Abstract
Cardioprotection and preconditioning mediated via G-protein-coupled receptors may be lost or impaired with advancing age, limiting ischemic tolerance and the ability to pharmacologically protect older hearts from ischemic injury. Our preliminary findings indicated a loss of δ-opioid receptor-mediated protection in aged vs. young mouse hearts, which may involve alterations in protective kinase signaling. In the present study, we tested the hypothesis that aging-related loss of opioid-triggered cardioprotection involves failure to activate p38 MAPK and its distal signaling targets. Langendorff-perfused hearts from young (10–14 weeks) or aged (24–26 months) C57 mice underwent 25-min ischemia and 45-min reperfusion in the presence or absence of 1 µmol/l DPDPE (δ-opioid agonist) or 1 µmol/l anisomycin (activator of p38 MAPK), and functional recovery and protein activation/phosphorylation were assessed. Contractile recovery was similar in untreated young and aged hearts (50±2% and 53±5%, respectively), and was enhanced by DPDPE in young hearts only (67±3%). Immunoblot analysis revealed that DPDPE comparably activated or phosphorylated GRK2, Akt, ERK1/2 and p70S6 kinase in young and aged hearts, whereas aging abrogated the stimulatory effects of DPDPE on p38 MAPK and HSP27. Treatment with anisomycin elicited comparable activation of p38 MAPK and HSP27 in both young and aged hearts, coupled with a pronounced and equivalent cardioprotection in the two groups (73±3% and 77±2%, respectively), an effect abolished by the p38 MAPK inhibitor, SB203580. These data indicate that aging-related loss of δ-opioid-mediated cardioprotection involves failure to activate p38 MAPK and HSP27. Direct targeting of this pathway elicits comparable protection in both age groups.
Keywords: Aging, Preconditioning, Signal transduction
1. Introduction
Ischemic heart disease is a leading cause of death and disability in developed countries, and is the single leading cause of death in those over 65 years. It is well documented that, experimentally, aged myocardium possesses a reduced intrinsic resistance to injury associated with ischemia–reperfusion [1–3]. In addition to the anatomical, mechanical, and biochemical alterations that may be responsible for ischemic intolerance in senescent hearts [4,5], there may also be a deficiency in endogenous cardioprotective responses. For example, the ischemic intolerance observed in aged animals may be attributed to dysfunction of post-receptor signaling pathways [6]. We, and others, have reported that preconditioning and other protective stimuli are ineffective in aged or senescent rodent hearts [7–10], while clinical equivalents of preconditioning also appear to be of limited benefit in aged subjects [11,12].
In recent years it has become clear that opioid receptors are involved in triggering acute and delayed cardioprotection or preconditioning [13–15]. Such a response may be amenable to therapeutic manipulation, providing benefit in hearts at risk of ischemia–reperfusion injury. However, very little is known regarding alterations to opioids and their signaling pathways in myocardium of older patients, which are most likely to suffer ischemic events and require therapeutic intervention. Curiously, opioid peptides, along with their message, have been shown to increase in aging hearts and in certain disease states [16]. Research in nervous tissue indicates shifts in opioid receptor sensitivity with age [17–19], which do not appear to involve changes in receptor density [20]. In investigating cardiac opioid responses, we recently found that acute opioid receptor-mediated cardioprotection is lost with aging, whereas delayed cardioprotection during chronic morphine exposure is retained in both young and aged hearts [7].
In the present study, we sought to identify the site(s) of failure in protective signaling underlying loss of acute opioid-induced protection in aged myocardium. We specifically focused on activation/phosphorylation of GRK2, Akt, p70s6 kinase, ERK1/2, p38 MAPK and HSP27, all of which have been implicated in cardioprotection. Given some degree of commonality in protective signaling for GPCR-triggered protective responses and preconditioning responses [21,22], abnormalities identified may be applicable to the more general observation of impaired protective responses (and intrinsic tolerance) in aged myocardium.
2. Methods
This investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Hearts were isolated from fed male wild-type C57/BL6 mice (young: 10–14 weeks; aged: 24–26 months).
2.1. Perfused heart preparation
Mice were anesthetized with 60 mg/kg sodium pentobarbital administered intraperitoneally, and perfused as described previously [23]. After anesthesia, a thoracotomy was performed and hearts rapidly excised into ice-cold perfusion buffer. The aorta was cannulated and the coronary circulation of all hearts perfused in a Langendorff mode at a constant pressure of 80 mm Hg with modified Krebs–Henseleit buffer containing (in mmol/l): NaCl, 120; NaHCO3, 25; KCl, 4.7; CaCl2, 2.5; MgCl2 1.2; KH2PO4 1.2, d-glucose, 15; and EDTA, 0.5. The perfusate was equilibrated with 95% O2, 5% CO2 at 37 °C to give a pH of 7.4 and PO2 of ~600 mm Hg at the tip of the aortic cannula over a 1–5 ml/min flow range. All perfusate delivered to the heart was passed through an in-line 0.22 Sterivex-HV filter cartridge (Millipore, Bedford, MA, USA) to continuously remove micro-particulates. The left ventricle was vented with a polyethylene apical drain and hearts instrumented for functional measurements as described below. They were then immersed in warmed perfusate inside a water jacketed bath maintained at 37 °C. The temperature of the perfusion fluid was monitored by a needle thermistor at the entry into the aortic cannula, and the temperature of the water bath was assessed using a second thermistor probe. Temperature was recorded using a 3-channel Physitemp TH-8 digital thermometer (Physitemp Instruments Inc., Clifton, NJ, USA).
For the assessment of isovolumic function, fluid-filled balloons constructed of polyvinyl chloride plastic film were inserted into the left ventricle via the mitral valve. Balloons were connected to a P23 XL pressure transducer (Viggo-Spectramed, Oxnard, CA, USA) by fluid-filled polyethylene tubing permitting continuous measurement of left ventricular pressure. Balloon volume was increased to an end-diastolic pressure of 5 mm Hg. Coronary flow was monitored via a cannulating Doppler flow-probe (Transonic Systems Inc., Ithaca, NY, USA) placed in the aortic perfusion line and connected to a T206 flowmeter (Transonic Systems Inc., Ithaca, NY, USA). All functional data were recorded at 1 kHz on an 8/s MacLab data acquisition system (ADInstruments, Castle Hill, Australia) connected to an Apple 7300/180 computer. The ventricular pressure signal was digitally processed to yield peak systolic pressure, diastolic pressure, +dP/dt, −dP/dt, and heart rate.
