), and right parahippocampal gyrus with color imagery (Howard et al., 1998). As average activity in the right parahippocampal correlation cluster was negative, down-regulating activity related to processing the task-irrelevant stimulus dimension may have facilitated performance of Sel-A.
The above findings may help explain the difference in RT between Sel-A and Sel-C. Although the visual stimulation length needed to detect a difference was adjusted to be identical between the angle and color discrimination, post-stimulus processing appears to differ. In Sel-A, further processing of the task-irrelevant stimulus dimension may be suppressed and the need for prefrontal control functions reduced, enabling, overall, more automatic and faster responding.
Specificity for DIV was found only for a left cerebellar correlation cluster. Again, greater activity was associated with slower responding, suggesting a possible role of the cerebellum in mediating functions that have progressed to a more automatic processing stage. Efferents from similar cerebellar areas project via the dentate nucleus to the thalamus, among others, partly terminating upon mediodorsal thalamic neurons that project to prefrontal cortex (Parent, 1996). Previous functional neuroimaging studies have indicated cerebellar involvement in information processing; the cerebellum has been suggested to aid flexible cognitive operations that may be of particular importance in DIV by modeling prefrontal information processing (Leiner et al., 1993; 1995; Cabeza and Nyberg, 2000; Ramnani, 2006). Furthermore, analogous to motor functions, a cortico-cerebellar-thalamic-cortical circuit has been suggested to facilitate a fluid coordination of cognitive activities by fine-tuning the timing and sequencing of the information flow (Andreasen et al., 1999). Again, this would accord with a particular importance of cerebellar functions for the more complex task condition (DIV).
Exploring correlations with RT provided insight into performance strategies beneficial for Sel-A, but apart from a rather speculative role of cerebellar functions for DIV, this approach did not uncover activity reflective of processes specific to DIV. A convincing case would have been made by DIV-selective correlations reflecting greater activation with faster performance, but these were not seen. Of note, also DT did not display any inverse correlations with BOLD activity. Overall, four separate analysis approaches (voxel-wise ANOVA, ANOVA on ROIs, voxel-wise paired t-tests, and voxel-wise correlations) did not indicate brain functions specific to DIV. The study was well powered; analyses were based on data from 25 individuals and 50 scan sessions. Despite the difficulty interpreting negative results as a true absence of effects, the current findings suggest that neuroanatomical mechanisms of dividing attention between two sets of input were qualitatively no different from focusing attention on a single set.
Among previous studies aimed at identifying brain mechanisms specific to divided attention, only a small number reported task-specific regional activation (see Introduction). Each employed a different paradigm, and activations specific to the divided attention condition differed between studies, suggesting that additional cognitive demands recruited by the divided as compared with the selective attention condition are paradigm-specific. It may thus be advantageous to operationalize cognitive requirements characteristic for each task.
Clearly, dual-task conditions engage more attentional control than single-task conditions, due to greater and more complex demands on voluntary attentional resource allocation. Not surprisingly, brain regions displaying strongest activation during DIV included those taxed by top-down control of spatial and non-spatial (e.g. based on color) attentional selection, such as intraparietal sulcus and MFG (Hopfinger et al., 2000; Corbetta et al., 2000; Giesbrecht et al., 2003). However, control demands can differ greatly between different divided attention conditions. Conditions with highly complex control demands may tax executive control functions. When aiming at identifying neural substrates of dividing attention, minimizing executive control demands may yield a clearer picture, since these operations have been defined and studied as separate constructs (D’Esposito et al., 1995; Posner and DiGirolamo, 1998; Koechlin et al., 1999). We tried to minimize such demands by employing a single stimulus with two stimulus dimensions that remained the same throughout. We also equated response accuracy between tasks, thus minimizing differences in error processing, response conflict and uncertainty. As such, the absence of DIV-specific BOLD activity may reflect an absence of processing demands beyond the requirement to divide attention between two stimulus aspects.
In conclusion, despite employing several different analytical approaches, regional activity specific to conditions of divided attention was conspicuous by its absence, despite some indication for a role of cerebellar functions. Overall, the findings support the notion that dividing attention between two stimulus dimensions intensifies demands on processes recruited when attending to a single dimension. While task conditions created to tax divided attention may often recruit additional and qualitatively different neurocognitive processes, the mere requirement to process an additional set of input may mostly recruit additional resources of attentional selection. Increased brain activity during divided attention thus appears to reflect additional processing demands that may not qualitatively differ from processes of selective attention.
