Abstract
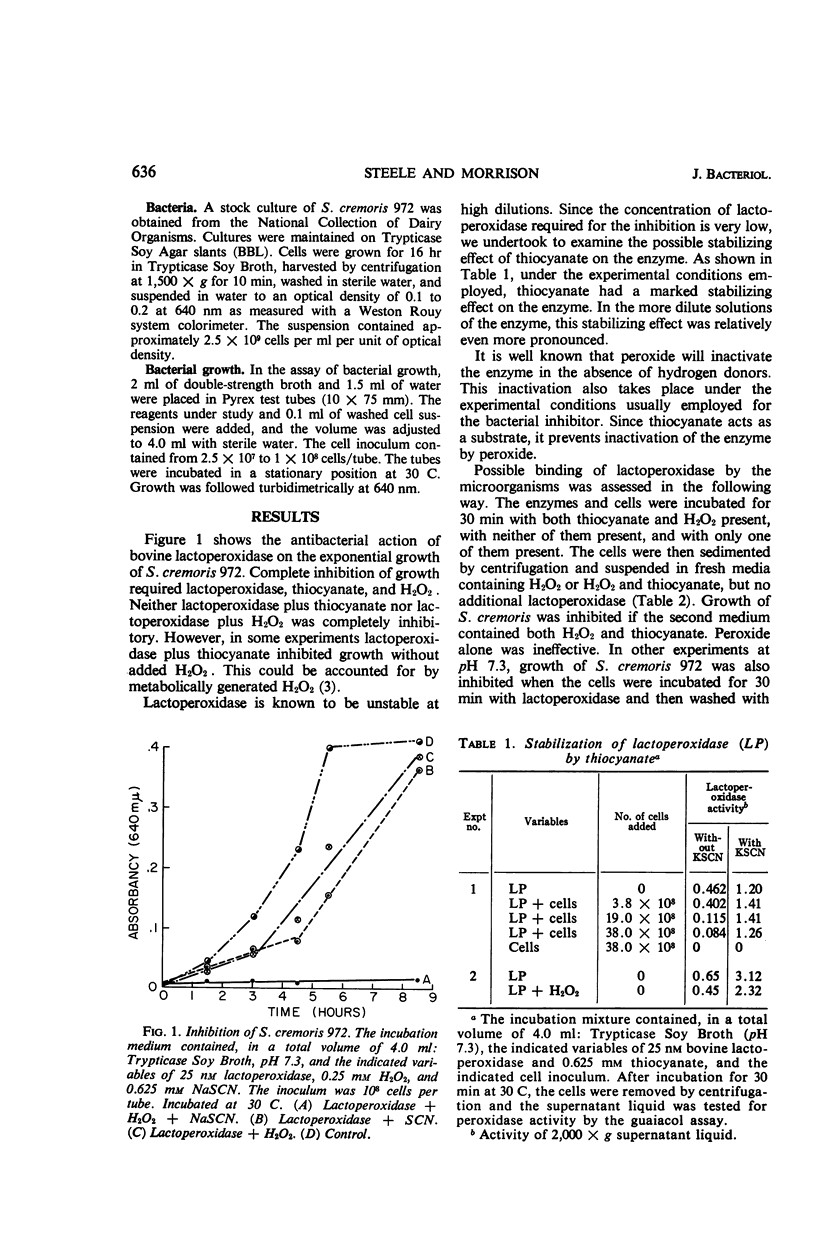
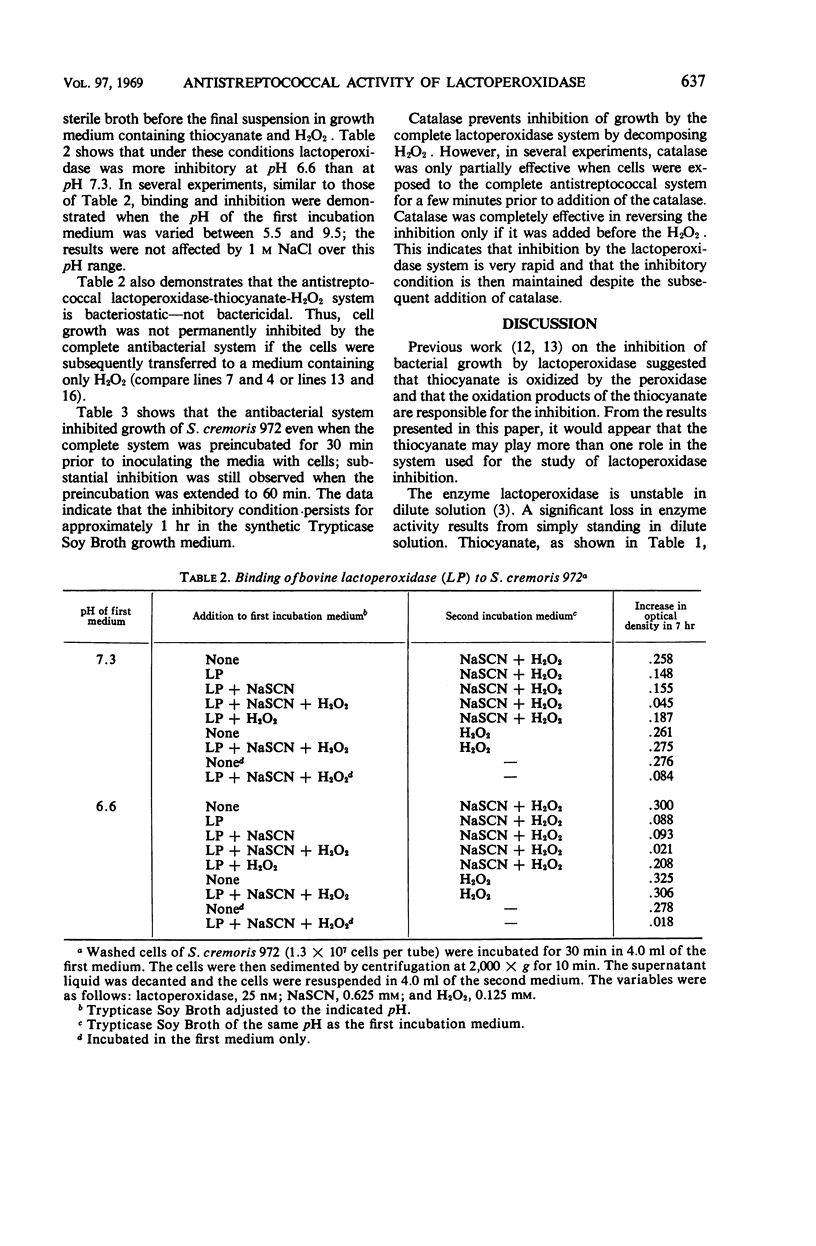
A study of the inhibition of the growth of Streptococcus cremoris 972 by the enzyme lactoperoxidase has shown, in agreement with previous investigations, that the inhibition requires a source of both peroxide and thiocyanate. The thiocyanate may play more than one role. It stabilizes the very dilute solutions of lactoperoxidase employed in these studies, and its oxidation products may be involved in the inhibition. Binding of the enzyme by the microorganism is suggested by the fact that when the organism was preincubated with the enzyme and then in a medium free from the enzyme, but containing peroxide and thiocyanate, the growth of the organism was inhibited. This inhibition has all the properties of the enzyme-containing system. Although no dialyzable factor could be demonstrated to cause the inhibition, the inhibitory state involving peroxide, the enzyme, and thiocyanate survived for at least 60 min before cells were added to the medium. When catalase was present in the medium prior to the addition of the cells, the inhibition was completely reversed. It was only partially reversed if catalase was added a few moments after the addition of the cells. The data have been interpreted as indicating that the inhibition takes place rapidly and requires the formation of a quaternary complex of the cells, thiocyanate, peroxide, and the enzyme lactoperoxidase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- JAGO G. R., MORRISON M. Anti-streptococcal activity of lactoperoxidase III. Proc Soc Exp Biol Med. 1962 Dec;111:585–588. doi: 10.3181/00379727-111-27862. [DOI] [PubMed] [Google Scholar]