Hearts were excluded from the study after the initial 20-min stabilization period if they met one of the following functional criteria: (i) coronary flow >5 ml/min (near maximal dilation, or an aortic tear), (ii) unstable (fluctuating) contractile function, (iii) left ventricular systolic pressure below 100 mm Hg, or (iv) significant cardiac arrhythmias. After 20-min stabilization at the intrinsic heart rate all hearts were switched to ventricular pacing at 420 beats/min (Grass S9 stimulator, Quincy, MA, USA) for a further 10 min.
3. Experimental protocol
3.1. Ischemia–reperfusion studies
After making baseline measurements, hearts were subjected to 25 min of ischemia followed by 45 min of normoxic reperfusion. Pacing was terminated upon initiation of ischemia and resumed after 1.5 min of reperfusion in all groups. Infusion of either saline, DPDPE, anisomycin, SB203580, or anisomycin with SB203580 was commenced 10 min prior to ischemia. Infusions were stopped on induction of ischemia and resumed at the onset of reperfusion and continued throughout the 45-min reperfusion period.
3.2. Signaling studies in normoxic hearts
In order to identify and contrast the effects of opioid receptor activation and p38 MAPK activation on key signaling elements, hearts perfused under normoxic conditions were treated for 10 min with either DPDPE (1 µmol/l), anisomycin (1 µmol/l) or saline. At the end of these incubation times the hearts were immediately frozen in liquid nitrogen and stored at −80 °C for subsequent immunoblot analysis.
3.3. Western immunoblotting
Western immunoblots were employed in quantifying the extent of phosphorylation of signaling kinases in DPDPE or anisomycin-treated vs. vehicle-treated hearts. Following the normoxic perfusion protocol, whole hearts were homogenized in lysis buffer (50 mmol/l HEPES, 150 mmol/l NaCl, 1.5 mmol/l MgCl2, 1 mmol/l EGTA, 1% Triton X, 10% glycerol plus 8.6 µmol/l leupeptin, 5.8 µmol/l pepstatin A, 4 mmol/l phenyl-methylsulfonyl fluoride (PMSF), 0.6 µmol/l aprotinin, 4 mmol/l sodium fluoride, and 0.8 mmol/l sodium orthovanadate), and centrifuged at 10000×g for 15 min to remove nuclei and debris. The supernatant was centrifuged at 100000×g to enrich for the cytosolic fraction. The resultant pellet (membrane fraction) was re-suspended with ultrasonication. Protein concentration of the two fractions was determined by the Pierce assay. Protein (35 µg) was then loaded onto 10% Tris–HCl gels and after electrophoresis (150 V, 1.5 h), transferred to a PVDF membrane (50 V, 2 h). Equal loading of samples was confirmed by Ponceau S staining. Membranes were blocked with 3% bovine serum albumin solution followed by probing overnight with an antibody for either GRK2 (Santa Cruz Biotechnology), Akt (Ser473), p70S6K (Thr389), p42/44 MAPK (Thr202/Tyr204), p38 MAPK (Thr180/Tyr182), or HSP27 (Ser82, Cell Signaling), followed by secondary antibody application (1:2500 Cell Signaling, 1:10000 for Santa Cruz) and ECL (Amersham). Protein was detected by X-ray film and densitometry assessed by NIH image 1.62.
3.4. Chemicals
Anisomycin and SB203580 were purchased through Sigma (St. Louis, MO), while [d-pen2,5] enkephalin (DPDPE) was acquired through Tocris Bioscience (St. Louis, MO). Aniso-mycin and DPDPE were dissolved in distilled water, with SB203580 being solubilized in DMSO. All solutions were diluted in Kreb’s buffer prior to infusion.
3.5. Statistical analysis
Data are expressed as mean±SEM. For all groups, n≥5. Functional responses to ischemia–reperfusion were compared by one-way analysis of variance, as were relative densities in Western immunoblots. When significance was detected, a Newman–Keuls post hoc test was employed for individual comparisons. For all tests significance was accepted for P<0.05.
4. Results
4.1. Ischemic responses to DPDPE in young and aged hearts
No differences in baseline (normoxic) function were noted across age and treatment groups (Young hearts: LVEDP, 3.1±0.6 mm Hg; SYS, 138±0.2 mm Hg; LVDP, 135.3±2.0 mm Hg; HR, 347.1±13.1 BPM. Aged hearts: LVEDP, 4.5±0.7 mm Hg; SYS, 136.6±7.0mmHg; LVDP, 134.0±7.3mmHg; HR, 357.4±15.3 BPM) with the exception of coronary flow rate, which was significantly higher in the aged hearts (3.2±0.2 vs. 2.6±0.2 ml/min, P<0.05), likely due to the larger body mass (37.6±1.1 vs. 23.1±0.4 g, P<0.05), and thus heart weight, in the aged mice. Upon termination of 45-min reperfusion (following 25-min ischemia), young untreated hearts exhibited poor recovery of left ventricular contractile function (ventricular developed pressure of 50±3% pre-ischemia) and reduced −dP/dt (54±4% of pre-ischemia), reflecting impaired ability to generate force and to relax adequately (Fig. 1). Aged hearts displayed a similar degree of post-ischemic contractile dysfunction. Treatment with 1 µmol/l of the selective δ-opioid receptor agonist, DPDPE, led to improved contractile recovery in hearts obtained from young mice, significantly improving pressure development to 67±3% (P<0.05 vs. untreated), and −dP/dt to 66±6% (P<0.05 vs. untreated). However, hearts from aged animals were unresponsive to improvements in contractile function in the presence of DPDPE (Fig. 1).
Fig. 1.
Post-ischemic functional recovery of (A) left ventricular developed pressure and (B) −dP/dt, following 25 min of global ischemia and 45 min of reperfusion. Recovery is expressed as %Baseline. Hearts from both young and aged groups were perfused in the presence or absence of 1 µM DPDPE, a δ-selective opioid receptor agonist. n=6 for all groups. Values are mean±SEM. *P<0.05.
