Abstract
Studies have shown that N-methyl-D-Aspartate (NMDA) receptors play a critical role in morphine analgesia and motoric processes at different levels of the central nervous system. In this study, we used cortex specific NR1 knockout mice (C57BL/6 strain) to elucidate the role of cortical NMDA receptors in morphine analgesia and motor coordination. On post-natal day 20, mice (CTL and KO) received vehicle (saline) or morphine (10mg/kg) and paw withdrawal latency (PWL) to a noxious thermal stimulus was measured. On post-natal day 21, motor coordination was measured using the rotating pole test. No differences in KO mice were found with respect to PWL following administration of saline or morphine (p > 0.05). However, sex-dependent differences were in motor coordination, with male KO mice showing a greater motor impairment in the rotating pole test than female KO mice (p < 0.05). The present results demonstrate that NMDA receptors are involved in both the analgesic effects of morphine and motor coordination, with the existence of sex-related differences in motor coordination.
Keywords: Motor coordination, morphine, analgesia, NMDA receptor, knockout, Noxious thermal stimuli
N-methyl-D-Aspartate (NMDA) receptors are hetero-oligomers ligand-gated ion channel that interacts with diverse intracellular proteins [26], composed of subunits NR1, NR2 (A,B,C,D) and NR3 (A,B) [8,26]. Functionally, NMDA receptors are involved in coordinating motor responses [4,5,28], and are suggested to play a critical role in mediating pain and analgesia [29], including the analgesic effects of morphine [13,19,20,27].
The present study was designed to elucidate further the role of NMDA receptors in mediating morphine analgesia and motor coordination by using NR1 - NMDA cortex-specific KO mice [6,7,12]. Previous studies on the role of NMDA receptors subunits in morphine analgesia have shown that antagonism of NR2B receptors increase the analgesic effects of morphine on phasic pain conditions [3,9]. Specifically, Fischer et al. [9] reported in adult male C57BL/6 mice that antagonism of NR2B by ifenprodil (dose of 10mg/kg) increased significantly the analgesic effect of morphine (doses of 1mg/kg, 3.2mg/kg and 10mg/kg) in the hot plate test. Similarly, another study in adult male Swiss Albino mice [3] showed that antagonism of NR2B by ifenprodil (dose of 30mg/kg) enhance the antinociceptive effect of morphine (dose of 5mg/kg) in the hot plate test.
Prior studies on the role of NMDA receptors subunits in motor coordination suggest that these are necessary for adequate motor coordination [14,28,34]. For instance, Niemann et al. [28] reported that adult male NR3B KO mice display deficits in motor learning and coordination, in the rotarod motor test. Moreover, Sprengel et al. [34] showed that adult male mice with mutations (truncation of C terminal domain) of NR2C or NR2A subunits (NR2CΔC/ΔC, NR2AΔC/ΔC) display deficits in motor coordination, measured in the rotarod motor test. Furthermore, in adult double NR2A and NR2C KO mice motor deficits in the quick rotarod test (25 rpm) have been demonstrated, whereas single NR2A or NR2C KO mice did not displayed such motor deficits [14]. In addition, double KO’s mice (NR2A and NR2C subunits), but not the single KO’s mice of these subunits, displayed different motor movements in the fixed pole test characterized by grasping/pulling with forepaws and dragging its hindlimbs [14]. Studies performed in other mutant mice, including NR1KO [10,22], NR2BΔC/ΔC (C-terminal truncated of NR2B subunit) [34] and NR2B KO [21] reported that these mice die at perinatal stage, being not possible to evaluate morphine analgesia and motor coordination aspects.
