Abstract
Objective
To analyse antiphospholipid (aPL) antibody‐positive patients using the 2006 revised antiphospholipid syndrome (APS) classification criteria.
Methods
A descriptive study of 200 aPL‐positive patients identified in a local, hospital‐based registry, analysing demographic, clinical and aPL characteristics. Patients were analysed for (1) fulfilment of the 1999 original (Sapporo) and 2006 revised APS classification criteria; (2) non‐criteria aPL features (for all aPL‐positive patients, based on the 2006 revised criteria definitions); and (3) non‐aPL thrombosis risk factors at the time of the clinical events (for patients with APS, based on the 2006 revised criteria stratifications).
Results
Of the 200 patients, 183 patients had sufficient data for analysis. Of these, 39 (21%) patients did not meet the laboratory requirement of the original 1999 criteria. Of 81 patients with APS who met the 1999 classification criteria, 47 (58%) also met the 2006 revised criteria. Of 63 asymptomatic (no vascular or pregnancy events) aPL‐positive patients who met the laboratory requirement of the 1999 classification criteria, 38 (60%) also met the laboratory requirement of the 2006 revised criteria. More than 50% of the patients with APS with vascular events had identifiable non‐aPL thrombosis risk factors at the time of clinical events.
Conclusions
Only 59% of the patients meeting the 1999 APS Sapporo classification criteria met the 2006 APS classification criteria. The revised criteria will have positive implications in APS research by way of limiting the inclusion of a heterogeneous group of patients and also by way of providing a risk‐stratified approach.
The original classification criteria for antiphospholipid syndrome (APS) were formulated at a workshop in Sapporo, Japan, in 1998, during the Eighth International Congress on antiphospholipid antibodies (aPLs), and subsequently published in 1999.1 The Sapporo Criteria, as they are often called, were revised at another workshop in Sydney, Australia, in 2004, during the Eleventh International Congress on aPL, and published as a consensus statement in 2006.2 The revised APS classification criteria provide a more uniform basis for selecting patients for APS research by emphasising risk stratification. As with the original classification criteria, at least one clinical (vascular thrombosis or pregnancy morbidity) and one laboratory (aPL) criterion had to be met for the diagnosis of APS.
The clinical criterion remains mostly unchanged, except for inclusion of transient cerebral ischaemia and stroke as forms of vascular thrombosis.3 The revised APS classification criteria strongly recommend investigating coexisting inherited and acquired thrombosis risk factors in patients with APS, especially in patients who are included in clinical trials. These non‐aPL thrombosis risk factors include, but are not limited to, traditional cardiovascular risk factors, inherited thrombophilias, oral contraceptive use, surgery, malignancy and nephrotic syndrome.
The laboratory criterion is substantially modified in the revised classification criteria. Anticardiolipin (aCL) antibodies and lupus anticoagulant (LA) test are required to be positive on ⩾2 occasions at least 12 weeks apart, as opposed to 6 weeks apart in the original criteria. Whereas in the original version aCL IgG/M must be present in medium or high titre, in the revised criteria “medium or high titre” is more specifically defined as IgG/M titres of ⩾40 U or ⩾99th centile. Further, the revised classification criteria include anti‐β2‐glycoprotein‐I (aβ2GPI) antibody IgG/M isotype as a valid laboratory requirement if titres are ⩾99th centile, on more than two occasions 12 weeks apart. The consensus statement suggests avoiding classification of APS “if less than 12 weeks or more than 5 years separate the positive aPL tests and the clinical manifestation”.2 In addition to the APS classification criteria revision, the consensus paper provides specific definitions for commonly associated clinical manifestations of APS—namely, livedo reticularis, cardiac valve disease, thrombocytopenia and nephropathy.
Given that the revised criteria provide a new classification paradigm, the primary objective of this study was to analyse aPL‐positive patients using the revised Sapporo APS classification criteria. Secondarily, based on the definitions and stratifications outlined in the recent consensus statement,2 non‐criteria aPL features and non‐aPL thrombosis risk factors at the time of vascular events were also analysed.
Methods
In this descriptive study, 200 aPL‐positive patients (with or without APS diagnosis) were selected from a local, hospital‐based registry, which is primarily constituted of two databases at our institution: (1) the national Antiphospholipid Syndrome Collaborative Registry (APSCORE)4 and (2) the Asymptomatic (no vascular/pregnancy events) aPL‐positive Registry (APLASA).5 The inclusion criterion for these databases was positive aPL (LA) test, aCL IgG/M/A and/or aβ2GPI IgG/M/A) on two occasions with or without APS diagnosis. Although APSCORE is a national database, only patients from Hospital for Special Surgery, New York, USA were included in this study.