4.2. Kinase/Protein responses to DPDPE in young and aged hearts
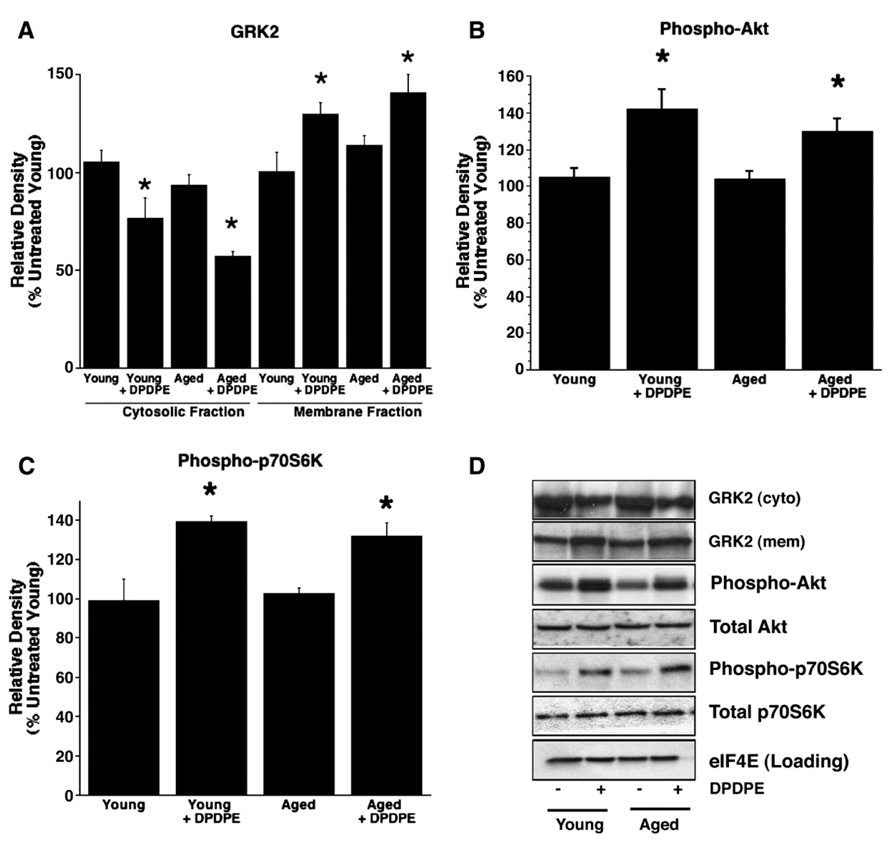
Based on the observed aging-related loss of protection with DPDPE, we further examined post-receptor signaling implicated in tissue protection (via Western immunoblot analysis). Specifically, we studied activation and/or phosphorylation of G-protein-coupled receptor kinase (GRK2), Akt (or protein kinase B, Ser473), ERK1/2 (or p42/44 MAPK, Thr202/Tyr204), p70S6 kinase (Thr389), p38 MAPK (Thr180/Tyr182), and heat shock protein 27 (HSP27, Ser82) following 10 min of acute δ-opioid activation with 1 µmol/l DPDPE in normoxic hearts from young and aged mice.
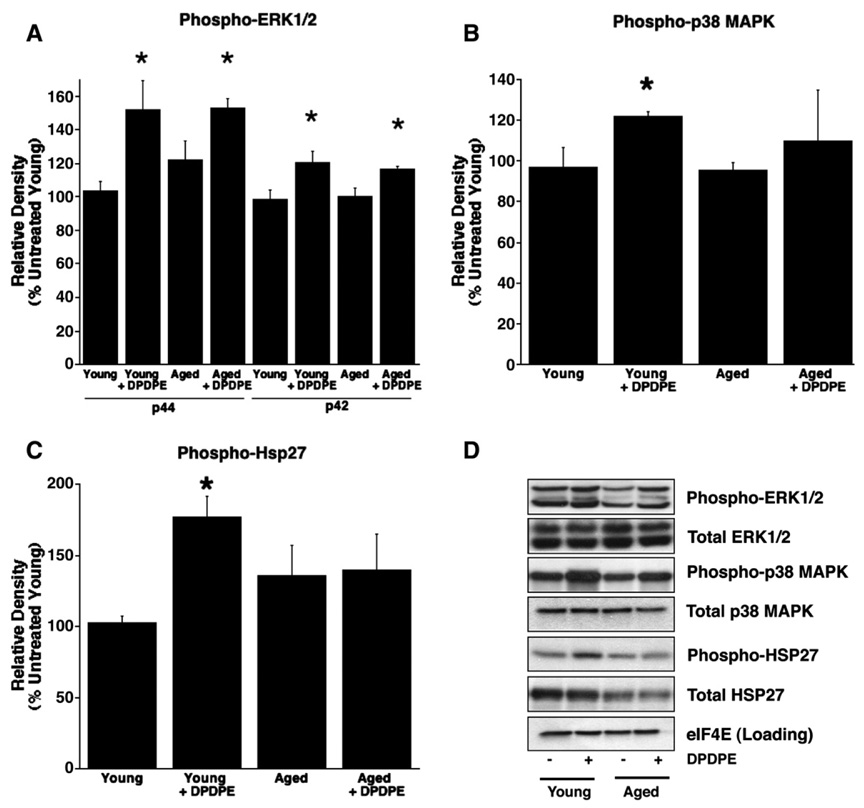
Following agonist binding to the receptor, GRK2 translocates from cytosol to sarcolemma, enhancing receptor phosphorylation. Subsequently, GRK2 facilitates receptor internalization. We found that DPDPE triggered equivalent GRK2 translocation in young and aged hearts. Similarly, kinases associated with protective signaling – Akt (Ser473), p70S6 kinase (Thr389), and ERK1/2 (Thr202/Tyr204) – were comparably phosphorylated/activated in young and aged hearts. These data suggest that both early receptor signaling and the mTOR pathway (Akt, ERK1/2 and p70S6 kinase) remain intact and effective in aged myocardium (Fig. 2, Fig. 3). No differences were noted in total protein content, with the exception of HSP27 which was lower in the aged heart. However, investigation of the p38 MAPK pathway revealed an age-dependent alteration in p38 MAPK (Thr180/Tyr182) phosphorylation, and failure to activate the downstream mediator HSP27 (Ser82, Fig. 3). This failure likely occurs proximal to p38 MAPK and could contribute to the failed opioid-mediated cardioprotection observed in the aged heart.
Fig. 2.
Relative densities (%untreated young control) of Western immunoblot bands of total (A) GRK2 and phosphorylated (B) Akt (Ser473), (C) p70S6K (Thr389) and (D) representative Western immunoblots of total GRK2 and both total and phosphorylated Akt Ser473 and p70S6K Thr389 from normoxic perfused young and aged hearts in the presence or absence of 1 µM DPDPE. n=6 for all observations. Values are mean±SEM. *P<0.05.
Fig. 3.
Relative densities (%untreated young control) of Western immunoblot bands of phosphorylated (A) ERK1/2 (Thr202/Tyr204), (B) p38 MAPK (Thr180/Tyr182) and (C) HSP27 (Ser82) and representative immunoblots of the same observations from tissue derived from normoxic perfused young and aged hearts with or without DPDPE (1 µM). n=6 for all groups. Values are mean±SEM. *P<0.05.