Western blot and in situ hybridization analysis reveals that, as compared with controls, NR1 – NMDA cortex-specific KO mice have a 95% reduction of NR1 subunit levels in cortex and hippocampus [12]. Using x-gal staining, the spatial restriction of Cre recombination has been verified at cortical levels [12]. Cortex-specific NR1 KO mice were originally generated by Iwasato et al. [12] by inserting the Cre recombinase gene downstream from the Emx1 promoter via homologous recombination in embronic stem cells. Emx1 is a homeobox gene expressed only in dorsal telencephalon from embryonic stages to adulthood. Emx1Cre/Cre mice were crossed with heterozygous NR1 null mutant mice (NR1+/−), then further crossed with homozygous floxed NR1 mice (NR1flox/flox). This breeding scheme generated one cortex-specific NR1 KO genotype (Emx1Cre/+NR1flox/−) and three control genotypes. For the present study, we adopted a modified breeding strategy in which half of the progeny were KO mice (of the genotype described above) and the other half were of one of the three original control genotypes, that differs by only one allele from the KOs. Genetically unmodified C57Bl/6 mice (the background strain) were used as an additional control in the tests of motor coordination. Genotype was determined by the Polymerase Chain Reaction method (PCR) on genomic DNA purified from tail biopsies.
Male and female cortex-specific NR1KO mice, genetically modified mice with normal NR1 expression, and C57BL/6 mice were bred, housed and maintained in the animal care facilities of the Louisiana State University Health Sciences Center according to PHS guidelines. Animals were housed in light/dark cycles of 12 × 12 hours (light onset at 06:00), with food and water available ad libitum. To evaluate the role of NMDA receptors in mediating morphine analgesia and motor coordination we used the Hargreaves test (paw withdrawal to thermal stimuli) [11] and the rotating pole test [23], respectively. Behavioral testing was performed when mice were P20 (Hargreaves test) and P21 (Rotating Pole test) days of age. This range in age was chosen because of upper and lower time constraints. NR1 KO mice have a very short lifespan, most dying between 30 and 40 days of age; moreover, pain responses reach full maturity by postnatal day 20, but not sooner [1,35].
On post-natal day 20, male and female mice (CTL and KO) received vehicle (saline) or morphine (10mg/kg) treatment (i.p.), and were tested on the Hargreaves test at 30, 60, and 90 mins. after injection (i.p.). Paw withdrawal latency (PWL) to thermal stimuli was measured according to the techniques previously described [11,31]. Briefly, PWL from a focused beam of light applied to the ventral paw surface was measured using an analgesiometer (IITC Life Sciences, Woodland Hills, CA), and a 10-sec cutoff was imposed to prevent tissue damage. The mean of four PWL (2 left and 2 right) measured at 3–5 min intervals was recorded for each mouse. Pain assays were performed in Plexiglas boxes (10 × 10 × 18 cm) following a 40 min habituation period.
On post-natal day 21, motor coordination assays were performed in a rotating pole test [23] (55 inch length, 1.6 inch diameter) set at a height of 46 inches from the floor, with a rotating speed of 5.2 rotations/minute. The total distance walked by the mice on the pole was measured. A total of 5 non-consecutive trials per mouse were performed and a cut off time of 60 seconds was set per mouse in each trial. The following rating scale was used, as adapted to that previously described by Mattiasson et al. [23]: 0 = 0 to 2 inches or 0 to 2 seconds; 1 = 2 to 11 inches or 2 to 12 seconds; 2 = 11 to 22 inches or 12 to 24 seconds; 3 = 22 to 33 inches or 24 to 36 seconds; 4 = 33 to 44 inches or 36 to 48 seconds and 5 = 44 to 55 inches or 48 to 60 seconds. In this scale, therefore, “0” represents the poorest motor coordination, and “5” represents the best motor coordination. All testing was performed under “blind” conditions (i.e., genotyping of mice was performed after behavioral testing was completed) between 07:00 and 12:00.
