Abstract
Cocaine- and amphetamine-regulated transcript (CART) peptides appear to modulate various effects of psychostimulant drugs. Injections of CART peptide into the nucleus accumbens (NAcc) inhibit locomotion produced by systemic injections of the psychostimulants cocaine and amphetamine. Intra-NAcc injections of CART peptide also inhibit locomotion produced by microinfusions of dopamine into the NAcc, suggesting that the effects of CART peptides may be due to an interaction with the dopaminergic system in the NAcc. We sought to determine if this inhibitory effect of CART peptide generalizes to other measures of dopaminergic function such as reward/reinforcement by testing the effect of bilateral intra-NAcc CART infusions (0, 0.25, 1.0 and 2.5 µg per side) on cocaine and food self-administration. One group of rats self-administered cocaine (0.75 mg/kg per 140 µl IV infusion) on a progressive ratio schedule. A separate group received 45 mg food pellets on the same progressive ratio schedule. Bilateral intra-NAcc injections of CART peptide dose-dependently decreased the number of cocaine infusions, the breakpoint of cocaine self-administration, and the total number of bar presses on the cocaine-associated lever. There were no effects of CART injections on the break point for food reward. Thus, we conclude that injections of CART into the NAcc appear to functionally antagonize a major site of action for cocaine self-administration in rats.
Introduction
CART (cocaine- and amphetamine-regulated transcript) peptides are brain/gut neurotransmitters with potential physiological functions including a role in feeding, anxiety and stress, sensory processing, and reward/reinforcement, especially pertaining to psychostimulant drug abuse [For reviews see11,16,25] and August 2007 issue of Peptides). Anatomically, CART mRNA and peptides are present in reinforcement and drug abuse-related brain areas including the VTA, NAcc, amygdala, and ventral pallidum, [9,23].
Regarding CART and psychostimulants, Douglas et al. (1995) reported increased CART mRNA expression in rat striatum after cocaine or amphetamine exposure. While the exact nature of this regulation is unclear and the effect is not always seen [28,37], it has been recently shown that acute cocaine increased the number of CART cells staining for c-Fos [12]. It has also been shown that repeated dosing of amphetamine and cocaine in a manner reminiscent of human drug use increases CART mRNA and peptide in the brain [6,10,15]. Additionally, CART mRNA levels are decreased in the ventral tegmental area (VTA) and increased in the nucleus accumbens (NAcc) of human cocaine overdose victims [3,34].
Weak psychostimulant-related effects are seen after injections of CART peptide into the VTA [17,22]. Intra-NAcc injections of CART alone have no effect on locomotion, but inhibit locomotion produced by systemic injections of amphetamine [21] and cocaine [18]. Because dopamine is thought to be a critical mediator of locomotor activity [29] and the actions of psychostimulants [38], CART may modulate locomotor activity and influence the action of psychostimulants by altering dopamine activity. Indeed, the slight increase in locomotion observed after intra-VTA injections of CART peptide has been shown to be dose-dependently blocked by the dopamine receptor antagonist haloperidol [22]. In addition, intra-NAcc injection of CART peptide dose-dependently blocked locomotion produced by intra-NAcc injection of dopamine [18]. The fact that intra-NAcc CART blocked the effects of exogenously applied dopamine strongly suggests that CART reduces dopamine-mediated events through a post-synaptic mechanism.
The current literature indicates that intra-NAcc injection of CART peptide can reduce the locomotor stimulating effect of several psychostimulant drugs as well as dopamine itself. It is still unclear, however, whether CART peptide can alter other measures of drug reward such as voluntary drug intake. Therefore, this study sought to determine if intra-NAcc injections of CART peptide would alter cocaine self-administration in rats. We also examined the effect of intra-NAcc CART injections on lever-pressing for food reward, in order to determine if injection of CART peptide selectively affected drug motivation.
Methods
Subjects
A total of seventeen male Sprague Dawley rats (Charles River, Wilmington, MA), weighing 350 – 550 gm at the time of the experiments, were used in these experiments (N=8 in the food experiment and N=8 in the cocaine experiment). One rat was excluded from the cocaine experiment because the site of the injections would not be histologically verified. The rats were maintained on a 12/12 h light/dark cycle with lights on at 0600 hr. The rats were housed in groups of two prior to surgery and individually housed thereafter and always had food and water freely available in their home cages. Animal husbandry conformed to the guidelines set forth in the NIH publication regarding the principles of laboratory animal care [1] and in a manner approved by the University’s Institutional Animal Care and Use Committee.
Cocaine and food self-administration training and testing
The rats began training for self-administration one week after arrival at the OHSU vivarium. Animals were trained in 60 min daily sessions in operant conditioning chambers equipped with two stimulus lights located above two retractable levers (an active and non-active lever), a food pellet dispenser and a houselight (Med Associates Inc., St Albans, VT). During daily training sessions animals were allowed to press an active lever on an FR1 schedule of reinforcement for 45 mg food pellets (Bioserve Inc., Frenchtown NJ). The lever on the opposite side of the food dispenser was inactive. The reinforcement schedule for food reward was gradually increased to FR12 over 3–5 days. Rats were then switched to a progressive ratio (PR) schedule of reinforcement. Under this schedule, response requirements for each successive presentation of a reinforcer increased progressively in the following series: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 603, 737, 901. Break point (BP) was determined in the cocaine experiment as the last ration achieved before 1 hr had lapsed without reinforcement with a maximum session length of 5 hr.