4.3. Kinase/Protein response to anisomycin
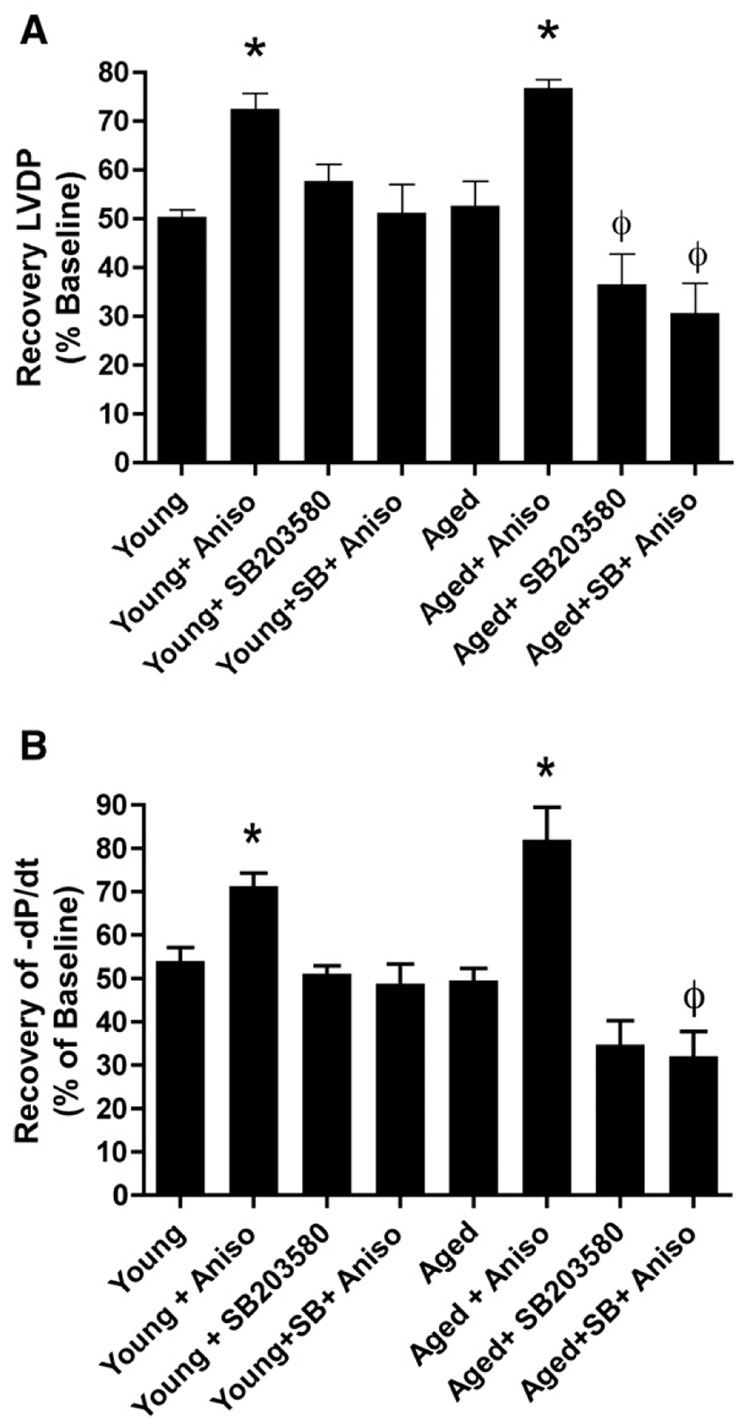
Further supporting a causal link between altered p38 MAPK activation and failed tissue protection, Western immunoblot analysis revealed that (as opposed to DPDPE) 1 µmol/l anisomycin significantly enhanced p38 MAPK and HSP27 activation in both young and aged hearts. Thus, while δ-opioid agonism fails to protect aged hearts and activate p38 MAPK and HSP27, a pharmacological p38 MAPK activator triggers p38 MAPK and HSP27 activation as assessed by Western analysis (Fig. 4), together with tissue protection, in terms of functional recovery, in aged myocardium (Fig. 5).
Fig. 4.
Representative blots of phosphorylated p38 MAPK and HSP27 from young and aged normoxic hearts following treatment with 1 µM anisomycin, a putative p38 MAPK activator. Values are mean±SEM. *P<0.05.
Fig. 5.
Recovery of Left ventricular developed pressure (A) and −dP/dt (B) in hearts following 25-min ischemia and 45-min reperfusion. Hearts from young and aged mice were untreated, or treated with 1 µM anisomycin in the presence or absence of 1 µM SB203580. n=6 for all groups, except young+SB203580 (n=5) and young+anisomycin (n=7). Values are mean±SEM. *P<0.05 vs. untreated young, ϕP<0.05 vs. untreated aged heart.
4.4. Effects of anisomycin on responses to ischemia
Given the above association between failed cardioprotection and failed activation of p38 MAPK in the aged heart, we sought to determine if “direct” activation of this pathway would improve post-ischemic function in aged hearts. Hearts from both age groups were treated with 1 µmol/l anisomycin, a putative p38 MAPK activator, in a protocol that mimicked that used with DPDPE. Data revealed that anisomycin substantially improved post-ischemic recovery of left ventricular pressure development and −dP/dt in young hearts (Fig. 5). Supporting a causal link between failed p38 MAPK activation and loss of tissue protection, anisomycin also significantly enhanced contractile function in aged hearts (77±2% recovery of pressure development; P<0.05 vs. untreated, Fig. 5).
4.5. Anisomycin and inhibition of p38 MAPK
Following the observation of both anisomycin-induced p38 MAPK/HSP27 phosphorylation and cardioprotection, coupled with the potential non-specific effects of anisomycin, we sought to further clarify the role of p38 MAPK and HSP27. To this end, we employed the putative p38 MAPK inhibitor, SB203580, a pyridinyl imidazole that inhibits phosphorylation of p38 MAPK and activation of MAPKAP kinase-2 and phosphorylation of p38 MAPK/HSP27. With no selectivity for JNK or p42 MAP kinase, it is useful for studying the roles/targets of p38 MAPK.
As shown in Fig. 5, pretreatment with 1 µM SB203580 completely abolished the protective effects of anisomycin in both young and aged hearts. Interestingly, SB203580 alone limited ischemic tolerance in the aged hearts (with no effect in the young). This result may imply that the degree of signaling redundancy often seen in the young heart is reduced, with the aged hearts being more dependent on p38 MAPK.