Patients were classified into three groups: vascular events (VE) with/without pregnancy morbidity (PM); PM alone; and asymptomatic (no VE/PM) aPL‐positive patients. Patient demographics, aPL‐related clinical manifestations and aPL profile (the results and testing dates of LA test, aCL ELISA and aβ2GPI ELISA) were systematically reviewed. Each group was analysed for: (1) fulfilment of the 1999 original (Sapporo) and 2006 revised APS classification criteria (or, in the case of the asymptomatic patients, fulfilment of the 1999 and 2006 laboratory criteria); (2) non‐criteria aPL features (for all aPL‐positive patients, based on the 2006 revised criteria definitions); and (3) non‐aPL thrombosis risk factors at the time of clinical events (for patients with APS, based on the 2006 revised criteria stratifications).
Patients who met the 2006 revised criteria were stratified into newly recommended categories based on aPL profiles, defined as: group I—more than one laboratory criterion present (any combination); group IIa—LA present alone; group IIb—aCL present alone; and group IIc—aβ2GPI present alone. If patients met only one of the classification criteria, either the 1999 original or 2006 revised, reasons for why they did not meet the other were defined. Patients who met pregnancy morbidity criteria were also stratified into the recommended categories.
We also analysed non‐criteria aPL features outlined in the revised classification criteria—namely, livedo reticularis, cardiac valve disease, thrombocytopenia, nephropathy, neurological manifestations, IgA aCL, IgA aβ2GPI, antiphosphatidylserine antibodies, antiphosphatidylethanolamine antibodies, antiprothrombin antibodies and antiphosphatidylserine‐prothrombin antibodies.
Thrombosis risk factors at the time of either the vascular or the pregnancy events were recorded wherever identifiable. These risk factors were inherited thrombophilias with documented laboratory confirmation (factor V Leiden mutation, methyltetrahydrofolate reductase mutation, prothrombin 20 210A mutation, protein C/S deficiencies and antithrombin III deficiency), other risk factors for hypercoagulability (oral contraceptive/hormone replacement use, malignancy and renal failure) and traditional cardiovascular risk factors (hypertension, diabetes mellitus, high low‐density lipoprotein or low high‐density lipoprotein cholesterol, smoking and family history of premature cardiovascular disease).
Results
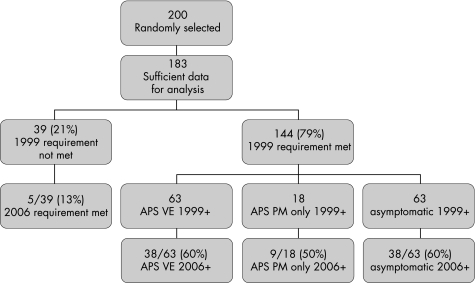
Out of 200 aPL‐positive patients, 183 patients had sufficient data for analysis (fig 1). More than 90% of the 183 patients were women, and over 50% had other coexisting systemic autoimmune diseases (table 1).
Figure 1 Distribution of antiphospholipid antibody‐positive patients who fulfil the 1999 and/or 2006 criteria: APS, antiphospholipid syndrome; VE, vascular event with or without PM; PM, pregnancy morbidity; 1999+, 1999 original APS classification criteria met. 2006+, 2006 revised APS classification criteria met.
Table 1 Demographics of persistently antiphospholipid antibody‐positive patients (n = 183).
| VE with or without PM n = 82 (%) | PM only n = 19 (%) | Asymptomatic n = 82 (%) | |
|---|---|---|---|
| Gender | |||
| Female | 70 (85) | 19 (100) | 75 (91) |
| Race | |||
| White | 68 (83) | 16 (84) | 69 (84) |
| Black | 5(6) | 1(5) | 4 (5) |
| Hispanic | 7 (9) | 2 (11) | 8 (10) |
| Asian | 2 (2) | 0 | 1 (1) |
| Systemic AI diseases | |||
| None | 47 (57) | 9 (47) | 25 (30) |
| SLE | 28 (34) | 5 (3) | 35 (43) |
| Other | 7(9) | 5 (3) | 22 (27) |
AI, autoimmune; PM, pregnancy morbidity; SLE, systemic lupus erythematosus; VE, vascular event.
