Abstract
Background
Nurses’ ability to rapidly detect drops in cerebral perfusion pressure (CPP) that may contribute to secondary brain injury may be limited by poor visibility of CPP displays.
Objective
To evaluate the impact of a highly visible CPP display on functional outcome in individuals with cerebral aneurysms.
Methods
Patients with cerebral aneurysms (n = 100) who underwent continuous CPP monitoring were enrolled and randomized to beds with or without the additional CPP display. Six-month outcome was assessed.
Results
Functional outcome was not significantly different between control and intervention groups, after controlling for initial neurologic condition (odds ratio 0.904, 95% confidence intervals 0.317 – 2.573). However, greater time below CPP thresholds (55 mm Hg to 70 mm Hg) was significantly associated with poorer outcome (p = 0.005 – 0.010).
Conclusions
Although the enhanced CPP display was not associated with significantly better outcome, longer periods of CPP below set levels were associated with poorer outcome.
Following aneurysmal subarachnoid hemorrhage (SAH) brain injury beyond the initial bleed may occur as a result of secondary insults, such as inadequate cerebral blood flow and cerebral perfusion. These insults can contribute to cerebral ischemia and infarction, and are associated with poorer outcome1–4. The prevention of ischemia is thus one of the challenges of the acute management of SAH. Cerebral perfusion pressure (CPP), the difference between mean arterial blood pressure (ABP) and mean intracranial pressure (ICP) is commonly monitored in the critical care setting to assess global cerebral blood delivery. Regional cerebral blood flow is decreased following SAH, particularly in those with delayed neurologic deficit5–9. As a result of cerebral vasospasm and impaired cerebral autoregulation following SAH10–12, cerebral blood flow becomes particularly dependent on CPP. Thus maintaining adequate CPP following SAH is one component of preventing or minimizing cerebral ischemia.
Nurses have a key role in the monitoring and management of CPP. Nursing care activities, such as positioning or suctioning, can affect CPP, with transient or sustained activity-related drops occurring that may contribute to secondary ischemic brain injury. Moreover, nursing monitoring of CPP is essential for titration of medical management, such as administration of intravenous fluids, mannitol, or vasopressors. Nurses are aware of the need to keep CPP above a critical level but interventions to do so are often based on hourly or half-hourly recordings of CPP. Although continuous CPP information may be available with current clinical bedside monitoring systems, these systems generally do not provide a highly visible, continuous display of CPP that brings immediate attention to care-related drops in CPP or clearly displays a trend of declining CPP. Nursing maneuvers to manage CPP may be enhanced by heightened awareness of these alterations in CPP allowing for immediate intervention, such as head elevation to decrease ICP, or titration of sedation or vasopressor medication. Thus, a highly visible display of CPP may be expected to contribute to fewer episodes of CPP drops below the critical level. To the degree that inadequate CPP contributes to secondary ischemic brain injury, preventing or minimizing these drops in CPP may contribute to improved short and long-term functional outcome.
This study was a randomized controlled trial to evaluate, in the context of optimal medical and surgical management, the impact of a highly visible continuous CPP display on CPP management and functional outcome in patients undergoing treatment for cerebral aneurysms. We hypothesized that patients randomized to the highly visible CPP display would have significantly better Extended Glasgow Outcome Scale (GOSE) and Functional Status Examination (FSE) scores at six months than those randomized to the control (blank) CPP display. A secondary aim of the study was to examine the effect of the percent time that CPP was below various threshold levels on outcome following SAH. The hypothesis for this aim was that an increased percent time below CPP thresholds would be associated with poorer outcome.
Methods
Participants
Approval for the study was received from the local Institutional Review Board. Patients 16 years of age or older who were admitted to an intensive care unit at an academic medical center between 2000 and 2002 with a cerebral aneurysm and who underwent invasive ICP and ABP monitoring were enrolled in the study. The majority presented with aneurysms that had ruptured resulting in SAH. During the period of this study a parallel study was carried out with a traumatic brain injury sample. Findings from that sample have been published separately13. Subjects were enrolled within 24 hours of ICP monitor insertion. Exclusion criteria were bilateral fixed pupils and impending death. Written consent for follow-up was obtained from the patients and/or a surrogate, as appropriate. Outcome assessment was carried out at discharge, three, and six months by trained interviewers.
During the time of this study standard medical management of CPP was targeted at both ICP and ABP. There was not a specific CPP management protocol used at the time of the study and targets for CPP management were typically set on an individual basis. Specific SAH management included administration of nimodipine, cerebrospinal fluid drainage, early aneurysm obliteration whenever possible, daily transcranial Doppler sonography, and the use of pulmonary artery catheters with hypertensive, hypervolemic, hemodilution therapy, and balloon angioplasty as indicated for cerebral vasospasm.
Interventions
Patients were randomized to beds with either a highly visible CPP display (intervention) or a blank CPP display (control), positioned on a stand at the head of the bed. The active display consisted of a bar graph reflecting CPP over the previous 30 minutes, with bars displayed in blue or red depending on whether CPP was above or below a threshold of 70 mm Hg, respectively. Given the absence of an established critical lower CPP threshold for patients with SAH, 70 mm Hg was selected. This was the recommended lower threshold for the critical care management of traumatic brain injury at the time of the study14. The numeric CPP value was also visible on the active CPP display. Update of the display occurred every 5 seconds. The blank display consisted of a black screen with the message “Program Running. Please Do Not Adjust.”