5. Discussion
The results of the current study demonstrate that while early receptor signaling, and the signaling cascade incorporating GRK, Akt, ERK1/2 and p70S6k, appears to be functionally intact and responsive in aged myocardium, there is an age-related loss of activation of p38 MAPK and HSP27, likely due to some unknown failure in proximal signaling. This is associated with the inability of opioid agonism to induce its anti-stunning effect in these hearts. When this failure in aged hearts is targeted, with a p38 MAPK activator, activation/phosphorylation of p38 MAPK and HSP27 occurs in concert with significant cardio-protection. Overall, the current data implicate abnormal or failed signaling proximal to p38 MAPK/HSP27 in the aging-related failure in opioid-mediated cardioprotection, an effect which may be relevant to reductions in responsiveness to other protective stimuli with age, such as IPC [10].
5.1. Cardioprotection and protective signaling in aged vs. young hearts
Failure in cardioprotection/preconditioning with aging has been well documented in human and animal studies [7–10], though this has not been universally observed [24]. The molecular basis of these changes remains poorly understood. Given that ischemic disorders are more prevalent in aged subjects, it is crucial that we enhance our understanding of the changes that underlie failed tissue protection and ischemic intolerance in older hearts. Opioid peptides, and agonists at opioid receptors (comprising μ, κ and δ subtypes), have been shown to afford acute, delayed and chronic protection or preconditioning in the myocardium [21,25,26]. Acute protective responses to opioids, primarily triggered by κ or δ receptors, appear to involve similar triggers, mediators and end-effectors involved in ischemic preconditioning and in cardioprotection mediated by other GPCRs [21,22]. These responses typically involve shifts in ROS generation and the activities of several kinases including Akt, PKC, PI3K, and MAPKs, which may ultimately converge on mitochondrial targets including mitochondrial KATP channels and the mitochondrial permeability transition pore (for reviews, see [21,22]). Our prior work implicates an aging-related failure in protective signaling proximal to mitochondrial KATP channels [3]. Alterations or failure in protective kinase pathways, upstream of mitochondrial effectors [22], therefore, seems a likely explanation for failed cardioprotection.
Our functional data confirm prior reports that acute protective responses are reduced or absent in aged myocardium [7–10]. Extending this work, we show that despite an absence of protection, a 10-min stimulation with the δ-opioid agonist, DPDPE, nonetheless triggers comparable translocation of GRK2 and phosphorylation of Akt (Ser473), ERK1/2 (Thr202/Tyr204), and p70s6k (Thr389, Fig. 2, Fig. 3) in young and aged hearts. Thus, it is unlikely that failed protection stems from a reduction in the roles of these proteins. On the other hand, activation/phosphorylation of p38 MAPK and its target HSP27, which is evident in young hearts treated with DPDPE, did not occur in aged myocardium (Fig. 3). Given evidence of a key role for p38 MAPK in protecting rat [27], mouse [28], porcine [29], canine [30] and human myocardium [31], this inability to trigger p38 MAPK activation may well underlie the failure in cardioprotection. Confirming this possibility, a more direct activator of p38 MAPK signaling (anisomycin) successfully activates both p38 MAPK and HSP27 in aged myocardium (Fig. 4) and triggers a cardioprotected state, reducing post-ischemic stunning (Fig. 5). Taken together, these observations provide strong support for abnormal p38 MAPK/HSP27 signaling as the basis of failed cardioprotection in the aged heart. Our observation of an association between impaired p38 MAPK/HSP27 activation and failed cardioprotection is relevant to the recent work of Zheng and colleagues [32]. They found that platelet-derived growth factor reverses the aging-related loss of ischemic preconditioning triggered cardioprotection, and this agent is known to activate both p38 MAPK [33] and HSP27 [34].
The role of MAPKs in preconditioning, and particularly p38 MAPK distal to PKC, is well established (for review, see Steenbergen [35]). Ischemic stress itself triggers p38 MAPK and HSP27 activation [30,36], and p38 MAPK appears to play a role in mediating cardioprotection triggered by different stimuli [30,37,38]. Interestingly, effects of p38 MAPK in ischemic-reperfused myocardium are extremely time-dependent, as revealed in the work of Marais et al. [38] and Sanada et al. [30]. These groups both show that while p38 MAPK activation prior to ischemia is protective, activation during ischemia itself may in fact be detrimental. Similarly, Schwartz and Lagranha [39] show that post-ischemic activation of other protective kinases (ERK and Akt) fails to modify the outcome. Thus, as exemplified by the work of Sanada et al. [30] and Marais et al. [38], the extent of MAPK activation in post-ischemic hearts may have little relevance to their role in protecting hearts from ischemic injury.
HSP27, the terminal substrate of the p38 MAPK cascade, affords a functional linkage between p38 MAPK and the actin cytoskeleton. Additionally, HSP27 (which is highly expressed in the heart) acts as an endogenous cytoprotective stress response protein, eliciting cardioprotection via its role as a molecular chaperone and in phosphorylation-dependent stabilization of actin. Ischemia induces early changes in actin and other myofibrillar proteins in human myocardium [40], with post-ischemic contractile dysfunction linked to both oxidation [41], and glutathionylation [42] of actin. Thus, actin stabilization by HSP27 may be important in functional protection conferred by p38 MAPK/HSP27 phosphorylation. This is internally consistent with our observation that protective effects of opioid receptor activation are lost in parallel with failed phosphorylation of p38 MAPK and HSP27.
Anisomycin is a potent activator of kinase cascades, particularly SAPK/MAPK pathways. Anisomycin activates immediate upstream kinases SEK1/JNKK/MKK4 (to activate JNK) and MKK6 (to activate p38 MAPK and subsequently MAPKAPK-2), effects which may be mediated via even more upstream kinases. Additionally, anisomycin activates a p70/85 S6 kinase to phosphorylate ribosomal protein S6. Thus, two stress response pathways (JNK/p38 MAPK) and the p70/85 S6 kinase pathway may all be activated by anisomycin. However, we show that of the proteins studied, only p38 MAPK and its downstream target HSP27 are clearly unresponsive to opioid activation in aged hearts (Fig. 3). Since anisomycin effectively stimulated phosphorylation of p38 MAPK (and HSP27) and produced an anti-stunning effect in aged hearts which was reversed by p38 MAPK inhibition (Fig. 5), inability of opioid receptor activation to trigger this process likely involves an abnormality in kinase signaling upstream of p38 MAPK (i.e., upstream of MKK3 or 6). If aging had altered the ability of MKKs to phosphorylate p38 MAPK, anisomycin would also have been ineffective in stimulating phosphorylation and cardioprotection in aged tissue. Regardless, as JNK may undergo an age-related decline [43], coupled with the selectivity of both anisomycin and SB203580 for JNK, the role for JNK cannot be entirely discounted in our observations, and this warrants further studies.