Once rats displayed stable responding on the PR schedule (criterion was 2 consecutive days within ± 2BP), the rats underwent surgery (see below). All of the rats in both the cocaine and the food self–administration experiments underwent the same surgery. The rats that had failed catheters prior to receiving any cocaine infusions were used in the food self-administration experiments (N=6).
After recovering from surgery, rats were placed in the operant conditioning chambers, an infusion line was attached to their i.v. catheters and a spring tether was attached to the headmount. In the case of the cocaine self-administration group, the infusion line (PE20) was attached to a syringe containing a cocaine solution loaded onto a syringe pump (Med Associates inc, St. Albans VT). Rats received an acquisition dose of 0.75 mg/kg/140 µl of cocaine delivered over 4 sec on an FR3 schedule of reinforcement during twice daily 1 hr drug access sessions. When rats achieved an acquisition criterion of at least 5 cocaine infusions in 1 hr sessions (approximately 3–4 days), they were switched to the PR schedule described above. They remained on the PR schedule or the rest of the experiment.
Rats in the food self-administration experiment were not food deprived at any time during training or the experiment. They were placed in operant conditioning chambers and allowed to lever press for 45 mg food pellets on the same PR schedule as rat in the cocaine self-administration group. The only difference in reinforcement schedule for the food self-administration rats was that BP was defined as the last ratio achieved before a 30 min period had lapsed without receiving a pellet and the maximum session length was 3 hr.
Surgery
Each rat was anesthetized with a cocktail of ketamine (45 mg/kg) acepromazine (1.0 mg/kg) and xylazine (5.5 mg/kg) and implanted with a jugular catheter and bilateral guide cannulae aimed at the nucleus accumbens.
Catheters were constructed similar to a method previously described in detail by Caine et al. [7]. Briefly, catheters were made by connecting micro-renathane tubing (0.012 mm ID × 0.025 mm OD; Braintree Scientific Inc., Braintree, MA) to an L-shaped external guide cannula (Plastics One Inc., Roanoke, VA) glued onto polypropylene mesh with cranioplastic cement. This cannula-mesh assembly was inserted under the skin between the scapulae and served as a catheter exit point. The other end of the tubing was tunneled subcutaneously and inserted approximately 27 mm into either the right or left jugular vein. After surgery, each rat was given the analgesic carprofen (50 mg/ml 0.08 ml s.c.; Rimadyl®; Pfiser Inc., New York, NY). Intravenous catheters were flushed daily with 0.2 ml isotonic saline containing heparin (70 U/ml) in the morning and 0.2 ml of the antibiotic ticarcillin (Timentin, 100 mg/ml; Smithkline Beecham, Philadelphia, PA) dissolved in sterile saline in the evening. Catheter patency was tested when necessary by infusion via the catheter of the fast acting barbiturate Brevital (methohexital sodium; 1 mg/0.1 ml; Lilly, Indianapolis, IN). Catheters were deemed patent in animals showing a profound loss of muscle tone within 5 sec.
Bilateral guide cannulae (23 ga. thin-walled stainless steel tubing cut to 10 mm length) were lowered into place 4.0 mm above the nucleus accumbens using aseptic stereotaxic surgery. Cannula coordinates were: A/P +1.2, M/L ±1.5, D/V −4.4 mm relative to bregma, according to the atlas of Paxinos and Watson (1998) [31]. The ventral coordinate for microinjection was −8.4 mm (see below). An aluminum head shield was placed onto the skull surface just anterior to the guide cannulae, and the shield and cannulae were anchored in place using cranioplastic cement and two 5 mm stainless steel screws (size 00–80; Small Parts Inc., Miami Lakes FL). Wire stylets (10mm, 26 ga.) were inserted into the cannulae to keep them unobstructed.
Drug Injections
Artificial cerebral spinal fluid (aCSF) and CART peptide injections were made using silica microinjectors. The microinjectors were constructed using fused silica glass tubing (75 µm i.d., 150 µm o.d.) glued into a 28 ga. stainless steel tubing which in turn was soldered inside a 10 mm piece of thin-walled 23 ga. tubing. The microinjector was constructed in such a way that the 28 ga. piece extended 2 mm past the end of the guide cannula and the fused silica tubing extended an additional 2 mm past the end of the 28 ga tubing to the target coordinates of the NAcc.
The microinjectors were connected to 10 µl syringes via PE20 tubing, and the syringes were mounted onto pumps (Braintree Scientific, Braintree MA). Bilateral infusions were given in a volume of 0.25 µl over 75 sec and the injectors were left in place for an additional 30 sec to allow for drug diffusion and prevent backflow through the cannulae. Drug self-administration sessions were started 3 minutes after the microinjectors were removed and the stylets were replaced.
Drugs
CART peptide (Peptide Institute, Louisville, KY) was dissolved in aCSF consisting of (in mM)120 NaCl, 4.8 KCl, 2.5 CaCl2, 1.2 MgCl2, 25 NaHCO3, 1.2 KH2PO4, and 10 glucose (pH adjusted to 7.3). Cocaine HCl was supplied by the Research Triangle Institute (Research Triangle Park, NC) under the National Institute on Drug Abuse Drug Supply Program. The cocaine was dissolved in physiological saline (0.9%) and the pH was adjusted to 6.0.
Results
The data from one rat was excluded from the analysis because the proper placement of the injectors could not be verified. All the other rats had the injectors properly located in the accumbens and their data were included in the analysis. Drug injections occurred predominantly in the transition region between the NAcc core and shell (Figure 1).