Measurement
ABP was measured using intra-arterial catheters connected to Abbott Transpect pressure transducers (Abbott Laboratories, Abbott Park, IL). ICP was measured using intraparenchymal Camino transducer-tipped catheters (Integra LifeSciences, Plainsboro, NJ). Analog signals were input to the display computer from the bedside monitoring system (Spacelabs Medical, Redmond, WA). CPP was calculated as mean ABP minus mean ICP.
Data Collection
Demographic (age, gender), diagnostic (computed tomography, angiography, transcranial Doppler sonography) and management (pharmacologic, surgical, or radiologic intervention) data were recorded. Hunt and Hess grade was documented. This grade reflects SAH severity based on clinical neurologic condition, with higher grades indicating more severe SAH15. Grade I reflects no symptoms or mild headache while grade V reflects deep coma and moribund appearance. Individuals with unruptured aneurysms were assigned a grade of 0. Glasgow Coma Scale (GCS) scores were recorded. The GCS provides a means to quantify level of consciousness and ranges from 3 (unresponsive) to 15 (alert and oriented)16. Five-second summaries of ICP and ABP data were saved to the display computers.
Outcomes
The primary outcome measure was functional outcome, as evaluated using the Extended Glasgow Outcome Scale (GOSE) and Functional Status Examination (FSE) at six months. A secondary endpoint was survival at six months. The GOSE divides each of the severe disability, moderate disability, and good recovery categories of the original Glasgow Outcome Scale into an upper and lower category17–18. This results in an eight-point scale. A GOSE score of 1 represents death and a score of 8 represents upper good recovery. The FSE assesses changes in physical, social, and psychologic functioning, and financial independence that are attributable to acute brain injury19. Ten categories reflecting functional status are each scored from 0 to 3, with 0 indicating no change from pre brain injury functioning and 3 indicating complete dependence. Scores are summed to give a score ranging from 0 to 30, with a score of 31 assigned for death. In contrast to the GOSE, where lower scores reflect poorer functioning, higher scores on the FSE reflect poorer functioning. Both GOSE and FSE scores are obtained using a standardized interview format.
Sample Size
A sample size of 50 per group allowed for detection of a true group difference of 0.8 or greater on the GOSE with 80% power, controlling for age, gender, and SAH severity.
Randomization
Randomization was carried out using a block randomization procedure, with block size randomly varying between four and six. Randomization assignments were generated, placed in consecutively numbered envelopes, and sealed. As patients were enrolled the research nurse selected the next consecutive envelope and set up the active or blank display as indicated. Given the visibility of the display computer, care providers, patients, and families could not be blinded to randomization group assignment. However, individuals assessing outcome were blinded.
Statistical Methods
Group differences in continuous variables reflecting baseline characteristics were assessed using the independent sample Student’s t test. Chi-square was used to assess group differences in categorical variables. The percent time that CPP was below the thresholds was first calculated per day and then the values for the first 4 days were averaged into a single value. This data collection period encompassed a time in which subjects were at risk for low CPP and was thus a period when it would be expected that the CPP display would be of greatest value. Binary logistic regression and analysis of variance were used to examine the primary intervention effect on outcome, controlling for age, gender, and measures reflecting SAH severity. Covariates used to statistically control for SAH severity included the initial GCS score, Hunt & Hess grade, and initial presence of intraventricular hemorrhage and hydrocephalus. The outcome variables used in the binary logistic regression analyses were survival and GOSE, dichotomized to favorable outcome (lower and upper moderate disability, lower and upper good recovery) and unfavorable outcome (dead, vegetative, lower and upper severe disability). There is no established cutoff point at which to dichotomize the FSE to reflect favorable versus unfavorable outcome. The FSE is therefore treated as an interval variable for the purpose of the statistical analysis.
The secondary purpose of this study was to explore the relationship between CPP and outcome, regardless of intervention or control group assignment. There is little literature specifically addressing the impact of CPP levels on outcome following SAH so thresholds were selected within a range of CPP levels likely to be encountered clinically. The degree to which CPP below a particular threshold contributes to cerebral ischemia may depend not only on the threshold but also on the length of time below the threshold. We therefore examined the impact on outcome of the length of time that CPP was below the thresholds. For each CPP threshold level (55 mmHg to 80 mmHg, in 5 mm Hg increments) a dichotomous variable was created using receiver operating characteristic analysis to identify a cutoff percent time below the threshold that identified subgroups at lower risk or higher risk for poorer outcome. The cutoff percent times used to create the dichotomized variable of greater or less time below the thresholds are presented in Appendix 1. As the CPP threshold decreases, the cutoff percent time also decreases, reflecting the greater risk for cerebral ischemia with shorter excursions below the lower thresholds. Although the percent times are relatively low they reflect cumulative time over four days of monitoring. Times below the different thresholds ranged from 16.3 hours (17%) to six minutes (0.1%).
For each CPP threshold level, analysis of variance was conducted, modeling the GOSE and the FSE (dependent variables) by greater or less percent time below CPP threshold, controlling for age, gender, and initial severity. In addition, binary logistic regression was performed using the dichotomous dependent variable survival (alive/dead) by greater or less percent time below CPP threshold subgroup, again controlling for age, gender, and the measures of SAH severity.
Results
A total of 100 patients were enrolled. Patient demographics and clinical variables are presented in Table 1. Control and intervention groups did not differ significantly in relation to age or gender. However, despite randomization, SAH severity differed between the intervention and control groups, with greater severity in the intervention group. The intervention group had a significantly higher mean Hunt and Hess grade (p = 0.021). The mean initial Glasgow Coma Scale (GCS) score for the intervention group was lower than for the control group, although the difference was not statistically significant. Whereas 59% of the control group presented with a GCS score of 15, only 39% of the intervention group had an initial GCS score of 15 (p = 0.046). Almost one-half (49%) of the intervention group was intubated on admission versus 29% of the control group (p = 0.036). The intervention group was significantly more likely to have a ventriculostomy over their course of treatment (p = 0.002). In addition, the intervention group was more likely to have intraventricular hemorrhage, cerebral vasospasm, and angioplasty, although these differences were not statistically significant. These variables all reflect poorer initial neurologic status, greater SAH severity, and a higher incidence of subsequent complications associated with more severe SAH in the intervention group.
