Abstract
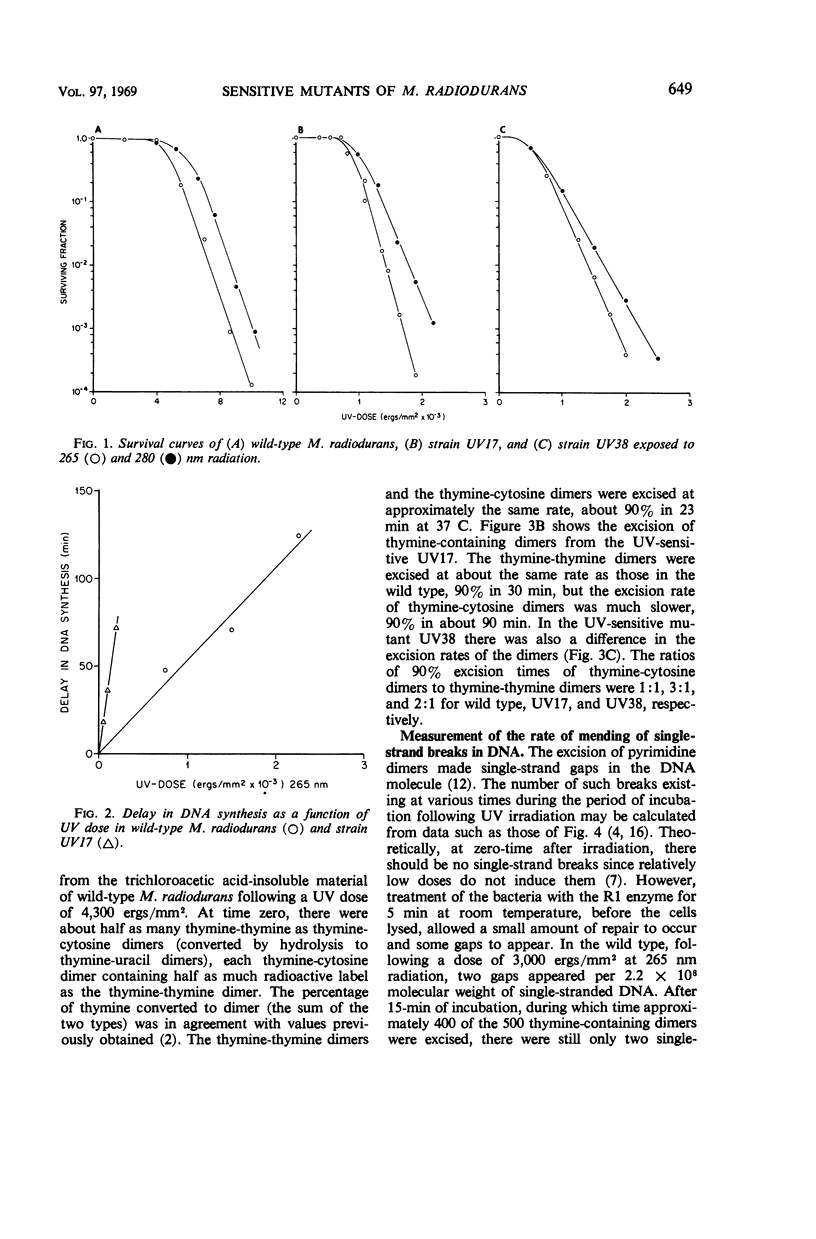
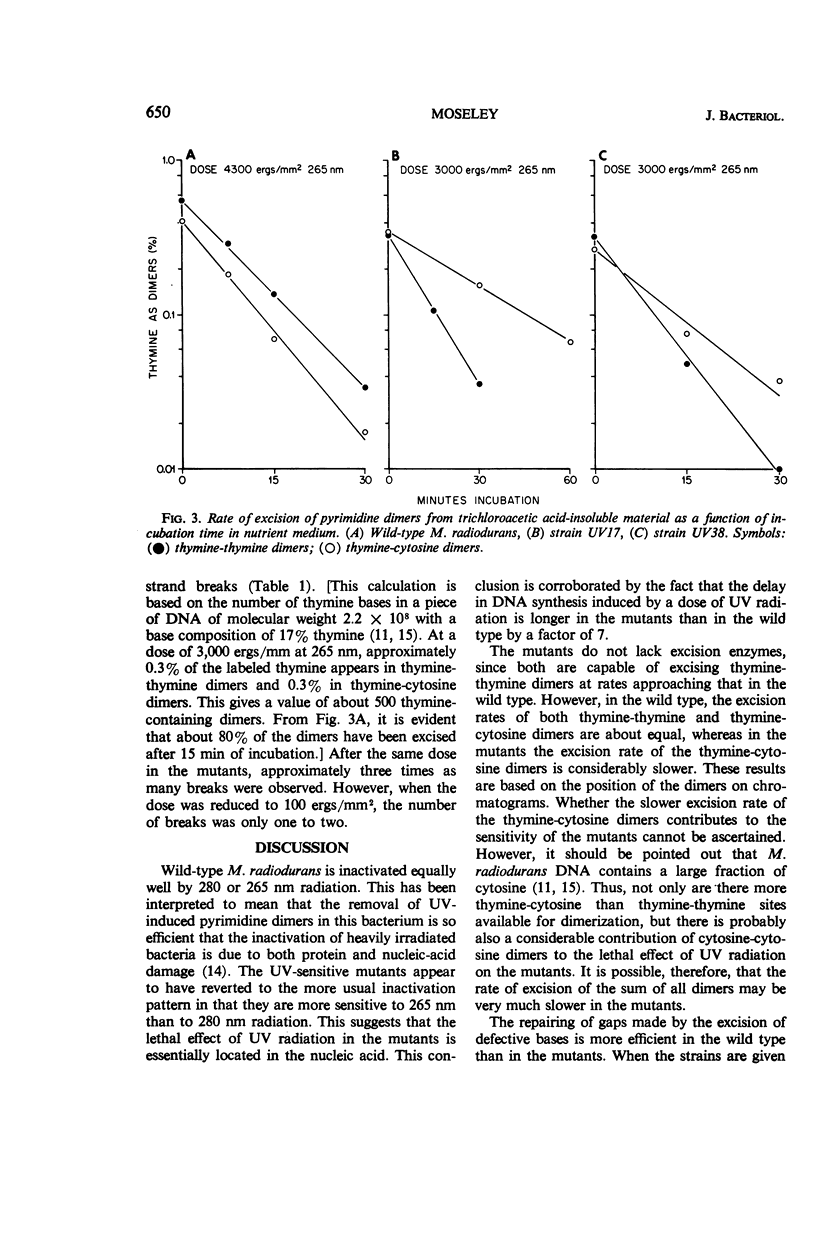
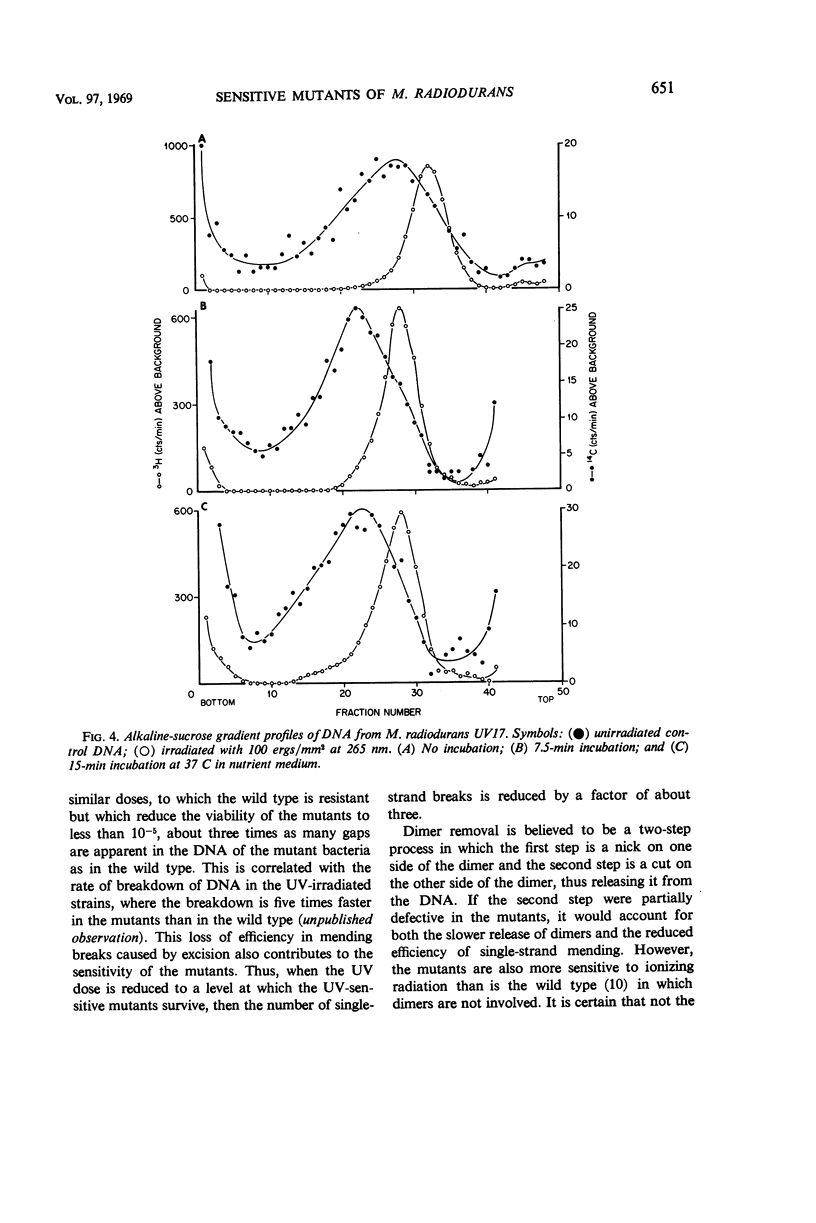
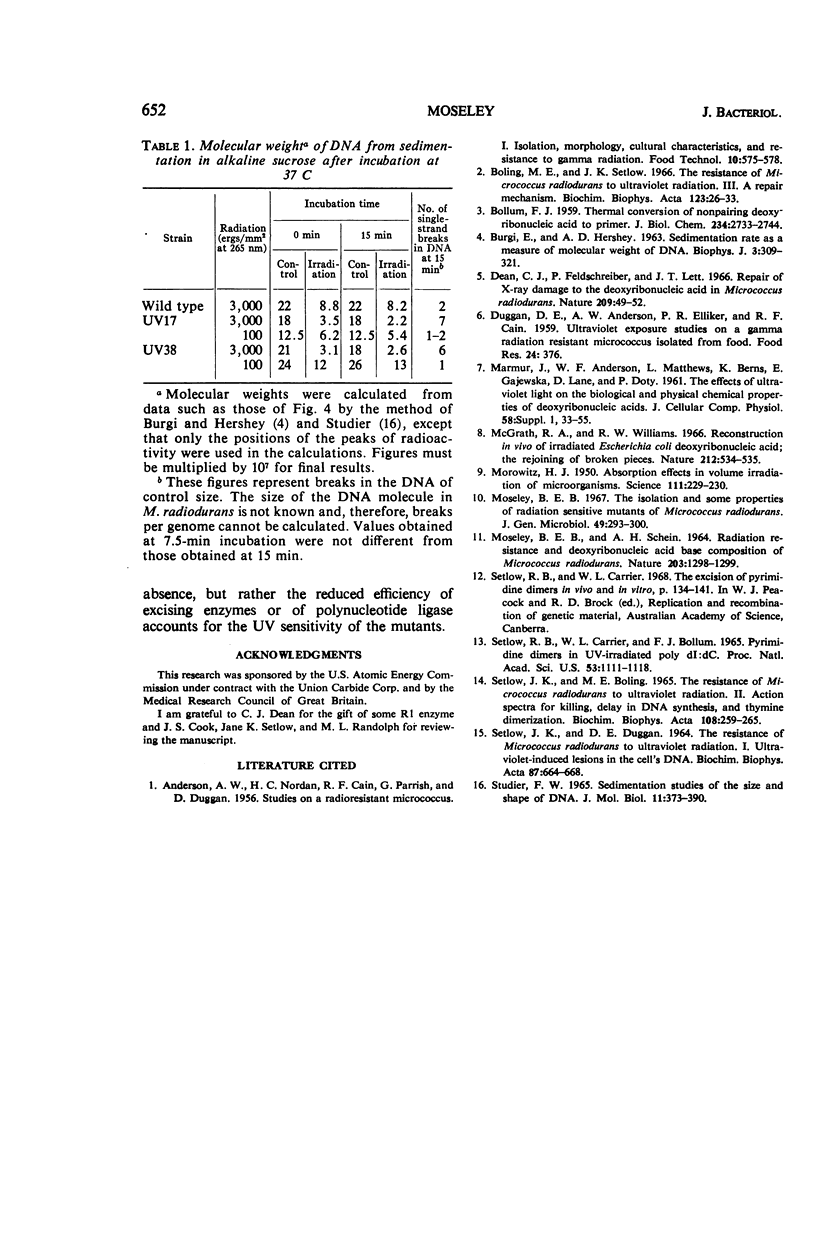
Various aspects of the repair of ultraviolet (UV) radiation-induced damage were compared in wild-type Micrococcus radiodurans and two UV-sensitive mutants. Unlike the wild type, the mutants are more sensitive to radiation at 265 nm than at 280 nm. The delay in deoxyribonucleic acid (DNA) synthesis following exposure to UV is about seven times as long in the mutants as in the wild type. All three strains excise UV-induced pyrimidine dimers from their DNA, although the rate at which cytosine-thymine dimers are excised is slower in the mutants. The three strains also mend the single-strand breaks that appear in the irradiated DNA as a result of dimer excision, although the process is less efficient in the mutants. It is