Figure 1.
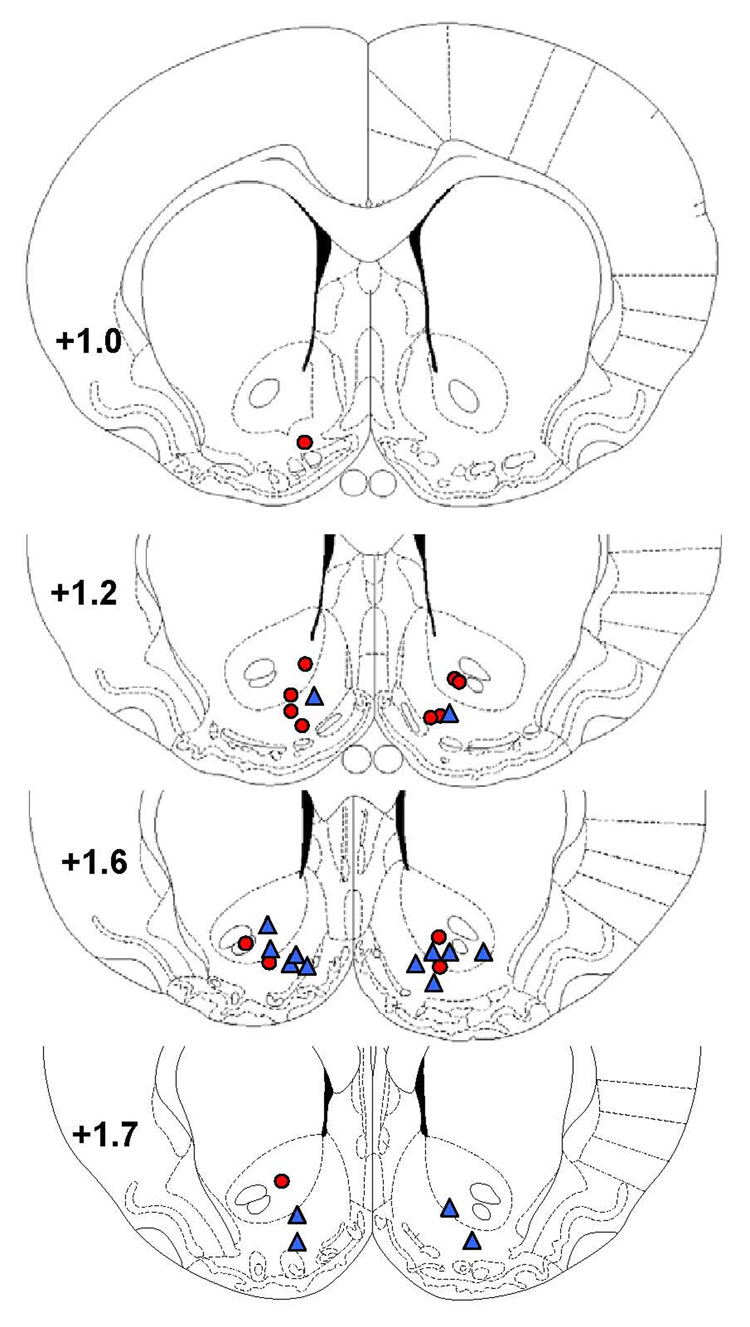
Locations of the intra-NAcc injection sites are depicted on schematic drawings of coronal slices. Numbers represent mm from Bregma according to the atlas of Paxinos & Watson (1998). Injection sites in rats that completed the cocaine self-administration study are depicted by red circles. Injection sites in rats used in the food self-administration study are shown in blue triangles.
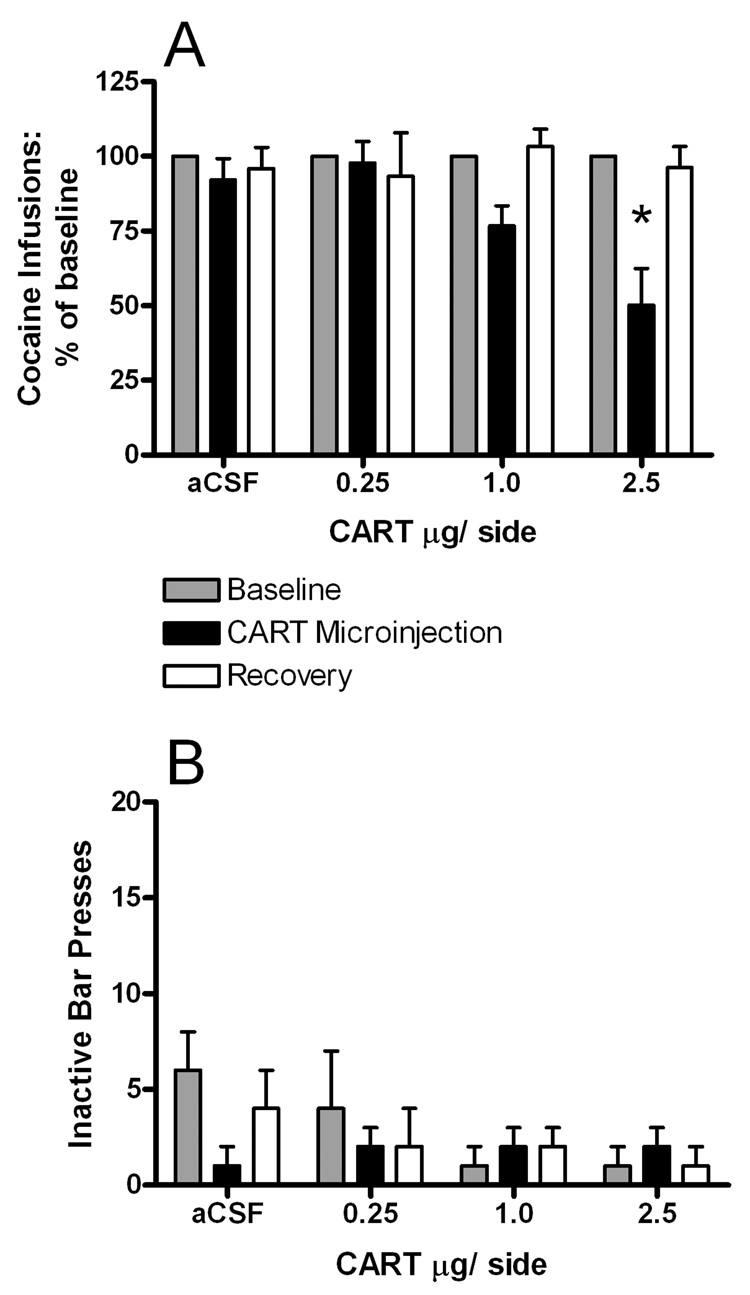
The effect of intra-NAcc CART peptide (and aCSF) infusions on the number of cocaine infusions taken relative to baseline is shown in Figure 2A. Cocaine infusions on the progressive ratio schedule were normalized to baseline values because the breakpoints for individual rats varied considerably. CART peptide, but not aCSF, decreased the number of infusions taken relative to baseline (for each rat, baseline was defined as the average number of infusions taken in the two consecutive days prior to testing). The effect of CART infusion dissipated by the next day (labeled “Recovery” in Fig. 2A), when rats achieved normal breakpoints for cocaine self-administration. There were main effects for time (F2,83 = 10, p = 0.0001) and CART dose (F3,83 = 3.1, p < 0.05) and a significant dose by time interaction (F6,83 = 3.5, p <0.005; Fig. 2A). Bonfferoni post-tests revealed that the highest dose of CART peptide (2.5 µg/side) decreased the number of cocaine infusions (and BP) compared to both the aCSF treatment and the baseline value for the 2.5 µg/side group (p<0.001 for both), while the other comparisons were not statistically significant. CART peptide dose-dependently decreased the BP for cocaine self-administration versus baseline levels (see Table 1). A one-way ANOVA revealed significant differences between groups (F3,30 = 4.6, p < 0.01; Fig. 2A). Bonfferoni post-tests revealed that the 2.5 µg/side dose of CART peptide decreased the BP versus aCSF (p<0.05), while the other doses had no statistically significant effects.
Figure 2.
A. The effect of intra-NAcc infusions of CART peptide on the number of cocaine infusions taken relative to baseline (grey bars; average of the 2 days prior to testing), CART microinjection day (black bars) and recovery (average of the 2 days after treatment; white bars). Data are MEAN±SEM. The n/group was 8,7,8, and 11 for the aCSF, 0.25, 1.0 and 2.5 µg CART injection groups, respectively. The