Table 1.
Demographic and Clinical Characteristics
| Control | Intervention | ||
|---|---|---|---|
| (n = 49) | (n =51) | p-value | |
| Age, yrs, mean ± SD | 55 ± 12 | 52 ± 13 | 0.37 |
| Sex, % female | 76 | 77 | NS |
| GCS-PR, mean ± SD | 12.4 ± 3.9 | 11.6 ± 3.8 | 0.26 |
| GCS-PR, median | 15 | 13 | |
| Hunt & Hess, mean ± SD | 2.1 ± 1.4 | 2.9 ± 1.3 | 0.010 |
| Hunt & Hess, n (%) | |||
| 0 | 6 (12) | 3 (6) | |
| I | 10 (20) | 4 (8) | |
| II | 16 (33) | 13 (26) | |
| III | 8 (16) | 14 (29) | |
| IV | 6 (12) | 11 (22) | |
| V | 3 (6) | 6 (12) | |
| CT findings, n (%) | |||
| IVH | 33 (67) | 38 (75) | |
| ICH | 10 (20) | 11 (22) | |
| Hydrocephalus | 30 (61) | 34 (67) | |
| Aneurysm site, % | |||
| Anterior circulation | 86 | 77 | |
| Posterior circulation | 14 | 24 | |
| Aneurysm management, n (%) | |||
| Ventriculostomy | 14 (29) | 30 (59) | |
| Clipping | 44 (90) | 44 (87) | |
| Coiling | 5 (10) | 4 (8) | |
| Angioplasty | 9 (18) | 16 (31) | |
| Vasospasm, n (%) | 34 (69) | 42 (82) |
GCS-PR, post-resuscitation Glasgow Coma Scale score; IVH, intraventricular hemorrhage; ICH, intracerebral hemorrhage
Discharge disposition of study participants is presented in Table 2. The actual numbers in each category are presented along with the predicted numbers. The distribution of the predicted values was determined using multinomial logistic regression, controlling for baseline demographic and SAH severity information, the latter which differed significantly between the control and intervention groups.
Table 2.
Discharge Status
| Control | Intervention | |||
|---|---|---|---|---|
| (n = 49) | (n = 51) | |||
| Disposition, n | Actual | Predicted | Actual | Predicted |
| Home | 22 | 22 | 7 | 10 |
| Rehabilitation | 11 | 12 | 18 | 20 |
| Skilled nursing facility | 10 | 7 | 8 | 5 |
| Died | 4 | 8 | 14 | 16 |
| Other | 2 | 0 | 4 | 0 |
Predicted values were determined using multinomial logistic regression and reflect the numbers that would be expected in each category controlling for age, gender, and subarachnoid hemorrhage severity.
Six-month mean adjusted GOSE and FSE scores are presented in Table 3. These values indicate, on average, recovery to a level requiring daily dependence on others for at least some activities. Analysis of variance shows no significant difference in GOSE or FSE scores between the control and intervention groups after controlling for age, gender, and SAH severity. For the logistic regression analyses, the odds ratios reflect the odds of a favorable outcome or survival at six months for subjects in the intervention group as compared to the control group. There is not a statistically significant difference between groups in relation to those achieving a favorable outcome (OR 0.904, 95% Confidence Interval 0.317 to 2.573), nor in six-month survival (OR 0.600, 95% Confidence Interval 0.157 – 2.296).
Table 3.
Outcome at six months by randomization group
| Analysis of variance of Extended Glasgow Outcome Scale scores at six months by randomization group. | ||||
|---|---|---|---|---|
| Control | Intervention | |||
| (n = 49) | (n = 51) | F | p Value | |
| GOSE, mean (SEM) | 4.13 (0.25) | 3.82 (0.24) | 0.749 | 0.389 |
| FSE, mean (SEM) | 18.46 (1.24) | 19.02 (1.12) | 0.103 | 0.749 |
| Binary logistic regression prediction of favorable outcome at six months by randomization group. | ||
|---|---|---|
| Odds Ratio | 95% Confidence Interval | p Value |
| 0.904 | 0.317 – 2.573 | 0.850 |
| Binary logistic regression prediction of six-month survival by randomization group. | ||
|---|---|---|
| Odds Ratio | 95% Confidence Interval | p Value |
| 0.600 | 0.157 – 2.296 | 0.456 |
Values are adjusted for age, gender, and subarachnoid hemorrhage severity.
Controlling for age, gender, and subarachnoid hemorrhage severity.
Intermediate variables that would reflect interventions carried out by nurses to manage CPP were examined for differences between the control and intervention groups. Mean ABP, ICP, and CPP, and the percent time below various CPP thresholds over the first four days of monitoring are presented in Table 4. There are no statistically significant differences in any of these measures between the intervention and control groups. However, individuals in the intervention group received on average significantly greater amounts of mannitol and packed red blood cells.
Table 4.