Of the 183 patients, 39 (21%) patients did not meet the laboratory requirement of the 1999 original classification criteria, for the following reasons: (1) positive aCL IgA only (n = 9); (2) positive aCL IgG/M only in the range of 10–20 U (n = 17); (3) two positive aPL, but not within the recommended time frame of >6 weeks apart (n = 3); (4) single positive LA test and negative aCL, with strong clinical suspicion for APS (n = 5); and (5) isolated aβ2GPI positivity (n = 5).
Of the 183 patients, 144 (79%) patients met the laboratory requirement for the 1999 original classification criteria (fig 1). Of these, 81 were patients with APS (63 in the VE with or without PM group and 18 in the PM only group) and 63 were asymptomatic. Of the 81 patients with APS, 47 (58%) patients also met the revised 2006 classification criteria. When these patients were stratified according to the recommended subgroups, over half of them were in subgroup I, fulfilling more than one of the laboratory requirements (table 2). Also, 34 (42%) patients did not fulfil the revised 2006 classification criteria, because of the following new requirements: (1) aCL medium‐to‐high titre cut‐off ⩾40 U (n = 15); (2) two positive aPL, but not within the recommended time frame of >12 weeks apart (n = 2); and (3) >5 years time gap between the aPL test and the clinical event (n = 17). Patients with pregnancy morbidities were categorised in the proposed subgroups (table 3). Of note, there were 15 patients in the VE with or without PM group who had pregnancy morbidity, and were mostly stratified to subgroup “B” with one or more premature births of a morphologically normal neonate before the 34th week of gestation, because of either eclampsia or placental insufficiency.
Table 2 2006 Revised antiphospholipid syndrome classification criteria subgroups based on antiphospholipid antibody positivity (n = 90).
| Subgroups | VE with or without PM n = 42 (%) | PM only n = 9 (%) | Asymptomatic n = 39 (%) |
|---|---|---|---|
| I: more than one aPL | 25 (60) | 5 (56) | 15 (38) |
| IIa: LA alone | 1 (2) | 1 (11) | 2 (5) |
| IIb: aCL alone | 12 (28) | 3 (33) | 21 (54) |
| IIc: aβ2GPI alone | 4 (10) | 0 | 1 (3) |
aCL, anticardiolipin; aβ2GPI, anti‐β2‐glycoprotein‐I antibodies; aPL, antiphospholipid; LA, lupus anticoagulant; PM, pregnancy morbidity; VE, vascular event.
Table 3 2006 Revised antiphospholipid syndrome classification criteria for pregnancy morbidity subgroups.
| Subgroups | VE with or without PM n = 15 (%) | PM only n = 19 (%) | |
|---|---|---|---|
| (A) One or more unexplained deaths of a morphologically normal neonate, or beyond the 10th week of gestation, with normal fetal morphology documented by ultrasound or by direct examination of the fetus | 6 (40) | 9 (47) | |
| (B) One or more premature births of a morphologically normal neonate before the 34th week of gestation because of: (1) eclampsia or severe pre‐eclampsia defined according to standard definition or (2) recognised features of placental insufficiency | 9 (60) | 7 (37) | |
| (C) Three or more unexplained consecutive spontaneous abortions before the 10th week of gestation, with maternal anatomic or hormonal abnormalities and paternal and maternal chromosomal causes excluded | 0 | 3 (16) |
PM, pregnancy morbidity; VE, vascular event.
In the group of 63 asymptomatic patients who met the 1999 original classification laboratory criterion, 38 (60%) patients fulfilled the laboratory requirement of the revised 2006 criteria (fig 1). Over 50% of the patients who met the laboratory requirement of the revised criteria were in subgroup IIb (aCL present alone; table 2). Of 63 patients, 25 (40%) did not fulfil the 2006 revised classification criteria, because of (1) aCL medium‐to‐high titre cut‐off ⩾40 U (n = 24) and (2) two positive aPL, but not within the recommended time frame of >12 weeks apart (n = 1).
Non‐criteria aPL features of 183 patients are shown in table 4. Approximately 25% of the VE with or without PM group with echocardiograms had aPL‐associated cardiac valve disease as defined by the new guidelines. Livedo reticularis was the most common non‐criteria aPL feature, which was identified most commonly in patients with vascular events and least commonly in asymptomatic patients.