asterisk denotes a significant difference from the treatment day value obtained for aCSF (p<0.05). B. The effect of the CART peptide infusions on the absolute number of inactive lever presses on baseline (grey bars), CART microinjection day (black bars) and recovery (white bars). There were no significant differences between the treatment groups. These data are not depicted as a percent of baseline because in some animals, the number of inactive lever presses was 0. Data are MEAN±SEM. The n/group was 8,7,8, and 11 for aCSF, 0.25, 1.0 and 2.5 µg CART peptide injection groups, respectively.
Table 1.
Numbers represent mean absolute numbers (± SEM) of cocaine infusions, break points and active bar presses for each treatment group. Values for the Baseline condition represent the numbers obtained on the day before the microinjection test. Values under Recovery represent the numbers obtained on the day after the microinjection.
| Group Mean (± SEM) | Baseline | CART Microinjection | Recovery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coc Infusions | Break Point | Bar Press | Coc Infusions | Break Point | Bar Press | Coc Infusions | Break Point | Bar Press | |
| aCSF | 16 (1) | 176 (64) | 669 (153) | 15 (2) | 146 (46) | 679 (209) | 15 (1) | 169 (66) | 729 (234) |
| 0.25 CART | 13 (1) | 65 (14) | 323 (69) | 12 (1) | 62 (22) | 322 (105) | 11 (1) | 39 (3) | 269 (57) |
| 1.0 CART | 16 (1) | 137 (70) | 754 (132) | 11 (2) | 77 (74) | 387 (152) | 16 (1) | 119 (36) | 749 (123) |
| 2.5 CART | 15 (1) | 113 (20) | 547 (130) | 8 (2) * | 40 (13)* | 246 (79) | 15 (2) | 141 (138) | 609 (286) |
Asterisk indicates significant difference from aCSF control (P<0.05).
The effect of aCSF and CART peptide injections on the number of inactive lever presses on the test day is shown in Figure 2B. There were no significant differences in the numbers of inactive lever presses between treatment conditions. Because the total number of inactive lever presses was quite low and was occasionally 0 for any given session, we did not transform the data to a percent of baseline, but instead left it untransformed (total number of lever presses on the drug treatment day). Regardless, there were no statistically significant differences in the number of inactive lever presses between groups. The absolute numbers of cocaine infusions and BP values for baseline, treatment and post-treatment days are summarized in Table 1.