Physiologic variables, based on averages over the first four days of monitoring
| Control | Intervention | p Value | |
|---|---|---|---|
| (n = 47) | (n = 50) | ||
| CPP, mean (SD) | 91.5 (13.6) | 92.5 (11.0) | NS |
| % time CPP < 80 mm Hg | 25.1 (24.2) | 24.70 (23.8) | NS |
| % time CPP < 75 mm Hg | 16.0 (21.4) | 16.1 (18.7) | NS |
| % time CPP < 70 mm Hg | 9.7 (16.6) | 9.3 (12.5) | NS |
| % time CPP < 65 mm Hg | 5.3 (12.0) | 4.6 (6.9) | NS |
| % time CPP < 60 mm Hg | 3.4 (10.5) | 2.0 (3.1) | NS |
| % time CPP < 55 mm Hg | 2.5 (9.5) | 0.8 (1.5) | NS |
| ABP, mean (SD) | 102.3 (12.6) | 105.3 (10.2) | NS |
| ICP, mean (SD) | 10.7 (6.8) | 12.6 (7.2) | NS |
| Mannitol (ml), mean (SD) | 55 (104) | 144 (237) | 0.017 |
| Packed red cells (ml), mean (SD) | 36 (84) | 81 (114) | 0.026 |
For physiologic data the sample size is 97 due to incomplete data for three subjects.
Although the percent time that CPP was below the various thresholds was not statistically significantly different between the intervention and control groups, it was lower in the intervention group at the lower CPP thresholds, particularly 55 mm Hg and 60 mm Hg. For example, for a threshold of 60 mm Hg, CPP was below the threshold 3.4% of the time for the control group versus 2.0% of the time for the intervention group. Over a period of 4 days, this difference of 1.4% translates to the intervention group on average having CPP below the threshold for 80 minutes less than the intervention group. For a CPP threshold of 55 mm Hg, subjects in the intervention group on average had CPP below the threshold for 98 minutes less than those in the control group. The highly visible display may have been a contributing factor to these differences.
The effect on six-month survival of greater or lesser time with CPP below the specified CPP thresholds, controlling for age, gender, and SAH severity was assessed using binary logistic regression (Table 5). Odds ratios reflect the odds of survival for subjects in the subgroup with CPP below the threshold for the greater percent time, as compared to those with CPP below the threshold for less time. The odds of six-month survival were lower in those with greater time below the CPP thresholds for all threshold levels, although not reaching statistical significance at the 75 mm Hg and 80 mm Hg thresholds.
Table 5.
Binary logistic regression prediction of six-month survival by greater versus lesser percent time of cerebral perfusion pressure below specified thresholds.
| Odds Ratio | 95% Confidence Interval | p Value | |
|---|---|---|---|
| Time CPP < 80 mm Hg | 0.650 | 0.186 – 2.267 | 0.499 |
| Time CPP < 75 mm Hg | 0.337 | 0.097 – 1.169 | 0.086 |
| Time CPP < 70 mm Hg | 0.168 | 0.043 – 0.656 | 0.010 |
| Time CPP < 65 mm Hg | 0.136 | 0.033 – 0.555 | 0.005 |
| Time CPP < 60 mm Hg | 0.124 | 0.029 – 0.528 | 0.005 |
| Time CPP < 55 mm Hg | 0.131 | 0.032 – 0.534 | 0.005 |
Controlling for age, gender, and subarachnoid hemorrhage severity.
GOSE and FSE scores at six months for those with greater versus less percent time below the specified CPP thresholds are presented in Figure 1 and Figure 2, respectively. At all thresholds, average GOSE and FSE scores (controlling for age, gender, and SAH severity) reflected poorer outcome in the subgroups with greater time of CPP below the thresholds. Analysis of variance of six-month outcome by greater versus less time below the thresholds shows that the differences in six-month GOSE scores were statistically significant for thresholds up to and including 70 mm Hg (Table 6). Differences in FSE scores were not significantly different.
Figure 1.
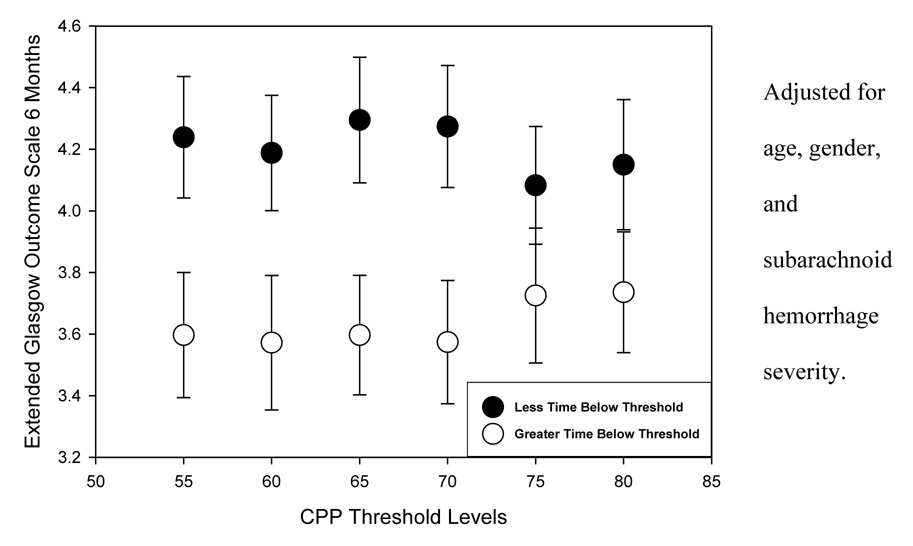
Extended Glasgow Outcome Scale scores at six months by greater versus lesser time below cerebral perfusion pressure thresholds
Figure 2.