- KLEBANOFF S. J., LUEBKE R. G. THE ANTILACTOBACILLUS SYSTEM OF SALIVA. ROLE OF SALIVARY PEROXIDASE. Proc Soc Exp Biol Med. 1965 Feb;118:483–486. doi: 10.3181/00379727-118-29882. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Clem W. H., Luebke R. G. The peroxidase-thiocyanate-hydrogen peroxide antimicrobial system. Biochim Biophys Acta. 1966 Mar 28;117(1):63–72. doi: 10.1016/0304-4165(66)90152-8. [DOI] [PubMed] [Google Scholar]
- MORRISON M., HAMILTON H. B., STOTZ E. The isolation and purification of lactoperoxidase by ion exchange chromatography. J Biol Chem. 1957 Oct;228(2):767–776. [PubMed] [Google Scholar]
- MORRISON M., HULTQUIST D. E. LACTOPEROXIDASE. II. ISOLATION. J Biol Chem. 1963 Aug;238:2843–2849. [PubMed] [Google Scholar]
- Morioka T., Zeldow B. J. A serum depressor of the human salivary antilactobacillus factor. J Dent Res. 1965 Sep-Oct;44(5):850–854. doi: 10.1177/00220345650440051701. [DOI] [PubMed] [Google Scholar]
- Morrison M., Allen P. Z., Bright J., Jayasinghe W. Lactoperoxidase. V. Identification and isolation of lactoperoxidase from salivary gland. Arch Biochem Biophys. 1965 Jul;111(1):126–133. doi: 10.1016/0003-9861(65)90330-9. [DOI] [PubMed] [Google Scholar]
- Oram J. D., Reiter B. The inhibition of streptococci by lactoperoxidase, thiocyanate and hydrogen peroxide. The effect of the inhibitory system on susceptible and resistant strains of group N streptococci. Biochem J. 1966 Aug;100(2):373–381. doi: 10.1042/bj1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram J. D., Reiter B. The inhibition of streptococci by lactoperoxidase, thiocyanate and hydrogen peroxide. The oxidation of thiocyanate and the nature of the inhibitory compound. Biochem J. 1966 Aug;100(2):382–388. doi: 10.1042/bj1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kesteren M., Bibby B. G., Berry G. P. Studies on the Antibacterial Factors of Human Saliva. J Bacteriol. 1942 May;43(5):573–583. doi: 10.1128/jb.43.5.573-583.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]