In contrast to the effect of CART on cocaine intake, intra-NAcc injections of CART did not affect the number of food pellets the non-food deprived rats self-administered on a progressive ratio reinforcement schedule (Figure 3).
Figure 3.
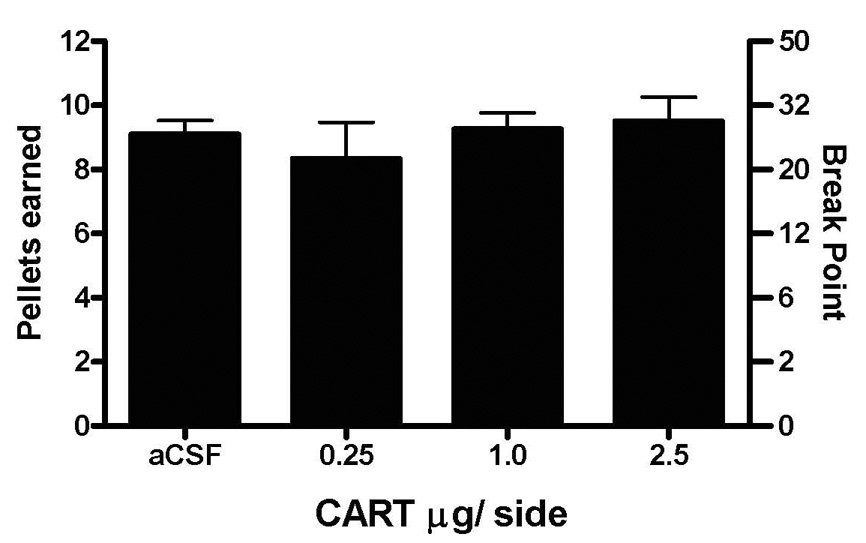
The effect of intra-NAc injection of CART peptide (and aCSF) on food pellets received on a progressive ratio reinforcement schedule. Data are MEAN±SEM. N=6 per group.
Discussion
We found that bilateral intra-NAcc injections of CART peptide 55–102 decreased several measures of cocaine self-administration. The total number of infusions, the BP and the total number of lever presses were all reduced suggesting a reduction in the rewarding value of cocaine. Our findings are in agreement with previous studies that found an inhibitory effect of intra-NAcc CART peptide on psychostimulant-induced behaviors. Intra-NAcc CART inhibits locomotion produced by systemic cocaine [18] and amphetamine [21]. Thus, CART peptide in the NAcc may function not only to modulate the motor properties but also the motivational/rewarding properties of psychostimulants.
Intra-NAcc infusions of CART peptide reduced the breakpoint for cocaine self-administration on a progressive ratio schedule. Breakpoint is a measure of work-effort an animal will expend for a reward and is positively and, to a point, linearly related to the perceived reinforcement value, or magnitude of a reinforcer. We therefore interpret the decrease in breakpoint that occurred after intra-NAcc injection of CART peptide to reflect a decrease in the reward value of self-administered cocaine. These results are consistent with the findings of previous studies that intra-NAcc CART infusions reduced locomotion produced by i.p. injections of amphetamine [21] and cocaine [18], and inhibits the expression of behavioral sensitization to cocaine [41]. Thus, CART in the NAcc appears to functionally inhibit the activating/reinforcing effects of psychostimulants.
The mechanism for the effect of intra-NAcc CART peptide injections on cocaine self-administration is currently unknown but presumably occurs through a CART receptor. A CART receptor has been revealed by CART’s induction of signaling. An inhibition of L-type voltage-gated calcium channels by CART peptide has been reported in cultured rat hippocampal neurons [40]. Specific CART peptide receptor binding has been identified in AtT20 cells [36], PC12 cells [27], HepG2 cells, hypothalamic dissociated cells [20], and in primary cultures of NAcc neurons [19]. Binding studies indicate that the CART receptor is a GPCR, which utilizes a pertussus toxin sensitive Gi/o coupling. In addition, based on the fact that different CART peptide fragments have varying potencies on different behaviors [4,35] there may be multiple CART receptors [36].
There is a growing literature documenting interactions between dopamine and CART peptide. Medium spiny GABAergic output neurons in the NAcc contain CART peptide and express dopamine D1, D2 and D3 receptors [5,13] and CART mRNA is regulated by D3 receptors [6,14]. We have previously reported that NAcc injections of CART peptide dose-dependently blocked locomotion caused by intra-NAcc injections of dopamine [18], which suggests that CART inhibits dopaminergic activity downstream from dopamine release. In contrast to its effect within the NAcc, a previous study showed that administration of CART into VTA caused a moderate increase in DA levels in the NAcc as measured by microdialysis [26]. Since CART receptors are coupled to Gi/o, we suspect the increase in DA resulted from disinhibition of VTA dopamine cells.