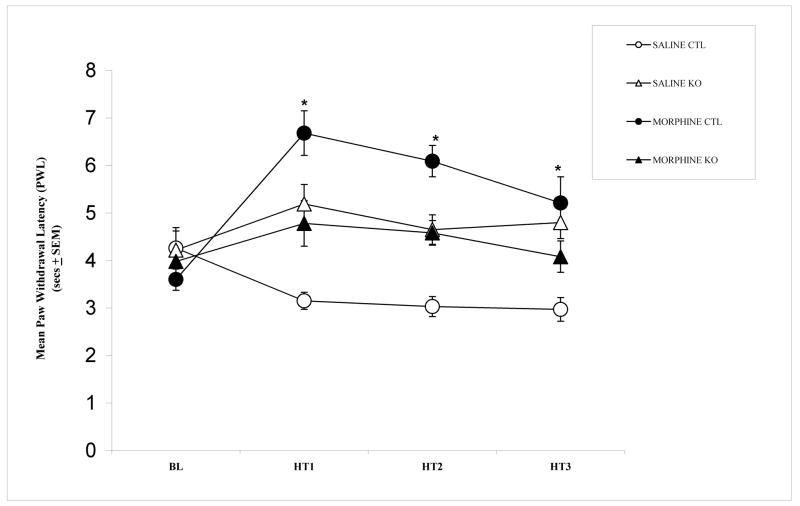
Figure 1 shows the mean PWL after administration of morphine or saline. Repeated measures analysis of variance (ANOVA) revealed significant differences between groups (F (3,36) = 12.855, p < 0.001). Post-hoc Tukey HSD comparisons showed that morphine treatment produced significant analgesic effect (i.e., increase PWL) compared to vehicle treatment, in CTL mice (p < 0.05). By contrast, in KO mice, there were no differences between morphine and vehicle treatment (p > 0.05). However, both KO groups (morphine and saline) showed higher latencies than CTL saline group after treatment administration (p < 0.05). A possible explanation for this is that some specific factors of KO mice alter their response to repeated thermal stimulation; it seems these factors are limited to repeated thermal stimulation since no differences in PWL exists in basal conditions (check also PWL in reference [31]). Nevertheless, the latency of morphine KO mice is still significantly lower than CTL morphine mice (p < 0.05) suggesting incomplete expression of morphine analgesia in morphine KO mice. Taken together, these results suggest that cortical NMDA receptors are critically involved in mediating the analgesic effects of morphine.
Figure 1.
Paw withdrawal latency (PWL) to thermal stimuli in cortex-specific NR1 knockout (KO) and genetic control (Ctrl) after vehicle or morphine treatment. Data are expressed as mean time (sec ± SEM) of PWL after application of a heat stimulus to the hindpaw. Significant differences were found between CTL group that received morphine and CTL group that received saline (p < 0.05). KO mice that received saline did not differ of KO mice that received morphine (p = 0.141). (Between subjects effects: F (3, 36) = 12.855, p = 0.000). Sample per groups: CTL saline (n = 13, 12 males and 1 female); KO saline (n = 7, 3 males and 4 females); CTL morphine (n = 10, 10 males) and KO morphine (n = 10, 5 males and 5 females).
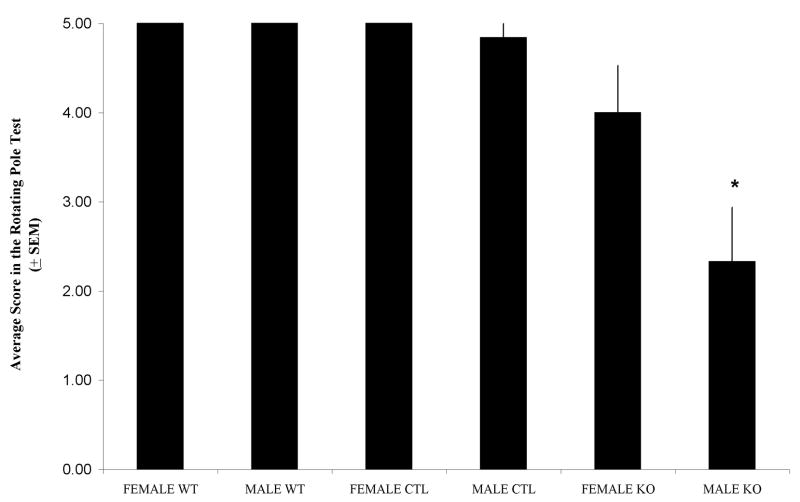
Figure 2 shows the mean of rotating pole scores. Analysis of Variance (ANOVA) revealed significant differences in motor coordination scores between male KO and the rest of the groups (F (5, 70) = 13.542, p < 0.001) suggesting sex-related differences in motor abilities may exist in the knock-outs.