5.2. Changes in p38 MAPK/HSP27 signaling with aging
While considerable data support roles for p38 MAPK (and subsequent activation of HSP27) in mediating cardioprotection, very little is known regarding effects of aging on this pathway. Aging has been shown to markedly reduce the activity of other MAPKs (JNK and ERK) in rat myocardium [43,44], and there is evidence of impaired MAPK activity with aging, in the absence of changes in protein content [45]. A small number of human studies, undertaken in diseased tissue of only moderate age [46,47], support reduced p38 MAPK activity/signaling with heart failure. In terms of stress-related p38 MAPK responses, aging impairs activation of MAPK-dependent early response genes during myocardial oxidative stress [48], muscle mechanotransduction processes triggering p38 MAPK phosphorylation appear to be lost in aged rats [49], and pressure-induced p38 MAPK activation in vascular muscle is also impaired with age [50]. Overall, these findings hint at repressed p38 MAPK activities and stress responses in aged tissue, though this is not a universal observation [51].
Even less is known regarding changes in myocardial HSP27 with aging. There are reports of elevations in HSP27 in failing myocardium [52], and shifts in HSP27 levels and degradation with cardiomyopathy [53]. Lymphocytes producing HSP27, and levels of HSP27 in lymphocytes, are both diminished with advancing age [54]. Moreover, the levels of HSP27 fall below that of detection in the aged rat heart [55]. These age-related failings of HSP27, combined with what we now know about the roles of HSP27 in cardioprotection (see above), could certainly be attributable to the loss of protection observed in the senescent heart. Thus, our findings, coupled with the mixed evidence for alterations in HSP27 activity with aging, reveal the need for further research into this potentially complex interaction between HSP27 (and p38 MAPK), aging, and the subsequent reduction in ischemic tolerance and cardioprotection.
6. Conclusions
In summary, the current study reveals that loss of opioid-mediated cardioprotection is associated with failure to activate p38 MAPK and HSP27 in aged myocardium. This failure in signaling may not only contribute to impaired protective responses to other stimuli, but also impaired ischemic tolerance in older hearts. Importantly, cardioprotection can still be achieved in aging hearts via pharmacological activation of p38 MAPK with anisomycin.
Acknowledgments
This work was supported by NIH grant (HL08311, G.J.G.), an NHMRC Howard Florey Centenary Research Fellowship (J.P.), and a National Heart Foundation of Australia grant-in-aid, #G 05B 2029 (J.P.).
References
- 1.Abete P, Testa G, Ferrara N, De Santis D, Capaccio P, Viati L, et al. Cardioprotective effect of ischemic preconditioning is preserved in food-restricted senescent rats. Am J Physiol: Heart Circ Physiol. 2002;282:H1978–H1987. doi: 10.1152/ajpheart.00929.2001. [DOI] [PubMed] [Google Scholar]
- 2.Headrick JP. Aging impairs functional, metabolic and ionic recovery from ischemia–reperfusion and hypoxia-reoxygenation. J Mol Cell Cardiol. 1998;30:1415–1430. doi: 10.1006/jmcc.1998.0710. [DOI] [PubMed] [Google Scholar]
- 3.Headrick JP, Willems L, Ashton KJ, Holmgren K, Peart J, Matherne GP. Ischaemic tolerance in aged mouse myocardium: the role of adenosine and effects of A1 adenosine receptor overexpression. J Physiol. 2003;549:823–833. doi: 10.1113/jphysiol.2003.041541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lakatta EG, Mitchell JH, Pomerance A, Rowe GG. Human aging: changes in structure and function. J Am Coll Cardiol. 1987;10:42A–47A. doi: 10.1016/s0735-1097(87)80447-3. [DOI] [PubMed] [Google Scholar]
- 5.Roberts WC. The aging heart. Mayo Clin Proc. 1988;63:205–206. doi: 10.1016/s0025-6196(12)64955-6. [DOI] [PubMed] [Google Scholar]
- 6.Bartling B, Hilgefort C, Friedrich I, Silber RE, Simm A. Cardio-protective determinants are conserved in aged human myocardium after ischemic preconditioning. FEBS Lett. 2003;555:539–544. doi: 10.1016/s0014-5793(03)01342-5. [DOI] [PubMed] [Google Scholar]
- 7.Peart JN, Gross GJ. Chronic exposure to morphine produces a marked cardioprotective phenotype in aged mouse hearts. Exp Gerontol. 2004;39:1021–1026. doi: 10.1016/j.exger.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 8.Bartling B, Friedrich I, Silber RE, Simm A. Ischemic preconditioning is not cardioprotective in senescent human myocardium. Ann Thorac Surg. 2003;76:105–111. doi: 10.1016/s0003-4975(03)00186-3. [DOI] [PubMed] [Google Scholar]
- 9.Lee TM, Su SF, Chou TF, Lee YT, Tsai CH. Loss of preconditioning by attenuated activation of myocardial ATP-sensitive potassium channels in elderly patients undergoing coronary angioplasty. Circulation. 2002;105:334–340. doi: 10.1161/hc0302.102572. [DOI] [PubMed] [Google Scholar]
- 10.Schulman D, Latchman DS, Yellon DM. Effect of aging on the ability of preconditioning to protect rat hearts from ischemia–reperfusion injury. Am J Physiol: Heart Circ Physiol. 2001;281:H1630–H1636. doi: 10.1152/ajpheart.2001.281.4.H1630. [DOI] [PubMed] [Google Scholar]
- 11.Abete P, Ferrara N, Cacciatore F, Madrid A, Bianco S, Calabrese C, et al. Angina-induced protection against myocardial infarction in adult and elderly patients: a loss of preconditioning mechanism in the aging heart? J Am Coll Cardiol. 1997;30:947–954. doi: 10.1016/s0735-1097(97)00256-8. [DOI] [PubMed] [Google Scholar]
- 12.Longobardi G, Abete P, Ferrara N, Papa A, Rosiello R, Furgi G, et al. “Warm-up” phenomenon in adult and elderly patients with coronary artery disease: further evidence of the loss of ”ischemic preconditioning” in the aging heart. J Gerontol, A Biol Sci Med Sci. 2000;55:24–29. doi: 10.1093/gerona/55.3.m124. [DOI] [PubMed] [Google Scholar]