Responses to psychostimulants have been tested in mice lacking the gene necessary for producing CART, however the results have been inconsistent. Couceyro et al. (2005) found a decrease in cocaine self-administration as well as altered amphetamine-induced locomotor activation and sensitization in CART knockout mice [8]. In contrast, another study found no differences in cocaine self-administration or cocaine-induced locomotor sensitization between wild type and CART knockout mice [33]. In the study by Steiner et al. (2006) psychostimulant-induced locomotor sensitization was measured using different stimulants (cocaine vs. amphetamine) and sex of the subjects; the study by Couceyro et al. (2005) used male mice, while Steiner et al. used female mice in their sensitization experiments. It is noteworthy that these labs used different groups of CART knockout mice with different backgrounds designed with different methodologies; the 2005 study by Couceyro et al. used knockout mice with all three CART exons deleted and expressed onto a mixed genetic background, while Steiner et al. (2006) used knockout mice with the first and second exons deleted and expressed onto a C57B1 background. The source(s) of the disparate results remain to be resolved, particularly in light of another recent study by Moffett et al (2006) who reported decreased self-administration of low doses of cocaine (0.125 & 0.25 mg/kg/infusion) but not a higher dose (0.5 mg/kg) vs. wild-type in the same CART knockout mice used by Steiner et al. [30]. Thus the effect of a CART knockout on psychostimulant-related behavior, including cocaine self-administration, remains unresolved. Some, but not all groups have found a decrease in self-administration of cocaine using the knockout model. Nevertheless, due to the large amount of research in intact animals reporting CART peptide regulation of a number of psychostimulant behaviors (including the present study), it appears that CART peptide may serve a regulatory peptide of the mesolimbic dopamine system, although this modulation may be altered or lacking in CART knockout mice. It is apparent that additional research is needed to delineate the effect of CART using a genetic deletion model, but our studies using intact animals clearly indicate that CART has an inhibitory effect on cocaine reward in rats.
In contrast to the effect seen on cocaine self-administration, intra-NAcc CART peptide infusions did not alter food self-administration. The factors involved in the effect of intra-NAcc on cocaine-, but not food-self administration, remain to be clarified. For example, in contrast to the lack of an effect on food self-administration seen here, Yang et al (2005) found that intra-NAcc infusions of similar doses of CART peptide decreased free feeding in rats [39]. Interestingly, this effect was more pronounced in freely fed rats compared to fasted rats. Clearly more work is needed to discern if this is a fuel availability issue (i.e. fasted versus non fasted) or a motivational issue (e.g. normal rat chow in a non-operant setting versus more appetitive food pellets in an operant setting). Overall, our findings suggest that the effect of CART peptide on cocaine self-administration may have at least some degree of selectivity between various stimulus-seeking behaviors and different reinforcers.
It is worth noting that slight tremors were occasionally observed in a few rats that received the highest dose of CART peptide. These tremors were only seen when the rats were moving, not when they were at rest. Movement-associated tremors mainly involved the head and did not appear to impact the rest of the body or interfere with animals’ lever pressing ability. The majority of rats that received the highest dose of CART displayed bursts of high frequency lever pressing at various times during the test session although all showed a reduced break point. (For instance, the BP of one rat decreased from a baseline average of 178 to 95 after receiving the 2.5 µg/side dose of CART. The time required to complete each progressive cocaine infusion between 1 and 95 never took this particular animal longer than 13 minutes, including the PR95, however the rat did not complete the next ratio [118 lever presses] even though it had a full hour to attain it.) In general, this observation argues against a CART-induced deficit in movement ability, since rats were able to attain relatively high response levels after CART injection. In addition, even at the highest dose tested, CART did not affect responding for food reward on a PR reinforcement schedule. This is evidence that intra-NAcc injection of CART did not interfere with the animals’ ability to lever-press.
The neural source of the head tremors is not clear. Similar, although more severe, movement-associated tremors in head movement have been reported after CART injections into the lateral ventricle, apparently acting at a hindbrain site surrounding the 4th ventricle [2]. CART fibers exist in the nucleus of the solitary tract, parabrachial nucleus, locus coeruleus, and raphe nuclei [24], and activation of one or more of these sites may be responsible for the observed head tremors, potentially via enhancement of serotonergic function producing a “serotonin syndrome” [2]. Although our histological examination revealed no obvious signs of ventricular intrusion (e.g. a discontinuity in parenchymal cell junctions), there is a possibility that with the highest dose of CART, a small amount of drug entered the ventricular system and contributed to the advent of head tremor. However, locomotor studies by Jaworski et al (2003) did not reveal any locomotor changes in rats after this dose of CART and it is doubtful that CART interfered with self-administration through a motor process. Finally, intra-NAcc CART injections, even at the highest dose, did not affect progressive ratio responding for food pellets, which indicates that motor function was not likely to have been impaired by CART.
In summary, this study found that intra-accumbal injection of a 2.5 µg/side dose of CART reduced cocaine self-administration on a progressive ratio reinforcement schedule. Since progressive ratio schedules result in relatively linear cocaine dose-response functions [32], we interpret the reduction in work effort to self-administer cocaine following CART infusion to reflect a reduction in motivation to obtain drug. This study agrees with and extends the findings of previous studies showing that CART peptide opposes the actions of psychostimulants. Our findings are among the first to demonstrate that CART can regulate motivational processes governing drug-seeking behavior.