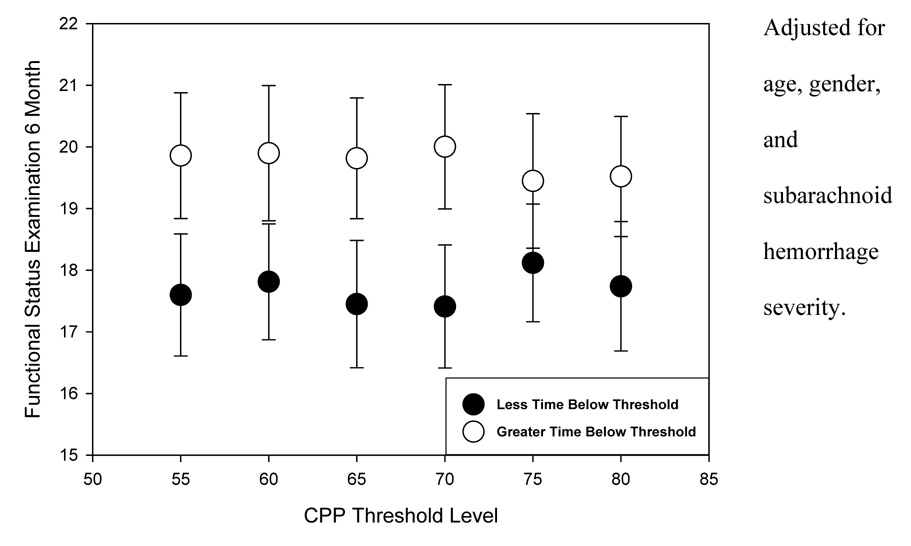
Functional Status Examination scores at six months by greater or lesser time below cerebral perfusion pressure thresholds.
Table 6.
Analysis of variance of six-month outcome by greater versus lesser time below cerebral perfusion pressure thresholds.
| Extended Glasgow Outcome Scale score | ||
|---|---|---|
| Cerebral perfusion pressure threshold | F | p Value |
| 55 mm Hg | 5.144 | 0.026 |
| 60 mm Hg | 4.569 | 0.035 |
| 65 mm Hg | 6.100 | 0.015 |
| 70 mm Hg | 6.153 | 0.015 |
| 75 mm Hg | 1.498 | 0.224 |
| 80 mm Hg | 2.068 | 0.154 |
| Functional Status Examination score | ||
|---|---|---|
| Cerebral perfusion pressure threshold | F | p Value |
| 55 mm Hg | 2.513 | 0.116 |
| 60 mm Hg | 2.073 | 0.153 |
| 65 mm Hg | 2.743 | 0.101 |
| 70 mm Hg | 3.320 | 0.072 |
| 75 mm Hg | 0.835 | 0.362 |
| 80 mm Hg | 1.541 | 0.218 |
Adjusted for age, gender, and subarachnoid hemorrhage severity.
Discussion
Critical care nurses have a key role in the monitoring and management of CPP following SAH. We are unaware of other studies that have examined the impact of the presentation of CPP information on nurses’ ability to manage CPP and on patient outcome. We hypothesized that the presence of a highly visible CPP display would enhance nurses’ ability to manage CPP, resulting in fewer episodes of CPP below a critical threshold and, to the extent that CPP below a critical threshold mediates poorer outcome, would contribute to better outcome. However, the presence of a highly visible, real-time display of CPP was not associated with significantly better functional outcome at six months. A number of factors may have contributed to the lack of a difference in outcome between subjects with the active and blank displays.
Despite the randomization protocol, there was a randomization imbalance in relation to SAH severity, which is an identified predictor of outcome. Several measures of initial clinical condition, including Hunt and Hess grade, initial Glasgow Coma Scale score, and initial intubation status, all reflected greater SAH severity in the intervention group. Although these variables were included as covariates in the statistical analyses, this control may have been inadequate and may not have captured additional relevant latent differences between the control and intervention groups, such as difficulty of aneurysm obliteration. In addition, outcome for those with the most severe SAH may be poor regardless of CPP management. Thus, the likelihood of an intervention effect would be decreased given the higher percentage in the intervention group of those with the most severe SAH. To prevent such an imbalance in future studies stratified randomization based on SAH severity is recommended. The majority of subjects had aneurysms that had ruptured, resulting in SAH. However, the percentage of subjects with unruptured aneurysms, who would be considered at lower risk for ischemic injury and poorer outcome, was higher in the control group. This may also have diluted an overall beneficial effect of the intervention.
The severity of SAH represented in this sample may have been impacted by concurrent intervention studies. The inclusion criteria for the CPP study were broader with fewer exclusion criteria than the other studies so some subjects were available for enrollment in the CPP study by virtue of not meeting inclusion criteria of other studies. This may have biased SAH severity in subjects in the CPP study in the direction of greater severity.
Whether patients were randomized to the active or blank CPP display, CPP was monitored in all patients in the study, although with the standard bedside monitoring system it was not readily visible during care due to poor ergonomics. The CPP display was thus an adjuvant to increase nurses’ awareness of and ability to rapidly respond to drops in CPP, not the sole source of CPP information. It is possible that as a result of the study nurses had a greater awareness of CPP in all patients, whether they had the active or blank display. The lack of direct measurement of nurses’ response to the CPP display is a limitation of this study and conclusions as to whether nursing management of CPP actually changed as a result of the CPP display are therefore speculative. A smaller study (n = 17) was carried out during this study to examine nursing response to CPP changes in patients in the traumatic brain injury sample of the study20. Patients with the active display, blank display, or usual bedside display were included. Drops in CPP that were sustained were found to occur mostly when the nurse was out of the patient’s room. Thus, although the active display provided information about drops occurring over the preceding 30 minutes that was unavailable with the blank or usual displays, it did not confer additional benefit to allow early responses to CPP drops if the nurses were not in the room to see the drops in real-time. Incorporating an audible alarm to alert nurses that CPP was below a critical threshold would help address this issue.