suggested that the increased sensitivity of the mutants to UV radiation may be caused by a partial defect in the second step of dimer excision.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOLLUM F. J. Thermal conversion of nonpriming deoxyribonucleic acid to primer. J Biol Chem. 1959 Oct;234:2733–2734. [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boling M. E., Setlow J. K. The resistance of Micrococcus radiodurans to ultraviolet radiation. 3. A repair mechanism. Biochim Biophys Acta. 1966 Jul 20;123(1):26–33. doi: 10.1016/0005-2787(66)90155-9. [DOI] [PubMed] [Google Scholar]
- Dean C. J., Feldschreiber P., Lett J. T. Repair of x-ray damage to the deoxyribonucleic acid in Micrococcus radiodurans. Nature. 1966 Jan 1;209(5018):49–52. doi: 10.1038/209049a0. [DOI] [PubMed] [Google Scholar]
- MARMUR J., ANDERSON W. F., MATTHEWS L., BERNS K., GAJEWSKA E., LANE D., DOTY P. The effects of ultraviolet light on the biological and physical chemical properties of deoxyribonucleic acids. J Cell Comp Physiol. 1961 Dec;58(3):33–55. doi: 10.1002/jcp.1030580406. [DOI] [PubMed] [Google Scholar]
- MOROWITZ H. J. Absorption effects in volume irradiation of microorganisms. Science. 1950 Mar 3;111(2879):229–229. doi: 10.1126/science.111.2879.229-a. [DOI] [PubMed] [Google Scholar]
- MOSELEY B. E., SCHEIN A. H. RADIATION RESISTANCE AND DEOXYRIBONUCLEIC ACID BASE COMPOSITION OF MICROCOCCUS RADIODURANS. Nature. 1964 Sep 19;203:1298–1299. doi: 10.1038/2031298a0. [DOI] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Reconstruction in vivo of irradiated Escherichia coli deoxyribonucleic acid; the rejoining of broken pieces. Nature. 1966 Oct 29;212(5061):534–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- Moseley B. E. The isolation and some properties of radiation-sensitive mutants of Micrococcus radiodurans. J Gen Microbiol. 1967 Nov;49(2):293–300. doi: 10.1099/00221287-49-2-293. [DOI] [PubMed] [Google Scholar]
- SETLOW J. K., DUGGAN D. E. THE RESISTANCE OF MICROCOCCUS RADIODURANS TO ULTRAVIOLET RADIATION. I. ULTRAVIOLET-INDUCED LESIONS IN THE CELL'S DNA. Biochim Biophys Acta. 1964 Aug 12;87:664–668. doi: 10.1016/0926-6550(64)90284-1. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E. The resistance of Micrococcus radiodurans to ultraviolet radiation. II. Action spectra for killing, delay in DNA synthesis, and thymine dimerization. Biochim Biophys Acta. 1965 Oct 11;108(2):259–265. doi: 10.1016/0005-2787(65)90010-9. [DOI] [PubMed] [Google Scholar]
- Setlow R. B., Carrier W. L., Bollum F. J. Pyrimidine dimers in UV-irradiated poly dI:dC. Proc Natl Acad Sci U S A. 1965 May;53(5):1111–1118. doi: 10.1073/pnas.53.5.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]