Figure 2.
Average scores in the rotating pole test (score ± SEM). Significant differences were found between male KO and the rest of the experimental groups. The rest of the groups did not differ from each other (F (5, 70) = 13.452, p < 0.001). Sample per groups: Female WT (n = 10), Male WT (n = 12), Female CTL (n = 11), Male CTL (n = 25), Female KO (n = 7) and Male KO (n = 6).
The transgenic mouse model used in this study has a knocking out of the NR1 subunit of the NMDA receptor, which is an essential functional subunit of the NMDA receptor [10]. As a consequence, these mice display non-functional NMDA receptors in the cerebral cortex, hippocampus and olfactory bulb, whereas NMDA receptors in the rest of the brain are fully functional and are expressed at normal levels [12]. The decrease in morphine analgesia found in the KO mice used in this study is consistent with others reports using pharmacological blockade of the NMDA receptor for morphine analgesia. For instance, dextromethorphan (a low affinity and non competitive antagonist) attenuates morphine analgesia at high dose, and the polyamine NR2B antagonist Ro 25-6981 attenuates morphine analgesia at all doses examined [27]. However, the present results also differ from other studies that evaluated the effect of NR2B receptor antagonism in morphine analgesia [3,9]. Those studies showed that antagonism of NR2B receptors increase the analgesic effects of morphine on phasic pain tests [3,9]. It can be appreciated that there is a high degree of variability in the effects of NMDA antagonists on morphine analgesia, as some studies have shown potentiation, whereas other studies attenuation or no effect [3,9,19,20,27,32]. Possible factors that could explain the lack of consistency in this kind of study include species, strain, opioid agonist used, dose, type of nociceptive stimulus, behavioral test, dose of NMDA antagonist, or interaction between factors [27]. To our knowledge this is the first study showing that cortical NMDA receptors are necessary for the development of morphine analgesia.
Previous studies have demonstrated sex-dependent differences in NMDA involvement in pain and analgesia [15,16,25]. For example, Mogil et al. [25] reported that sexually mature male and female mice have similar levels of analgesia after swim stress (swim stress induced analgesia, SSIA) at 15°C; however, there are differences in the underlying mechanisms. Specifically, NMDA antagonists (dizocilpine; MK-801) blocked the analgesic response in male but not in female. However, sensitivity to dizocilpine/MK-801 reducing effects on analgesia was reinstated by ovariectomy, and after estrogen replacement the females recovered the dizocilpine/MK-801 insensitivity during analgesic response, suggesting that these sex-differences in NMDA involvement are hormonally mediated [25]. In the present study, sex differences in morphine analgesia were not explored because at the age of testing (post natal day 20), mice were not in the stage of sexual maturity and its hormonal correlates.
The present results in motor coordination, found only in male KO, are in agreement with previous studies in which pharmacological [4,5] and genetic [14,28,34] disruption of NMDA receptor function resulted in motor coordination deficits. For instance, genetic manipulation of other NMDA receptor parts like knocking out of NR3B subunits [28], double knocking out of NR2A and NR2C subunits [14] and truncation of C terminal domain of NR2A (NR2AΔC/ΔC) or NR2C (NR2CΔC/ΔC) subunits resulted in motor coordination deficits in the rotarod motor tests. In the present study, the deficits in motor coordination were not found in females. For our knowledge this is the first report demonstrating possible sex-related variation in the role of cortical NMDA receptors in motor coordination; the previous reports in the field were limited to males [28,34] or did not specify sex [4,5,14].
In the present study, the sex-related difference found can not be explained by activational effects of hormones, as the age of test (P21) was still far ahead of sexual maturity stage. A possible explanation of these sex-related differences could be linked to the organizational effects [2,17,30] of androgens or estrogens in motor coordination circuitry. Different studies have suggested neuroprotective effects of estrogens on motor activity [18,24], however these studies were limited to adult stage of development. Indeed, according to McEwen [24], estrogen supply can improve motor coordination in women that undergo post-menopausic estrogen decrease. The present study suggests this possibility, as no studies to date have reported sex-related differences in motor coordination explicitly caused by organizational effects.