Although the differences in the percent time that CPP was below the various thresholds in the intervention and control groups were not statistically significantly, CPP was below the thresholds of 60 mmHg and 55 mmHg on average 80 and 98 minutes less, respectively, in the group with the highly visible display than in the control group. This amount of time could be clinically significant in relation to secondary brain injury. Whether the intervention group had less time with CPP below these thresholds related to action by nurses in response to presence of the active display or related to other physician or patient factors influencing CPP, however, is not known.
The effect of ICP and ABP following SAH have typically been examined independently, rather than as the combined parameter of CPP. Thus this study examining the impact of CPP on outcome adds to the research literature beyond the independent effects of ICP and ABP on outcome. Considered independently, increased ICP is known to contribute to cerebral ischemia and negatively influence outcome following SAH21. Mean arterial blood pressure of less than 70 mm Hg is also independently associated with death or severe disability at three months post-SAH22. The simplified acute physiology score (SAPS) II has been used to examine the ability of 12 physiologic variables, age, type of admission, and three underlying disease characteristics to predict outcome and the occurrence of delayed cerebral ischemia following SAH23. The SAPS II value was the strongest and only independent predictor of poor outcome at three months post-SAH in the multivariate analysis. In the univariate analysis, systolic blood pressure outside normal ranges was significantly associated with poor outcome. The SAPS II was also the only independent predictor of delayed cerebral ischemia. Enblad examined the occurrence and impact on outcome of secondary brain insults, including CPP less than or equal to 70 mm Hg, in patients with SAH24. CPP of 70 mm Hg or less was the most frequently occurring secondary insult in the first week post-SAH, with 48 episodes of at least three hours in length occurring (maximum count of one per day). The effect of each insult was not analyzed separately but the total number of insults was a significant independent predictor of outcome. The measures and analyses used in the above studies do not allow for a direct comparison of findings to those of the current study, however they do support a role of the components of CPP in outcome following SAH. In addition, the study by Enblad highlights the substantial number of potentially preventable secondary brain insults that occur despite intensive care management and the need for more precise monitoring and further examination of the impact of secondary insults on outcome. More visible and meaningful displays of relevant physiologic variables may contribute to increased detection and more rapid management by nurses so as to prevent or minimize the occurrence of secondary insults.
Medical management of this particular patient population was often targeted at maintaining specific levels of ICP, arterial blood pressure, and pulmonary capillary wedge pressure, rather than specifically targeting CPP. Hypertensive-hypovolemic-hemodilution (triple-H) therapy25–31 was used to treat cerebral vasospasm. Although hypertension and/or triple-H therapy have been shown to increase CBF9, 32 and reverse delayed ischemic deficits from cerebral vasospasm9, 33, triple-H therapy is not without complications and its value remains controversial26, 30. However, as a result of it use in this study sample, the overall mean CPP was greater than 90 mm Hg and subjects were not typically at risk for CPP drops to what would generally be considered critical ischemic levels. Thus the beneficial effect of the active display in this study may have been limited to those with low CPP levels who are at particular risk for cerebral ischemia.
Although most subjects did not have a high percentage time of below the various CPP thresholds examined, within subjects, a relatively small percentage of time below the thresholds up to 70 mm Hg was significantly associated with poorer outcome. In the presence of cerebral vasospasm, triple-H therapy generally dictates the upper target for ABP, and therefore, to a large degree, CPP. However, given the occurrence of low cerebral blood flow following SAH, the minimal CPP threshold is also of interest in defining risk for cerebral ischemia. This is particularly so when triple-H therapy is not used or has not yet been initiated. In this study the critical time below the CPP threshold levels that was associated with poorer outcome was threshold dependent, decreasing as the CPP threshold level decreased, reflecting less ability to tolerate CPP below lower thresholds. Whereas short excursions below CPP thresholds examined in this study may be tolerated in general, the study demonstrates that drops occurring over longer periods or cumulative drops are associated with poorer outcome. Individual patient circumstances are likely to determine the exact critical CPP threshold and critical time below the threshold that are associated with poorer outcome. The neurologic impact of a drop in CPP may not be readily apparent at the time of the drop, however, nurses must be aware of the potential cumulative effect beyond the immediate period.
Conclusions
Although the presence of a highly visible display of CPP was not associated with better outcome in this study, an association between periods of low CPP and poorer outcome was found. Overall, the incidence of poor outcomes in individuals suffering aneurysmal SAH remains significant and current management strategies are based on limited studies, many which suffer from methodologic weaknesses34. This leaves considerable room for improvement. Further research is needed to understand the impact of physiologic derangements, including low CPP, and their management, on outcome following SAH. Given the complex multimodality physiologic monitoring in current intensive care management and the high incidence of secondary insults, improving the ergonomics of clinical monitoring systems can potentially contribute to nurses’ ability to better control physiologic parameters, which may ultimately improve outcome following SAH.
Acknowledgements
This study was supported by NIH NINR R01 NR04901.
Appendix 1
The cutoff percent times used to create the dichotomized variable of greater or less time below the threshold for each CPP threshold level are as follows:
CPP < 80 mm Hg: 17% (54% had CPP below 80 mm Hg for > 17% of monitored time)
CPP < 75 mm Hg: 13% (43% had CPP below 75 mm Hg for > 13% of monitored time)
CPP < 70 mm Hg: 4% (50% had CPP below 70 mm Hg for > 4% of monitored time)
CPP < 65 mm Hg: 1.3% (53% had CPP below 65 mm Hg for > 1.3% of monitored time)
CPP < 60 mm Hg: 0.8 % (42% had CPP below 60 mm Hg for > 0.8% of monitored time)
CPP < 55 mm Hg: 0.1% (52% had CPP below 55 mm Hg for > 0.1% of monitored time).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Catherine J. Kirkness, Research Associate Professor, Biobehavioral Nursing and Health Systems, University of Washington, Seattle, WA.