4. Experimental Procedures
Participants
Twenty-five right-handed healthy individuals (7 females) participated in the study, aged 18–44 years (mean±stdev 28.5±6.36 years). Subjects were recruited from the general population through newspaper advertising, flyers and referrals and gave informed consent for a protocol approved by the NIDA-IRP Institutional Review Board. Subjects were screened for major medical illnesses, claustrophobia, history of neurological or psychiatric disorders and drug abuse, pregnancy and appropriateness for MRI. All participants were non-smokers.
Procedure
The protocol required three separate visits. During the first visit, participants gave written informed consent and were trained on the attention task, initially on a bench computer and then for 30 min in a mock scanner that mimicked all properties of the MRI scanner. Sessions 2 and 3 were identical and served as time controls for a pharmacological experiment not reported here. Prior to both MR scans, participants were tested for recent drug use (TRIAGE®) and for alcohol intake or smoking via breath analysis; a pregnancy test was given to female participants. Subjects then received a 9-min reminder task training on a bench computer. In subsequent MR scans, three runs of the selective/divided attention task (see below) were performed, separated by one-minute rest periods. Also an anatomical reference scan was acquired in each session.
Measurement of Selective and Divided Attention
The task stimulus consisted of a circle containing two wedges displayed against a grey background in the center of the screen (Fig. 1). The diameter of the circle, based on a viewing distance of 80 cm, was 3.6° of visual angle, thus allowing foveal stimulus processing without significant eye-movement. Each wedge was divided into three sections of an inner, middle and outer ring of color. Within each wedge, these three segments always had different colors (red, blue and purple). In three different tasks, subjects made a forced choice of whether specific feature(s) in the two wedges were the same or different. In the two selective attention tasks, subjects were instructed to attend either to the color order of the rings (SEL-C) or to the angles of the wedges (SEL-A) and decide if they were the same. The third was a divided attention (DIV) task, where subjects attended to both of these features and decided whether or not the wedges were identical in both features. A button for “same” was pressed with the right index finger and a button for “different” with the left index finger.
The visual properties of the wedged circles were identical in all three tasks, i.e. each task used the same colors and wedge angles. In the SEL-A and SEL-C tasks, the wedges differed on the task-relevant feature in 50% of trials. Also the task-irrelevant feature differed in 50% of trials, independent of the status of the task-relevant feature; thus, only task demands, not stimulus characteristics defined the two different conditions. In the DIV task, the wedges differed on either one of the stimulus features in 50% of trials, i.e. 25% on the angle and 25% on the color feature; in the other half, neither feature differed.
Performance accuracy was held at 75% by manipulating DT. The purpose was to equate accuracy across the three tasks and to account for individual differences in processing ability. Adjustments were made in 16 ms units in accordance with the screen refresh rate. Initial DT was determined during training for each individual subject. Early during the training procedure, the wedge angle difference was determined such that DT for SEL-A was identical to SEL-C at 75% accuracy. This difference-value was then adopted for all three tasks. Angle difference-values ranged from 4–14° across participants (mean±stdev 7.8±2.3). Throughout, DT was dynamically adjusted after every four trials. If a correct response was made in three out of the four preceding trials, DT stayed the same. If two or fewer trials were correct, DT increased by 16 ms, and if all four trials were correct, DT decreased by 16 ms. DT was increased as soon as two incorrect responses had been made, thus satisfying the criterion. During scan sessions, DT was independently adjusted in the same manner for SEL-A, SEL-C and DIV, starting with the values obtained at completion of the training. The same starting values were used for both scans.
In each scan session, three 8:42 min task runs were completed. Each run started with eight trials of a visuomotor reaction time (VRT) task that is not reported here. One block of SEL-A, SEL-C and DIV, each 16 trials long (trial length 4 s), was then performed in a randomized sequence, followed by eight more trials of the VRT task. Thus, the total number of trials in each condition was 96 (16 trials × 3 runs × 2 sessions). Each block began with the task instruction, displayed for 4 s, followed by a 6 s epoch where participants performed a forced choice test (“Press the button on the side that names this task”). Only blocks preceded by correct answers entered further analyses. Each task trial started with a 500-ms central fixation cross followed by 500 ms of blank screen. The wedged circle was then presented for the duration of DT, followed by 48 ms of a back mask consisting of the circle filled with colored dots, to erase any persisting afterimage of the task stimulus on the retina. The letters d and s for “different” and “same” then appeared on the left and right, respectively, of where the circle had just been presented and stayed on display until a response was made for a maximum duration of 2 s. Trials where no response was recorded within this time were excluded from further analysis (1.5% of all trials). Trials were separated by a variable inter-stimulus interval of 0, 2, 4 or 6 s duration. The inter-stimulus interval was extended by the length of time needed to complete the preceding TR.