It should be noted that the mouse model in the present study has a knockout condition restricted to cortex, hippocampus and olfactory bulb, in which the genetic modification is manifested from the beginning of the embryonic development. This model represents an advantage in comparison to the general NR1 knockout model, in which the mice die at postnatal day 0 (P0) [10,22]. Additionally, the model presented by South et al. [33] in which the genetic deletion is restricted in space and time represents a more precise approach that reduces the probability of non-controlled developmental deficits. In the future, it would therefore be helpful to perform a study disrupting cortical NMDA receptors, but adopting the South et al. approach [33]. Specifically, to employ mice in which the NR1 gene is flanked by flox sites combined with the administration of a vector (with inserted Cre recombinase) to cerebral cortex.
In conclusion, the present work proposes that NMDA receptors in the cerebral cortex are an important element in the generation of morphine analgesia under phasic pain conditions. Moreover, it suggests possible sex-related differences in variation in the role of cortical NMDA receptors in motor coordination.
Acknowledgments
This work was supported by National Institute of Health (NIH) NS 039050 (R.S.E.), LSUHSC Research Enhancement Fund, and IFARHU/SENACYT “Programa de Becas 2003 para formación de investigadores”, Republic of Panama. We thank Takuji Iwasato, PhD (RIKEN Brain Science Institute, Japan) for providing the transgenic mice and Ms. Barri King for breeding the mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barr GA. Maturation of the biphasic behavioral and heart rate response in the formalin test. Pharmacol Biochem Behav. 1998;60:329–335. doi: 10.1016/s0091-3057(97)00602-3. [DOI] [PubMed] [Google Scholar]
- 2.Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2006;146(4):1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 3.Bernardi M, Bertolini A, Szczawinska K, Genedani S. Blockade of the polyamine site of NMDA receptors produces antinociception and enhances the effect of morphine, in mice. Eur J Pharmacol. 1996;298:51–55. doi: 10.1016/0014-2999(95)00778-4. [DOI] [PubMed] [Google Scholar]
- 4.Carter AJ. Many agents that antagonize the NMDA receptor-channel complex in vivo also cause disturbances of motor coordination. J Pharmacol Exp Ther. 1994;269(2):573–580. [PubMed] [Google Scholar]
- 5.Carter AJ. Antagonists of the NMDA receptor-channel complex and motor coordination. Life Sci. 1995;57(10):917–929. doi: 10.1016/0024-3205(95)02027-g. [DOI] [PubMed] [Google Scholar]
- 6.Datwani A, Iwasato T, Itohara S, Erzurumlu RS. NMDA receptor-dependent pattern transfer from afferents to postsynaptic cells and dendritic differentiation in the barrel cortex. Mol Cel Neurosci. 2002a Nov 21;3:477–492. doi: 10.1006/mcne.2002.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datwani A, Iwasato T, Itohara S, Erzurumlu RS. Lesion-induced thalamocortical axonal plasticity in the S1 cortex is independent of NMDA receptor function in excitatory cortical neurons. J Neurosci. 2002b;22(21):9171–9175. doi: 10.1523/JNEUROSCI.22-21-09171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 9.Fischer BD, Carrigan KA, Dykstra LA. Effects of N-methyl-D-aspartate receptor antagonists on acute morphine-induced and l-methadone-induced antinociception in mice. J Pain. 2005;6:425–433. doi: 10.1016/j.jpain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Forrest D, Yuzaki M, Soares HD, Ng L, Luk DC, Sheng M, Stewart CL, Morgan JI, Connor JA, Curran T. Targeted disruption of NMDA receptor 1 gene abolishes NMDA response and results in neonatal death. Neuron. 1994;13:325–338. doi: 10.1016/0896-6273(94)90350-6. [DOI] [PubMed] [Google Scholar]