Table 4 Features associated with antiphospholipid (aPL) syndrome but not included in the revised classification criteria (non‐criteria aPL features) (n = 183).
| Non‐criteria features | VE with or without PM n = 82 (%) | PM only n = 19 (%) | Asymptomatic n = 82 (%) |
|---|---|---|---|
| Livedo reticularis | 16/82 (20) | 3/19 (16) | 3/82 (4) |
| Cardiac valve disease* | 11/44 (25) | 0/6 (0) | 2/28 (7) |
| Thrombocytopenia | 11/82 (13) | 0/19 (0) | 5/82 (6) |
| Nephropathy | 5/82 (6) | 1/19 (5) | 1/82 (1) |
| Positive aCL IgA† | 3/82 (4) | 2/19 (11) | 13/82(16) |
| Positive aβ2GPI IgA† | 1/53 (2) | 0/6 (0) | 3/46 (7) |
| Other aPL‡ | 5/6 (83) | 0/0 | 1/6 (17) |
aCL, anticardiolipin; aβ2GPI, anti‐β2‐glycoprotein‐I; aPL, antiphospholipid; PM, pregnancy morbidity; VE, vascular event.
*Based on 78 patients with echocardiogram reports.
†Titres above laboratory normal limits.
‡Titres above laboratory normal limits based on 12 patients who were tested for antiprothrombin antibody, antiphosphatidylserine antibody, antiphosphatidylserine‐prothrombin antibody or antiphosphatidylethanolamine antibody.
The most commonly identifiable thrombosis risk factor in the VE with or without PM group was smoking, followed by oral contraceptives and hormone replacement therapy (table 5). In this group, over 50% of the patients had at least one coexisting thrombosis risk factor at the time of the vascular event. In all, 45%, 4% and 5% of the patients had 1, 2 and 3 coexisting non‐aPL thrombosis risk factors at the time of the event, respectively. In the PM only group, besides pregnancy itself as a thrombosis risk factor, a single thrombosis risk factor at the time of pregnancy morbidity was identified in 16% of the patients.
Table 5 Coexisting inherited or acquired factors for thrombosis.
| Thrombosis risk factor | VE with or without PM n = 82 | PM only n = 19 |
|---|---|---|
| Oral contraceptive pills or hormone replacement therapy | 12 (15%) | 0 |
| Hypertension | 7 (9%) | 0 |
| Smoking | 13 (16%) | 1 (5%) |
| Surgery/immobilisation | 5 (6%) | 0 |
| Cholesterol* | 6 (7%) | 0 |
| Malignancy | 3(4%) | 0 |
| Postpartum | 3 (4%) | 0 |
| Other coagulopathy | ||
| FVL mutation | 1/21 (5%) | 0/3 |
| PT mutation | 3/19 (16%) | 0/2 |
| PC/S deficiency | 0/21 | 0/3 |
| AT3 deficiency | 1/19 (5%) | 0/3 |
| MTHFR mutation | 5/13(38%) | 2/4 (50%) |
| Renal failure | 1(1%) | 0 |
AT3, antithrombin III; FVL, factor V Leiden; MTHFR, methyltetrahydrofolate reductase; PC/S, protein C/protein S; PM, pregnancy morbidity; PT, prothrombin 20210A gene; VE, vascular events.
*Cholesterol: elevated low‐density lipoprotein or low high‐density lipoprotein
Discussion
This descriptive study, in which we analysed aPL‐positive registry patients using the 2006 revised APS classification criteria, demonstrates that the revised criteria are more stringent than the original version. The study also supports the consensus statement's emphasis on the importance of the identification of the non‐criteria aPL features and non‐aPL thrombosis risk factors in patients with APS.
Of the 144 aPL‐positive patients who met the laboratory requirement of the 1999 original classification criteria, only 59% met the laboratory requirement of the 2006 revised classification criteria, resulting in a more selective aPL‐positive patient population. Thus, we believe that the revision of the original APS classification criteria is a step towards formulating a comprehensive, yet selective and risk‐stratified framework for APS research.
Five patients in the study did not meet the 1999 original classification criteria, but did meet the revised 2006 classification criteria, because of isolated aβ2GPI positivity; four of these patients had APS and one was asymptomatic. The design of this study did not allow us to determine whether the inclusion of the aβ2GPI in the revised criteria would increase the overall number of patients who fulfil the APS classification. However, in another retrospective study of 107 patients by Pourrat et al,6 there was a 6% increase in the number of patients who met the revised criteria, when compared with the 1999 version, where persistent aβ2GPI antibody titres were taken into account.
Definitions for the non‐aPL features are new to the revised 2006 criteria, with the hope that their clinical and prognostic significance will be better defined in future APS research. In this cohort, non‐aPL features were more common in patients with APS with vascular events. The definitions for the non‐aPL features are based on expert consensus, and for the most part were consistent with usual clinical practice. However, we found the definition for aPL‐associated cardiac valve disease quite rigorous, as the interpretations of the echocardiograms are to be carried out by two expert echocardiographers. As this study was performed at an academic centre, echocardiogram reports that were evaluated by both a fellow and an attending physician were considered adequate.