Magnetic resonance imaging
Scanning was performed on a 3 Tesla Siemens Allegra scanner (Erlangen, Germany). Whole brain functional EPI images were acquired for measurement of T2*-weighted BOLD effects (4mm sagittal slices, 64×64 matrix, FOV=22×22cm, TR=2s, TE=27ms, FA=75°). In each scanning session, a whole-brain sagittal T1-weighted structural image (MPRAGE) was acquired for anatomical reference (1mm3 isotropic voxels, TR=2.5s, TE=4.38 ms, FA=8°).
Analysis of behavioral data
Data from the two scan sessions were analyzed. DT and reaction times (RT) were expressed as averages for each task condition. Response accuracy reflected the percentage of responses that correctly indicated a difference in the angle or color of the wedges or correctly indicated no difference. Accuracy was analyzed to verify that it was successfully equated between tasks. All three measures were analyzed, separately, by 2-factor ANOVA for repeated measures with TASK (Sel-A, Sel-C, DIV) and SESSION (1, 2) as within-subject factors, followed by paired t-tests where indicated.
Analysis of fMRI data
Data were processed using the AFNI software package (Cox, 1996). Motion correction was performed by registering each 3D volume to a base volume. The time series was then analyzed by voxel-wise multiple regression. Regressors were expressed as a delta function, time-locked to the onset of each circle stimulus, convolved with a model hemodynamic response function and its temporal derivative. Regressors corresponded to the four different tasks (Sel-A, Sel-C, DIV and VRT) and to the six motion parameters as nuisance regressors to help account for residual motion. Two further nuisance regressors corresponded to the display and retention test of the task instruction. If applicable, one additional nuisance regressor accounted for trials in which no response was registered, and one for blocks in which the task instruction was not correctly repeated. For each subject and each test session, the voxel-wise average amplitude of signal change (β-value) produced by each task type was determined relative to baseline. The resulting activation maps were resampled to a higher (1µl) resolution, converted to a standard stereotaxic coordinate system (Talairach and Tournoux, 1988) and spatially blurred using a Gaussian 4.2 mm FWHM isotropic kernel.
Regions of interest (ROIs) were derived functionally by second-level voxel-wise t-tests, ANOVA, or Pearson correlation coefficients across subjects, performed on β-values for each task. Monte Carlo simulations were performed for each analysis type, taking account of spatial covariations in the output dataset of each analysis type. For t-tests and ANOVA, a voxel-wise threshold of p<0.005 was applied to the activation maps and combined with a minimum cluster volume size of 444µl (t-tests) and 280µl (ANOVA). For correlation coefficients, a voxel-wise threshold of p<0.01 was combined with a minimum cluster volume size of 363µl. This yielded an overall false positive p<0.05 in each case.
Supplementary Material
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Institute on Drug Abuse. We thank William Rea and Loretta Spurgeon for their assistance in the conduct of the study.