- 13.Fryer RM, Pratt PF, Hsu AK, Gross GJ. Differential activation of extracellular signal regulated kinase isoforms in preconditioning and opioid-induced cardioprotection. J Pharmacol Exp Ther. 2001;296:642–649. [PubMed] [Google Scholar]
- 14.Kodani E, Xuan YT, Shinmura K, Takano H, Tang XL, Bolli R. Delta-opioid receptor-induced late preconditioning is mediated by cyclooxygenase-2 in conscious rabbits. Am J Physiol: Heart Circ Physiol. 2002;283:H1943–H1957. doi: 10.1152/ajpheart.00150.2002. [DOI] [PubMed] [Google Scholar]
- 15.Peart JN, Patel HH, Gross GJ. Delta-opioid receptor activation mimics ischemic preconditioning in the canine heart. J Cardiovasc Pharmacol. 2003;42:78–81. doi: 10.1097/00005344-200307000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Boluyt MO, Younes A, Caffrey JL, O’Neill L, Barron BA, Crow MT, et al. Age-associated increase in rat cardiac opioid production. Am J Physiol. 1993;265:H212–H218. doi: 10.1152/ajpheart.1993.265.1.H212. [DOI] [PubMed] [Google Scholar]
- 17.Crisp T, Stafinsky JL, Hoskins DL, Dayal B, Chinrock KM, Uram M. Effects of aging on spinal opioid-induced antinociception. Neurobiol Aging. 1994;15:169–174. doi: 10.1016/0197-4580(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 18.Smith MA, Gray JD. Age-related differences in sensitivity to the antinociceptive effects of opioids in male rats. Influence of nociceptive intensity and intrinsic efficacy at the mu receptor. Psychopharmacology (Berl) 2001;156:445–453. doi: 10.1007/s002130100750. [DOI] [PubMed] [Google Scholar]
- 19.Smith MA, French AM. Age-related differences in sensitivity to the antinociceptive effects of kappa opioids in adult male rats. Psychopharmacology (Berl) 2002;162:255–264. doi: 10.1007/s00213-002-1102-6. [DOI] [PubMed] [Google Scholar]
- 20.Hoskins DL, Gordon TL, Crisp T. The effects of aging on mu and delta opioid receptors in the spinal cord of Fischer-344 rats. Brain Res. 1998;791:299–302. doi: 10.1016/s0006-8993(98)00034-1. [DOI] [PubMed] [Google Scholar]
- 21.Gross GJ. Role of opioids in acute and delayed preconditioning. J Mol Cell Cardiol. 2003;35:709–718. doi: 10.1016/s0022-2828(03)00135-4. [DOI] [PubMed] [Google Scholar]
- 22.Murphy E. Primary and secondary signaling pathways in early preconditioning that converge on the mitochondria to produce cardioprotection. Circ Res. 2004;94:7–16. doi: 10.1161/01.RES.0000108082.76667.F4. [DOI] [PubMed] [Google Scholar]
- 23.Peart J, Headrick JP. Intrinsic A(1) adenosine receptor activation during ischemia or reperfusion improves recovery in mouse hearts. Am J Physiol: Heart Circ Physiol. 2000;279:H2166–H2175. doi: 10.1152/ajpheart.2000.279.5.H2166. [DOI] [PubMed] [Google Scholar]
- 24.Loubani M, Ghosh S, Galinanes M. The aging human myocardium: tolerance to ischemia and responsiveness to ischemic preconditioning. J Thorac Cardiovasc Surg. 2003;126:143–147. doi: 10.1016/s0022-5223(02)73601-5. [DOI] [PubMed] [Google Scholar]
- 25.Peart JN, Gross GJ. Morphine-tolerant mice exhibit a profound and persistent cardioprotective phenotype. Circulation. 2004;109:1219–1222. doi: 10.1161/01.CIR.0000121422.85989.BD. [DOI] [PubMed] [Google Scholar]
- 26.Peart JN, Gross ER, Gross GJ. Opioid-induced preconditioning: recent advances and future perspectives. Vascul Pharmacol. 2005;42:211–218. doi: 10.1016/j.vph.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Yue Y, Qin Q, Cohen MV, Downey JM, Critz SD. The relative order of mK (ATP) channels, free radicals and p38 MAPK in preconditioning’s protective pathway in rat heart. Cardiovasc Res. 2002;55:681–689. doi: 10.1016/s0008-6363(02)00452-2. [DOI] [PubMed] [Google Scholar]
- 28.Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, et al. Mitochondrial PKCepsilon and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCepsilon-MAPK interactions and differential MAPK activation in PKCepsilon-induced cardioprotection. Circ Res. 2002;90:390–397. doi: 10.1161/01.res.0000012702.90501.8d. [DOI] [PubMed] [Google Scholar]
- 29.Schulz R, Belosjorow S, Gres P, Jansen J, Michel MC, Heusch G. p38 MAP kinase is a mediator of ischemic preconditioning in pigs. Cardiovasc Res. 2002;55:690–700. doi: 10.1016/s0008-6363(02)00319-x. [DOI] [PubMed] [Google Scholar]
- 30.Sanada S, Kitakaze M, Papst PJ, Hatanaka K, Asanuma H, Aki T, et al. Role of phasic dynamism of p38 mitogen-activated protein kinase activation in ischemic preconditioning of the canine heart. Circ Res. 2001;88:175–180. doi: 10.1161/01.res.88.2.175. [DOI] [PubMed] [Google Scholar]
- 31.Loubani M, Galinanes M. Pharmacological and ischemic preconditioning of the human myocardium: mitoK(ATP) channels are upstream and p38MAPK is downstream of PKC. BMC Physiol. 2002;2:10. doi: 10.1186/1472-6793-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng J, Chin A, Duignan I, Won KH, Hong MK, Edelberg JM. Growth factor-mediated reversal of senescent dysfunction of ischemia-induced cardioprotection. Am J Physiol: Heart Circ Physiol. 2006;290:H525–H530. doi: 10.1152/ajpheart.00470.2005. [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi H, Igarashi M, Hirata A, Sugae N, Tsuchiya H, Jimbu Y, et al. Altered PDGF-BB-induced p38 MAP kinase activation in diabetic vascular smooth muscle cells: roles of protein kinase C-delta. Arterioscler, Thromb, Vasc Biol. 2004;24:2095–2101. doi: 10.1161/01.ATV.0000144009.35400.65. [DOI] [PubMed] [Google Scholar]
- 34.Takenaka M, Matsuno H, Ishisaki A, Nakajima K, Hirade K, Takei M, et al. Platelet-derived growth factor-BB phosphorylates heat shock protein 27 in cardiac myocytes. J Cell Biochem. 2004;91:316–324. doi: 10.1002/jcb.10717. [DOI] [PubMed] [Google Scholar]