Acknowledgements
This research was supported by PHS grants RR00165, DA00418 and DA10732 (MJK), DA014639 (GPM), National Research Service Award number F32 DA015279 (JNJ) and the Methamphetamine Abuse Research Center grant P50 DA018165.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Research Council of the National Academies. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. Washington, DC: The National Academies Press; 2003. [PubMed] [Google Scholar]
- 2.Aja S, Sahandy S, Ladenheim EE, Schwartz GJ, Moran TH. Intracerebroventricular CART peptide reduces food intake and alters motor behavior at a hindbrain site. Am J Physiol Regulat Integrat Comp Physiol. 2001;281:R1862–R1867. doi: 10.1152/ajpregu.2001.281.6.R1862. [DOI] [PubMed] [Google Scholar]
- 3.Albertson DN, Pruetz B, Schmidt CJ, Kuhn DM, Kapatos G, Bannon MJ. Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. J Neurochem. 2004;88:1211–1219. doi: 10.1046/j.1471-4159.2003.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bannon AW, Seda J, Carmouche M, Francis JM, Jarosinski MA, Douglass J. Multiple behavioral effects of cocaine- and amphetamine-regulated transcript (CART) peptides in mice: CART 42–89 and CART 49–89 differ in potency and activity. J Pharmacol Exp Ther. 2001;299:1021–1026. [PubMed] [Google Scholar]
- 5.Beaudry G, Zekki H, Rouillard C, Levesque D. Clozapine and dopamine D3 receptor antisense reduce cocaine- and amphetamine-regulated transcript expression in the rat nucleus accumbens shell. Synapse. 2004;51:233–240. doi: 10.1002/syn.10302. [DOI] [PubMed] [Google Scholar]
- 6.Brenz Verca MS, Widmer DA, Wagner GC, Dreyer J. Cocaine-induced expression of the tetraspanin CD81 and its relation to hypothalamic function. Mol Cell Neurosci. 2001;17:303–316. doi: 10.1006/mcne.2000.0942. [DOI] [PubMed] [Google Scholar]
- 7.Caine SB, Lintz R, Koob GF. Intravenous drug self-administration techniques in animals. In: Sahgal A, editor. Behavioral Neuroscience; A Practical Approach. New York: Oxford University Press; 1993. pp. 117–143. [Google Scholar]
- 8.Couceyro PR, Evans C, McKinzie A, Mitchell D, Dube M, Hagshenas L, White FJ, Douglass J, Richards WG, Bannon AW. Cocaine- and amphetamine-regulated transcript (CART) peptides modulate the locomotor and motivational properties of psychostimulants. J Pharmacol Exp Ther. 2005;315:1091–1100. doi: 10.1124/jpet.105.091678. [DOI] [PubMed] [Google Scholar]
- 9.Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15:2471–2481. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fagergren P, Hurd YL. Mesolimbic gender differences in peptide CART mRNA expression: effects of cocaine. Neuroreport. 1999;10:3449–3452. doi: 10.1097/00001756-199911080-00034. [DOI] [PubMed] [Google Scholar]
- 11.Hubert GW, Jones DC, Moffett MC, Rogge G, Kuhar MJ. CART peptides as modulators of dopamine and psychostimulants and interactions with the mesolimbic dopaminergic system. Biochem Pharmacol. 2008;75:57–62. doi: 10.1016/j.bcp.2007.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hubert GW, Kuhar MJ. Cocaine administration increases the fraction of CART cells in the rat nucleus accumbens that co-immunostain for c-Fos. Neuropeptides. 2008 doi: 10.1016/j.npep.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hubert GW, Kuhar MJ. Colocalization of CART peptide with prodynorphin and dopamine D1 receptors in the rat nucleus accumbens. Neuropeptides. 2006;40:409–415. doi: 10.1016/j.npep.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Hunter RG, Jones D, Vicentic A, Hue G, Rye D, Kuhar MJ. Regulation of CART mRNA in the rat nucleus accumbens via D3 dopamine receptors. Neuropharmacology. 2006;50:858–864. doi: 10.1016/j.neuropharm.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Hunter RG, Vicentic A, Rogge G, Kuhar MJ. The effects of cocaine on CART expression in the rat nucleus accumbens: a possible role for corticosterone. Eur J Pharmacol. 2005;517:45–50. doi: 10.1016/j.ejphar.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 16.Jaworski JN, Jones DC. The role of CART in the reward/reinforcing properties of psychostimulants. Peptides. 2006;27:1993–2004. doi: 10.1016/j.peptides.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 17.Jaworski JN, Kimmel HL, Mitrano DA, Tallarida RJ, Kuhar MJ. Intra-VTA CART 55–102 reduces the locomotor effect of systemic cocaine in rats: an isobolographic analysis. Neuropeptides. 2007;41:65–72. doi: 10.1016/j.npep.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaworski JN, Kozel MA, Philpot KB, Kuhar MJ. Intra-accumbal injection of CART (cocaine-amphetamine regulated transcript) peptide reduces cocaine-induced locomotor activity. J Pharmacol Exp Ther. 2003;307:1038–1044. doi: 10.1124/jpet.103.052332. [DOI] [PubMed] [Google Scholar]
- 19.Jones DC, Kuhar MJ. CART receptor binding in primary cell cultures of the rat nucleus accumbens. Synapse. 2008;62:122–127. doi: 10.1002/syn.20476. [DOI] [PubMed] [Google Scholar]