Robert L. Burr, Research Associate Professor, Biobehavioral Nursing and Health Systems, University of Washington, Seattle, WA.
Kevin C. Cain, Biostatistician, Biostatistics and Office for Nursing Research, University of Washington, Seattle, WA.
David W. Newell, Executive Director and Medical Director, Seattle Neuroscience Institute at Swedish Medical Center, Seattle, WA.
Pamela H. Mitchell, Elizabeth S. Soule Professor, Biobehavioral Nursing and Health Systems, University of Washington, Seattle, WA.
References
- 1.Rabinstein AA, Weigand S, Atkinson JL, Wijdicks EF. Patterns of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke. 2005;36(5):992–997. doi: 10.1161/01.STR.0000163090.59350.5a. [DOI] [PubMed] [Google Scholar]
- 2.Longstreth WT, Jr, Nelson LM, Koepsell TD, van Belle G. Clinical course of spontaneous subarachnoid hemorrhage: a population-based study in King County, Washington. Neurology. 1993;43(4):712–718. doi: 10.1212/wnl.43.4.712. [DOI] [PubMed] [Google Scholar]
- 3.Kalaria RN, Kalimo H. Introduction: Non-atherosclerotic cerebrovascular disorders. Brain Pathol. 2002;12(3):337–342. doi: 10.1111/j.1750-3639.2002.tb00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kassell NF, Torner JC, Haley C, et al. The International Cooperative Study on the timing of aneurysm surgery. Part 1: overall management results. Journal of Neurosurgery. 1990;73:18–36. doi: 10.3171/jns.1990.73.1.0018. [DOI] [PubMed] [Google Scholar]
- 5.Fukui MB, Johnson DW, Yonas H, Sekhar L, Latchaw RE, Pentheny S. Xe/CT cerebral blood flow evaluation of delayed symptomatic cerebral ischemia after subarachnoid hemorrhage. AJNR Am J Neuroradiol. 1992;13(1):265–270. [PMC free article] [PubMed] [Google Scholar]
- 6.Minhas PS, Menon DK, Smielewski P, et al. Positron emission tomographic cerebral perfusion disturbances and transcranial Doppler findings among patients with neurological deterioration after subarachnoid hemorrhage. Neurosurgery. 2003;52(5):1017–1022. discussion 1022-1014. [PubMed] [Google Scholar]
- 7.Frykholm P, Andersson JL, Langstrom B, Persson L, Enblad P. Haemodynamic and metabolic disturbances in the acute stage of subarachnoid haemorrhage demonstrated by PET. Acta Neurol Scand. 2004;109(1):25–32. doi: 10.1034/j.1600-0404.2003.00174.x. [DOI] [PubMed] [Google Scholar]
- 8.Horn P, Vajkoczy P, Bauhuf C, Munch E, Poeckler-Schoeniger C, Schmiedek P. Quantitative regional cerebral blood flow measurement techniques improve noninvasive detection of cerebrovascular vasospasm after aneurysmal subarachnoid hemorrhage. Cerebrovasc Dis. 2001;12(3):197–202. doi: 10.1159/000047704. [DOI] [PubMed] [Google Scholar]
- 9.Kim DH, Joseph M, Ziadi S, Nates J, Dannenbaum M, Malkoff M. Increases in cardiac output can reverse flow deficits from vasospasm independent of blood pressure: a study using xenon computed tomographic measurement of cerebral blood flow. Neurosurgery. 2003;53(5):1044–1051. doi: 10.1227/01.neu.0000088567.59324.78. discussion 1051-1042. [DOI] [PubMed] [Google Scholar]
- 10.Ratsep T, Asser T. Cerebral hemodynamic impairment after aneurysmal subarachnoid hemorrhage as evaluated using transcranial doppler ultrasonography: relationship to delayed cerebral ischemia and clinical outcome. J Neurosurg. 2001;95(3):393–401. doi: 10.3171/jns.2001.95.3.0393. [DOI] [PubMed] [Google Scholar]
- 11.Lang EW, Diehl RR, Mehdorn HM. Cerebral autoregulation testing after aneurysmal subarachnoid hemorrhage: the phase relationship between arterial blood pressure and cerebral blood flow velocity. Crit Care Med. 2001;29(1):158–163. doi: 10.1097/00003246-200101000-00031. [DOI] [PubMed] [Google Scholar]
- 12.Soehle M, Czosnyka M, Pickard JD, Kirkpatrick PJ. Continuous assessment of cerebral autoregulation in subarachnoid hemorrhage. Anesth Analg. 2004;98(4):1133–1139. doi: 10.1213/01.ANE.0000111101.41190.99. table of contents. [DOI] [PubMed] [Google Scholar]
- 13.Kirkness CJ, Burr RL, Cain KC, Newell DW, Mitchell PH. Effect of continuous display of cerebral perfusion pressure on outcomes in patients with traumatic brain injury. Am J Crit Care. 2006;15(6):600–609. [PubMed] [Google Scholar]
- 14.The Brain Trauma Foundation. The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. Guidelines for cerebral perfusion pressure. J Neurotrauma. 2000;17(6–7):507–511. doi: 10.1089/neu.2000.17.507. [DOI] [PubMed] [Google Scholar]
- 15.Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28(1):14–20. doi: 10.3171/jns.1968.28.1.0014. [DOI] [PubMed] [Google Scholar]