- 11.Hargreaves K, Dubner R, Brown C, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 12.Iwasato T, Datwani A, Wolf AM, Nishiyama I, Taguchi Y, Tonegawa S, Knopfel T, Erzurumlu RS, Itohara S. Cortex-restricted disruption of NMDAR1 impairs neuronal patterns in the barrel cortex. Nature. 2000;406:726–731. doi: 10.1038/35021059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacquet YF. The NMDA receptor: central role in pain inhibition in rat periaqueductal gray. Eur J Pharmacol. 1988;154(3):271–276. doi: 10.1016/0014-2999(88)90201-4. [DOI] [PubMed] [Google Scholar]
- 14.Kadotani H, Hirano T, Masugi M, Nakamura K, Nakao K, Katsuki M, Nakanishi S. Motor discoordination results from combined gene disruption of the NMDA receptor NR2A and NR2C subunits, but not from single disruption of the NR2A or NR2C subunit. J Neurosci. 1996;16:7859–7867. doi: 10.1523/JNEUROSCI.16-24-07859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kavaliers M, Choleris E. Sex differences in N-methyl-D-aspartate involvement in kappa opioid and non-opioid predator-induced analgesia in mice. Brain Res. 1997;768(1–2):30–36. doi: 10.1016/s0006-8993(97)00569-6. [DOI] [PubMed] [Google Scholar]
- 16.Kavaliers M, Colwell DD, Choleris E. Sex differences in opioid and N-methyl-D-aspartate mediated non-opioid biting fly exposure induced analgesia in deer mice. Pain. 1998;77(2):163–171. doi: 10.1016/S0304-3959(98)00092-X. [DOI] [PubMed] [Google Scholar]
- 17.Kimura D. Sex differences in the brain. Sci Am. 2002:32–37. doi: 10.1038/scientificamerican0992-118. [DOI] [PubMed] [Google Scholar]
- 18.Kipp M, Karakaya S, Pawlak J, Araujo-Wright G, Arnold S, Beyer C. Estrogen and the development and protection of nigrostriatal dopaminergic neurons: concerted action of a multitude of signals, protective molecules, and growth factors. Front Neuroendocrinol. 2006;27(4):3676–390. doi: 10.1016/j.yfrne.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Kozela E, Danysz W, Popik P. Uncompetitive NMDA receptor antagonists potentiate morphine antinociception recorded from the tail but not from the hind paw in rats. Eur J Pharmacol. 2001;423(1):17–26. doi: 10.1016/s0014-2999(01)01084-6. [DOI] [PubMed] [Google Scholar]
- 20.Kozela E, Popik P. The effects of NMDA receptor antagonists on acute morphine antinociception in mice. Amino Acids. 2002;23:163–168. doi: 10.1007/s00726-001-0123-5. [DOI] [PubMed] [Google Scholar]
- 21.Kutsuwada T, Sakimura K, Manabe T, Takayama C, Katakura N, Kushiya E, Natsume R, Watanabe M, Inoue Y, Yagi T, Aizawa S, Arakawa M, Takahashi T, Nakamura Y, Mori H, Mishina M. Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor epsilon 2 subunit mutant mice. Neuron. 1996;16(2):333–344. doi: 10.1016/s0896-6273(00)80051-3. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Erzurumlu RS, Chen C, Jhaveri S, Tonegawa S. Whisker-related neuronal patterns fail to develop in the trigeminal brainstem nuclei of NMDAR1 knockout mice. Cell. 1994;76:427–437. doi: 10.1016/0092-8674(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 23.Mattiasson GJ, Philips MF, Tomasevic G, Johansson BB, Wieloch T, McIntosh TK. The rotating pole test: evaluation of its effectiveness in assessing functional motor deficits following experimental head injury in the rat. J Neurosci Methods. 2000;95(1):75–82. doi: 10.1016/s0165-0270(99)00162-4. [DOI] [PubMed] [Google Scholar]