- 35.Steenbergen C. The role of p38 mitogen-activated protein kinase in myocardial ischemia/reperfusion injury; relationship to ischemic preconditioning. Basic Res Cardiol. 2002;97:276–285. doi: 10.1007/s00395-002-0364-9. [DOI] [PubMed] [Google Scholar]
- 36.Luss H, Neumann J, Schmitz W, Schulz R, Heusch G. The stress-responsive MAP kinase p38 is activated by low-flow ischemia in the in situ porcine heart. J Mol Cell Cardiol. 2000;32:1787–1794. doi: 10.1006/jmcc.2000.1213. [DOI] [PubMed] [Google Scholar]
- 37.Yoshimura Y, Kristo G, Keith BJ, Jahania SA, Mentzer RM, Jr, Lasley RD. The p38 MAPK inhibitor SB203580 blocks adenosine A(1) receptor-induced attenuation of in vivo myocardial stunning. Cardiovasc Drugs Ther. 2004;18:433–440. doi: 10.1007/s10557-004-6220-4. [DOI] [PubMed] [Google Scholar]
- 38.Marais E, Genade S, Strijdom H, Moolman JA, Lochner A. p38 MAPK activation triggers pharmacologically-induced beta-adrenergic preconditioning, but not ischaemic preconditioning. J Mol Cell Cardiol. 2001;33:2157–2177. doi: 10.1006/jmcc.2001.1478. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz LM, Lagranha CJ. Ischemic postconditioning during reperfusion activates Akt and ERK without protecting against lethal myocardial ischemia–reperfusion injury in pigs. Am J Physiol: Heart Circ Physiol. 2006;290:H1011–H1018. doi: 10.1152/ajpheart.00864.2005. [DOI] [PubMed] [Google Scholar]
- 40.Hein S, Scheffold T, Schaper J. Ischemia induces early changes to cyto-skeletal and contractile proteins in diseased human myocardium. J Thorac Cardiovasc Surg. 1995;110:89–98. doi: 10.1016/S0022-5223(05)80013-3. [DOI] [PubMed] [Google Scholar]
- 41.Powell SR, Gurzenda EM, Wahezi SE. Actin is oxidized during myocardial ischemia. Free Radic Biol Med. 2001;30:1171–1176. doi: 10.1016/s0891-5849(01)00514-7. [DOI] [PubMed] [Google Scholar]
- 42.Chen FC, Ogut O. Decline of contractility during ischemia–reperfusion injury: actin glutathionylation and its effect on allosteric interaction with tropomyosin. Am J Physiol: Cell Physiol. 2006;290:C719–C727. doi: 10.1152/ajpcell.00419.2005. [DOI] [PubMed] [Google Scholar]
- 43.Izumi Y, Kim S, Murakami T, Yamanaka S, Iwao H. Cardiac mitogen-activated protein kinase activities are chronically increased in stroke-prone hypertensive rats. Hypertension. 1998;31:50–56. doi: 10.1161/01.hyp.31.1.50. [DOI] [PubMed] [Google Scholar]
- 44.Aoyagi T, Izumo S. Hemodynamic overload-induced activation of myocardial mitogen-activated protein kinases in vivo: augmented responses in young spontaneously hypertensive rats and diminished responses in aged Fischer 344 Rats. Hypertension. 2001;37:52–57. doi: 10.1161/01.hyp.37.1.52. [DOI] [PubMed] [Google Scholar]
- 45.Yeo EJ, Park SC. Age-dependent agonist-specific dysregulation of membrane mediated signal transduction: emergence of the gate theory of aging. Mech Ageing Dev. 2002;123:1563–1578. doi: 10.1016/s0047-6374(02)00092-1. [DOI] [PubMed] [Google Scholar]
- 46.Lemke LE, Bloem LJ, Fouts R, Esterman M, Sandusky G, Vlahos CJ. Decreased p38 MAPK activity in end-stage failing human myocardium: p38 MAPK alpha is the predominant isoform expressed in human heart. J Mol Cell Cardiol. 2001;33:1527–1540. doi: 10.1006/jmcc.2001.1415. [DOI] [PubMed] [Google Scholar]
- 47.Communal C, Colucci WS, Remondino A, Sawyer DB, Port JD, Wichman SE, et al. Reciprocal modulation of mitogen-activated protein kinases and mitogen-activated protein kinase phosphatase 1 and 2 in failing human myocardium. J Card Fail. 2002;8:86–92. doi: 10.1054/jcaf.2002.32755. [DOI] [PubMed] [Google Scholar]
- 48.Edwards MG, Sarkar D, Klopp R, Morrow JD, Weindruch R, Prolla TA. Impairment of the transcriptional responses to oxidative stress in the heart of aged C57BL/6 mice. Ann N Y Acad Sci. 2004;1019:85–95. doi: 10.1196/annals.1297.017. [DOI] [PubMed] [Google Scholar]
- 49.Mylabathula DB, Rice KM, Wang Z, Uddemarri S, Kinnard RS, Blough ER. Age-associated changes in MAPK activation in fast- and slow-twitch skeletal muscle of the F344/NNiaHSD X Brown Norway/BiNia rat model. Exp Gerontol. 2006;41:205–214. doi: 10.1016/j.exger.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Rice KM, Kinnard RS, Harris R, Wright GL, Blough ER. Effects of aging on pressure-induced MAPK activation in the rat aorta. Pflugers Arch. 2005;450:192–199. doi: 10.1007/s00424-005-1383-9. [DOI] [PubMed] [Google Scholar]
- 51.Hornberger TA, Mateja RD, Chin ER, Andrews JL, Esser KA. Aging does not alter the mechanosensitivity of the p38, p70S6k, and JNK2 signaling pathways in skeletal muscle. J Appl Physiol. 2005;98:1562–1566. doi: 10.1152/japplphysiol.00870.2004. [DOI] [PubMed] [Google Scholar]
- 52.Knowlton AA, Kapadia S, Torre-Amione G, Durand JB, Bies R, Young J, et al. Differential expression of heat shock proteins in normal and failing human hearts. J Mol Cell Cardiol. 1998;30:811–818. doi: 10.1006/jmcc.1998.0646. [DOI] [PubMed] [Google Scholar]
- 53.Scheler C, Li XP, Salnikow J, Dunn MJ, Jungblut PR. Comparison of two-dimensional electrophoresis patterns of heat shock protein Hsp27 species in normal and cardiomyopathic hearts. Electrophoresis. 1999;20:3623–3628. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3623::AID-ELPS3623>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 54.Njemini R, Lambert M, Demanet C, Mets T. The effect of aging and inflammation on heat shock protein 27 in human monocytes and lymphocytes. Exp Gerontol. 2006;41:312–319. doi: 10.1016/j.exger.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 55.Colotti C, Cavallini G, Vitale RL, Donati A, Maltinti M, Del Ry S, et al. Effects of aging and anti-aging caloric restrictions on carbonyl and heat shock protein levels and expression. Biogerontology. 2005;6:397–406. doi: 10.1007/s10522-005-4906-z. [DOI] [PubMed] [Google Scholar]