- 20.Keller PA, Compan V, Bockaert J, Giacobino JP, Charnay Y, Bouras C, Assimacopoulos-Jeannet F. Characterization and localization of cocaine- and amphetamine-regulated transcript (CART) binding sites. Peptides. 2006;27:1328–1334. doi: 10.1016/j.peptides.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Kim JH, Creekmore E, Vezina P. Microinjection of CART peptide 55–102 into the nucleus accumbens blocks amphetamine-induced locomotion. Neuropeptides. 2003;37:369–373. doi: 10.1016/j.npep.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Kimmel HL, Gong W, Vechia SD, Hunter RG, Kuhar MJ. Intra-ventral tegmental area injection of rat cocaine and amphetamine-regulated transcript peptide 55–102 induces locomotor activity and promotes conditioned place preference. J Pharmacol Exp Ther. 2000;294:784–792. [PubMed] [Google Scholar]
- 23.Koylu EO, Couceyro PR, Lambert PD, Kuhar MJ. Cocaine and amphetamine-regulated transcript peptide immunohistochemical localization in the rat brain. J Comp Neurol. 1998;391:115–132. [PubMed] [Google Scholar]
- 24.Koylu EO, Smith Y, Couceyro PR, Kuhar MJ. CART peptides colocalize with tyrosine hydroxylase neurons in rat locus coeruleus. Synapse. 1999;31:309–311. doi: 10.1002/(SICI)1098-2396(19990315)31:4<309::AID-SYN10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Kuhar MJ, Dall Vechia SE. CART peptides: novel addiction and feeding-related neuropeptides. Trends in Neurosciences. 1999;22:316–320. doi: 10.1016/s0166-2236(98)01377-0. [DOI] [PubMed] [Google Scholar]
- 26.Kuhar MJ, Jaworski JN, Hubert GW, Philpot KB, Dominguez G. Cocaine- and amphetamine-regulated transcript peptides play a role in drug abuse and are potential therapeutic targets. Aaps J. 2005;7:E259–E265. doi: 10.1208/aapsj070125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maletinska L, Maixnerova J, Matyskova R, Haugvicova R, Sloncova E, Elbert T, Slaninova J, Zelezna B. Cocaine- and amphetamine-regulated transcript (CART) peptide specific binding in pheochromocytoma cells PC12. Eur J Pharmacol. 2007;559:109–114. doi: 10.1016/j.ejphar.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Marie-Claire C, Laurendeau I, Canestrelli C, Courtin C, Vidaud M, Roques B, Noble F. Fos but not CART (cocaine and amphetamine regulated transcript) is overexpressed by several drugs of abuse: a comparative study using real-time quantitative polymerase chain reaction in rat brain. Neuroscience Letters. 2003;345:77–80. doi: 10.1016/s0304-3940(03)00307-0. [DOI] [PubMed] [Google Scholar]
- 29.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiological Reviews. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 30.Moffett M, Stanek L, Harley J, Rogge G, Asnicar M, Hsiung H, Kuhar M. Studies of cocaine- and amphetamine-regulated transcript (CART) knockout mice. Peptides. 2006;27:2037–2045. doi: 10.1016/j.peptides.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 31.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th Edition. Sydney: Academic Press; 1998. 141 pp. [Google Scholar]
- 32.Roberts DCS, Richardson NR. Self-administration of psychomotor stimulants using progressive ratio schedules of reinforcement. In: Wu P, Boulton A, Baker GB, editors. Neuromethods: Animal Models of Drug Addiction. Vol. 24. Clifton, NJ: Humana Press; 1992. pp. 263–269. [Google Scholar]
- 33.Steiner RC, Hsiung HM, Picciotto MR. Cocaine self-administration and locomotor sensitization are not altered in CART knockout mice. Behav Brain Res. 2006;171:56–62. doi: 10.1016/j.bbr.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 34.Tang WX, Fasulo WH, Mash DC, Hemby SE. Molecular profiling of midbrain dopamine regions in cocaine overdose victims. J Neurochem. 2003;85:911–924. doi: 10.1046/j.1471-4159.2003.01740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thim L, Nielsen PF, Judge ME, Andersen AS, Diers I, Egel-Mitani M, Hastrup S. Purification and characterisation of a new hypothalamic satiety peptide, cocaine and amphetamine regulated transcript (CART), produced in yeast. FEBS Lett. 1998;428:263–268. doi: 10.1016/s0014-5793(98)00543-2. [DOI] [PubMed] [Google Scholar]
- 36.Vicentic A, Lakatos A, Kuhar MJ. CART (cocaine- and amphetamine-regulated transcript) peptide receptors: specific binding in AtT20 cells. Eur J Pharmacol. 2005;528:188–189. doi: 10.1016/j.ejphar.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 37.Vrang N, Larsen PJ, Kristensen P. Cocaine-amphetamine regulated transcript (CART) expression is not regulated by amphetamine. Neuroreport. 2002;13:1215–1218. doi: 10.1097/00001756-200207020-00029. [DOI] [PubMed] [Google Scholar]
- 38.Wise RA. Neurobiology of addiction. Current Opinions in Neurobiology. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- 39.Yang SC, Shieh KR, Li HY. Cocaine- and amphetamine-regulated transcript in the nucleus accumbens participates in the regulation of feeding behavior in rats. Neuroscience. 2005;133:841–851. doi: 10.1016/j.neuroscience.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 40.Yermolaieva O, Chen J, Couceyro PR, Hoshi T. Cocaine- and amphetamine-regulated transcript peptide modulation of voltage-gated Ca2+ signaling in hippocampal neurons. J Neurosci. 2001;21:7474–7480. doi: 10.1523/JNEUROSCI.21-19-07474.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoon HS, Kim S, Park HK, Kim JH. Microinjection of CART peptide 55–102 into the nucleus accumbens blocks both the expression of behavioral sensitization and ERK phosphorylation by cocaine. Neuropharmacology. 2007;53:344–351. doi: 10.1016/j.neuropharm.2007.05.014. [DOI] [PubMed] [Google Scholar]