- 24.McEwen BS. The molecular and neuroanatomical basis for estrogen effects in the central nervous system. J Clin Endocrinol Metab. 1999;84(6):1790–1797. doi: 10.1210/jcem.84.6.5761. [DOI] [PubMed] [Google Scholar]
- 25.Mogil JS, Sternberg WF, Kest B, Marek P, Liebeskind JC. Sex differences in the antagonism of swim stress-induced analgesia: effects of gonadectomy and estrogen replacement. Pain. 1993;53(1):17–25. doi: 10.1016/0304-3959(93)90050-Y. [DOI] [PubMed] [Google Scholar]
- 26.Narita M, Kato H, Miyoshi K, Aoki T, Yajima Y, Suzuki T. Treatment for psychological dependence on morphine: usefulness of inhibiting NMDA receptor and its associated protein kinase in the nucleus accumbens. Life Sci. 2005;77:2207–2220. doi: 10.1016/j.lfs.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 27.Nemmani KVS, Grisel JE, Stowe JR, Smith-Carliss R, Mogil JS. Modulation of morphine analgesia by site-specific N-methyl-D-aspartate receptor antagonists: dependence on sex, site of antagonism, morphine dose, and time. Pain. 2004;109:274–283. doi: 10.1016/j.pain.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 28.Niemann S, Kanki H, Fukui Y, Takao K, Fukaya M, Hynynen MN, Churchill MJ, Shefner JM, Bronson RT, Brown RH, Jr, Watanabe M, Miyakawa T, Itohara S, Hayashi Y. Genetic ablation of NMDA receptor subunit NR3B in mouse reveals motoneuronal and nonmotoneuronal phenotypes. Eur J Neurosci. 2007;26(6):1407–1420. doi: 10.1111/j.1460-9568.2007.05774.x. [DOI] [PubMed] [Google Scholar]
- 29.Petrenko AB, Yamakura T, Baba H, Shimoji K. The role of N-methyl-D-Aspartate (NMDA) receptors in pain: a review. Anesth Analg. 2003;97:1108–1116. doi: 10.1213/01.ANE.0000081061.12235.55. [DOI] [PubMed] [Google Scholar]
- 30.Piek JP, Gasson N, Barrett N, Case I. Limb and gender differences in the development of coordination in early infancy. Hum Mov Sci. 2002;21:621–639. doi: 10.1016/s0167-9457(02)00172-0. [DOI] [PubMed] [Google Scholar]
- 31.Quintero GC, Erzurumlu RS, Vaccarino AL. Decreased pain response in mice following cortex-specific knockout of the N-methyl-D-aspartate NR1 subunit. Neurosci Lett. 2007;425:89–93. doi: 10.1016/j.neulet.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Redwine KE, Trujillo KA. Effects of NMDA receptor antagonists on acute mu-opioid analgesia in the rat. Pharmacol Biochem Behav. 2003;76(2):361–372. doi: 10.1016/j.pbb.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 33.South SM, Kohno T, Kaspar BK, Hegarty D, Vissel B, Drake CT, Ohata M, Jenab S, Sailer AW, Malkmus S, Masuyama T, Horner P, Bogulaysky J, Gage FH, Yaksh TL, Woolf CJ, Heinemann SF, Inturrisi CE. A conditional deletion of the NR1 subunit of the NMDA receptor in adult spinal cord dorsal horn reduces NMDA current and injury-induced pain. J Neurosci. 2003;23(12):5031–5040. doi: 10.1523/JNEUROSCI.23-12-05031.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sprengel R, Suchanek B, Amico C, Brusa R, Burnashev N, Rozov A, Hvalby Ø, Jensen V, Paulsen O, Andersen P, Kim JJ, Thompson RF, Sun W, Webster LC, Grant SGN, Eilers J, Konnerth A, Li J, McNamara JO, Seeburg PH. Importance of the intracellular domain of NR2 subunits for NMDA receptor function in vivo. Cell. 1998;92:279–289. doi: 10.1016/s0092-8674(00)80921-6. [DOI] [PubMed] [Google Scholar]
- 35.Teng CJ, Abbott FV. The formalin test: a dose-response analysis at three developmental stages. Pain. 1998;76:337–347. doi: 10.1016/S0304-3959(98)00065-7. [DOI] [PubMed] [Google Scholar]