Abbreviations
- AC-PC
anterior commissure – posterior commissure
- AFNI
Analysis of Functional NeuroImages
- ANOVA
analysis of variance
- BOLD
Blood Oxygenation Level Dependent
- EPI
echo planar imaging
- FA
flip angle
- fMRI
functional Magnetic Resonance Imaging
- FOV
field of view
- MPRAGE
magnetization-prepared rapid acquisition with gradient-echo
- ROI
region of interest
- TE
echo time
- TR
repetition time
- Sel-A
selective attention – angle discrimination task
- Sel-C
selective attention – color discrimination task
- DIV
divided attention task
- DT
display time
- RT
reaction time
- VRT
visuomotor reaction time
- IFG
inferior frontal gyrus
- IOG
inferior occipital gyrus
- MFG
middle frontal gyri
- MOG
middle occipital gyrus
- SMA
supplementary motor area
- SOG
superior occipital gyrus
- SPL
superior parietal lobule
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen N, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol. Psychiatry. 1999;46:908–920. doi: 10.1016/s0006-3223(99)00152-3. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Braun J. Divided Attention: Narrowing the gap between brain and behavior. In: Parasuraman R, editor. The Attentive Brain. Cambridge, Massachusetts: MIT Press; 1998. pp. 327–351. [Google Scholar]
- Broadbent DE. Perception and Communication. London: Plenum Press; 1958. [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J. Cogn. Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc. Natl. Acad. Sci. U.S.A. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Selective and divided attention during visual discriminations of shape, color, and speed: functional anatomy by positron emission tomography. J. Neurosci. 1991a;11:2383–2402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Shulman GL, Petersen SE. Selective attention modulates extrastriate visual regions in humans during visual feature discrimination and recognition. Ciba Found. Symp. 1991b;163:165–175. doi: 10.1002/9780470514184.ch10. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat. Neurosci. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comp. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature. 1995;378:279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Giesbrecht B, Woldorff MG, Song AW, Mangun GR. Neural mechanisms of top-down control during spatial and feature attention. NeuroImage. 2003;19:496–512. doi: 10.1016/s1053-8119(03)00162-9. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat. Rev. Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Herath P, Klingberg T, Young J, Amunts K, Roland P. Neural correlates of dual task interference can be dissociated from those of divided attention: an fMRI study. Cereb. Cortex. 2001;11:796–805. doi: 10.1093/cercor/11.9.796. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat. Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Howard RJ, Ffytche DH, Barnes J, McKeefry D, Ha Y, Woodruff PW, Bullmore ET, Simmons A, Williams SC, David AS, Brammer M. The functional anatomy of imagining and perceiving colour. Neuroreport. 1998;9:1019–1023. doi: 10.1097/00001756-199804200-00012. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Zatorre RJ. Neural substrates for dividing and focusing attention between simultaneous auditory and visual events. Neuroimage. 2006;31:1673–1681. doi: 10.1016/j.neuroimage.2006.02.026. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Wojciulik E. Visual attention: insights from brain imaging. Nat. Rev. Neurosci. 2000;1:91–100. doi: 10.1038/35039043. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS. Cognitive and language functions of the human cerebellum. Trends Neurosci. 1993;16:444–447. doi: 10.1016/0166-2236(93)90072-t. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS. The underestimated cerebellum. Hum. Brain Mapp. 1995;2:244–254. [Google Scholar]
- Loose R, Kaufmann C, Auer DP, Lange KW. Human prefrontal and sensory cortical activity during divided attention tasks. Hum. Brain Mapp. 2003;18:249–259. doi: 10.1002/hbm.10082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BT, D'Esposito M. Searching for "the top" in top-down control. Neuron. 2005;48:535–538. doi: 10.1016/j.neuron.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Nebel K, Wiese H, Stude P, de Greiff A, Diener HC, Keidel M. On the neural basis of focused and divided attention. Brain Res. Cogn. Brain Res. 2005;25:760–776. doi: 10.1016/j.cogbrainres.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Parasuraman R. The attentive brain: issues and prospects. In: Parasuraman R, editor. The Attentive Brain. Cambridge, Massachusetts: MIT Press; 1998. pp. 3–15. [Google Scholar]
- Parent A. Carpenter's Human Neuroanatomy. 9th ed. Media, Pennsylvania: Williams & Wilkins; 1996. [Google Scholar]
- Posner MI, DiGirolamo GJ. Executive attention: conflict, target detection, and cognitive control. In: Parasuraman R, editor. The Attentive Brain. Cambridge, Massachusetts: MIT Press; 1998. pp. 401–423. [Google Scholar]
- Pulvermuller F, Hauk O. Category-specific conceptual processing of color and form in left fronto-temporal cortex. Cereb. Cortex. 2006;16:1193–1201. doi: 10.1093/cercor/bhj060. [DOI] [PubMed] [Google Scholar]
- Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat. Rev. Neurosci. 2006;7:511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- Rees G, Frackowiak R, Frith C. Two modulatory effects of attention that mediate object categorization in human cortex. Science. 1997;275:835–838. doi: 10.1126/science.275.5301.835. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- Vandenberghe R, Duncan J, Dupont P, Ward R, Poline JB, Bormans G, Michiels J, Mortelmans L, Orban GA. Attention to one or two features in left or right visual field: a positron emission tomography study. J. Neurosci. 1997;17:3739–3750. doi: 10.1523/JNEUROSCI.17-10-03739.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerda R, Vallines I, Thomas JP, Rutschmann RM, Greenlee MW. Effects of nonspatial selective and divided visual attention on fMRI BOLD responses. Exp. Brain Res. 2006;173:555–563. doi: 10.1007/s00221-006-0403-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.