- 16.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 17.Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15(8):573–585. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- 18.Teasdale GM, Pettigrew LE, Wilson JT, Murray G, Jennett B. Analyzing outcome of treatment of severe head injury: a review and update on advancing the use of the Glasgow Outcome Scale. J Neurotrauma. 1998;15(8):587–597. doi: 10.1089/neu.1998.15.587. [DOI] [PubMed] [Google Scholar]
- 19.Dikmen S, Machamer J, Miller B, Doctor J, Temkin N. Functional status examination: a new instrument for assessing outcome in traumatic brain injury. J Neurotrauma. 2001;18(2):127–140. doi: 10.1089/08977150150502578. [DOI] [PubMed] [Google Scholar]
- 20.Kenner GIA. Nursing care activities in traumatic brain injury patients with physiologic monitoring [Masters thesis] Seattle: School of Nursing, University of Washington; 2002. [Google Scholar]
- 21.Heuer GG, Smith MJ, Elliott JP, Winn HR, LeRoux PD. Relationship between intracranial pressure and other clinical variables in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2004;101(3):408–416. doi: 10.3171/jns.2004.101.3.0408. [DOI] [PubMed] [Google Scholar]
- 22.Claassen J, Vu A, Kreiter KT, et al. Effect of acute physiologic derangements on outcome after subarachnoid hemorrhage. Crit Care Med. 2004;32(3):832–838. doi: 10.1097/01.ccm.0000114830.48833.8a. [DOI] [PubMed] [Google Scholar]
- 23.Schuiling WJ, de Weerd AW, Dennesen PJ, Algra A, Rinkel GJ. The simplified acute physiology score to predict outcome in patients with subarachnoid hemorrhage. Neurosurgery. 2005;57(2):230–236. doi: 10.1227/01.neu.0000166536.42876.9c. discussion 230–236. [DOI] [PubMed] [Google Scholar]
- 24.Enblad P, Persson L. Impact on clinical outcome of secondary brain insults during the neurointensive care of patients with subarachnoid haemorrhage: a pilot study. J Neurol Neurosurg Psychiatry. 1997;62(5):512–516. doi: 10.1136/jnnp.62.5.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kosty T. Cerebral vasospasm after subarachnoid hemorrhage: an update. Crit Care Nurs Q. 2005;28(2):122–134. doi: 10.1097/00002727-200504000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Sen J, Belli A, Albon H, Morgan L, Petzold A, Kitchen N. Triple-H therapy in the management of aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2003;2(10):614–621. doi: 10.1016/s1474-4422(03)00531-3. [DOI] [PubMed] [Google Scholar]
- 27.Miller JA, Dacey RG, Jr, Diringer MN. Safety of hypertensive hypervolemic therapy with phenylephrine in the treatment of delayed ischemic deficits after subarachnoid hemorrhage. Stroke. 1995;26(12):2260–2266. doi: 10.1161/01.str.26.12.2260. [DOI] [PubMed] [Google Scholar]
- 28.Egge A, Waterloo K, Sjoholm H, Solberg T, Ingebrigtsen T, Romner B. Prophylactic hyperdynamic postoperative fluid therapy after aneurysmal subarachnoid hemorrhage: a clinical, prospective, randomized, controlled study. Neurosurgery. 2001;49(3):593–605. doi: 10.1097/00006123-200109000-00012. discussion 605-596. [DOI] [PubMed] [Google Scholar]
- 29.Gupta D, Sharma BS, Gupta SK, Bapuraj R, Khosla VK. Postoperative hypertensive-hypervolaemic-haemodilution (Triple H) therapy in the treatment of vasospasm following aneurysmal subarachnoid haemorrhage. Neurol India. 2000;48(2):126–131. [PubMed] [Google Scholar]
- 30.Treggiari MM, Walder B, Suter PM, Romand JA. Systematic review of the prevention of delayed ischemic neurological deficits with hypertension, hypervolemia, and hemodilution therapy following subarachnoid hemorrhage. J Neurosurg. 2003;98(5):978–984. doi: 10.3171/jns.2003.98.5.0978. [DOI] [PubMed] [Google Scholar]
- 31.Mayberg MR, Batjer HH, Dacey R, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage. A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1994;25(11):2315–2328. doi: 10.1161/01.str.25.11.2315. [DOI] [PubMed] [Google Scholar]
- 32.Origitano TC, Wascher TM, Reichman OH, Anderson DE. Sustained increased cerebral blood flow with prophylactic hypertensive hypervolemic hemodilution ("triple-H" therapy) after subarachnoid hemorrhage. Neurosurgery. 1990;27(5):729–739. doi: 10.1097/00006123-199011000-00010. discussion 739–740. [DOI] [PubMed] [Google Scholar]
- 33.Kassell NF, Peerless SJ, Durward QJ, Beck DW, Drake CG, Adams HP. Treatment of ischemic deficits from vasospasm with intravascular volume expansion and induced arterial hypertension. Neurosurgery. 1982;11(3):337–343. doi: 10.1227/00006123-198209000-00001. [DOI] [PubMed] [Google Scholar]
- 34.van der Schaaf IC, Ruigrok YM, Rinkel GJ, Algra A, van Gijn J. Study design and outcome measures in studies on aneurysmal subarachnoid hemorrhage. Stroke. 2002;33(8):2043–2046. doi: 10.1161/01.str.0000024110.82735.5a. [DOI] [PubMed] [Google Scholar]