Almost half of the patients with vascular events had at least one identifiable non‐aPL thrombosis risk factor at the time of their vascular events. This is comparable with the study by Giron‐Gonzalez et al,7 which found that half of the patients with APS had other non‐aPL thrombosis risk factors at the time of their events. Thus, this study further supports the “second hit”8 hypothesis and the important role of non‐aPL thrombosis risk factors in the development of thrombosis in aPL‐positive patients. We believe that the identification of the thrombosis risk profile, as strongly encouraged by the revised classification criteria, will help better risk stratify aPL‐positive patients in both research and clinical practice.
This study is limited by the usual constraints of a retrospective data analysis, but, as the patients were part of the aPL/APS registries at an academic institution, detailed information, including laboratory data, was available for analysis. The selection bias could not be excluded, as the study population was (1) composed of aPL‐positive patients who agreed to participate in aPL/APS registries in one academic centre and (2) under‐represented for certain ethnic groups. The inclusion criteria of aPL/APS registries were mostly based on aCL and/or LA positivity; thus, the contribution of the newly added “aβ2GPI positivity” in APS diagnosis could not be determined accurately. Finally, the inclusion of asymptomatic aPL‐positive patients who did not fulfil the APS classification criteria could be viewed as a limitation; however, we believe that inclusion of these asymptomatic patients provided useful information, as these patients are relatively common in clinical practice. Also, given that most changes in the criteria are regarding laboratory requirements, analysis of the laboratory data of asymptomatic aPL‐positive patients provided pertinent information.
In summary, we believe that the revised version of the classification criteria will have positive implications in APS research by limiting the inclusion of a heterogeneous group of patients and providing a risk‐stratified approach for evaluating them. Furthermore, although the APS classification criteria are not meant for clinical purposes, they are the best available tool to avoid overdiagnosis of APS in clinical practice.
Acknowledgements
This study was partially supported by the New York Chapter of the Arthritis Foundation, the New York Community Trust, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Mary Kirkland Center for Lupus Research and the Barbara Volcker Center for Women and Rheumatic Disease.
Abbreviations
aCL - anticardiolipin
aβ2GPI - anti‐β2‐glycoprotein‐I
aPL - antiphospholipid
APS - antiphospholipid syndrome
LA - lupus anticoagulant
Footnotes
Competing interests: None declared.
References
- 1.Wilson W A, Gharavi A E, Koike T, Lockshin M D, Branch D W, Piette J C.et al International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthritis Rheum 1999421309–1311. [DOI] [PubMed] [Google Scholar]
- 2.Miyakis S, Lockshin M D, Atsumi T, Branch D C, Brey R L, Cervera R.et al International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 20064295–306. [DOI] [PubMed] [Google Scholar]
- 3.Brey R L, Chapman J, Levine S R, Ruiz‐Irastorza G, Derksen R H W M, Khamashta M.et al Stoke and the antiphospholipid syndrome: consensus meeting Taormina 2002. Lupus 200312508–513. [DOI] [PubMed] [Google Scholar]
- 4.Roubey R A S, Buxton G, APSCORE Investigators The Antiphospholipid Syndrome Collaborative Registry (APSCORE): report on the first 546 subjects (abstract). Arthritis Rheum 200450S640 [Google Scholar]
- 5.Erkan D, Sammaritano L, Levy R, Harrison M J, Peterson M, Yazici Y.et al APLASA study update: primary thrombosis prevention in asymptomatic APL (+) patients with aspirin (ASA) [abstract]. Thromb Res 2004114P618 [Google Scholar]
- 6.Pourrat O, Jollit C, Gombert J, Boinot C, Pierre F. Clinical relevance of the recent update of the classification criteria for definite antiphospholipid syndrome: an obstetric medicine clinic series of 107 patients. J Thromb Haemost 200642276–2277. [DOI] [PubMed] [Google Scholar]
- 7.Giron‐Gonzalez J A, Garcia del Rio E, Rodriguez C, Rodriguez‐Martorell J, Serrano A. Antiphospholipid syndrome and asymptomatic carriers of antiphospholipid antibody: prospective analysis of 404 individuals. J Rheumatol 2004311560–1567. [PubMed] [Google Scholar]
- 8.Erkan D, Lockshin M D. What is antiphospholipid syndrome? Curr Rheumatol Rep 20046451–457. [DOI] [PubMed] [Google Scholar]