Abstract
Objectives and Goal
This study assessed the acceptability after the use of vaginal lubricants as surrogates for microbicides among women in Zambia and the role of cultural factors as facilitators or impediments to their potential use for HIV risk reduction within the Zambian context.
Study Design
HIV seronegative women (N = 301) recruited from the University Teaching Hospital HIV Voluntary Counseling and Testing Center were randomized into group, individual, or enhanced usual care arms. Participants attended pre- and post-HIV test counseling, followed by a 3-session, 2-hour once-a-month intervention introducing them to vaginal lubricants (2 types of gels, suppositories) in addition to male and female condoms. Supplies were offered at months 4 and 5; assessments were at baseline, 6, and 12 months.
Results
At baseline, the majority of women reported minimal previous exposure to vaginal products and low levels of condom use. Participants’ use of products was influenced by product characteristics and perceived partner acceptability; the majority of participants preferred drier products and suppository delivery systems. The basis for decisions regarding vaginal product acceptability changed over time and followed product exposure, and was greatly influenced by perceptions of partner acceptability.
Conclusion
Results illustrate the influence of male partners on Zambian seronegative women’s preferences for microbicidal products, and the change in preferred characteristics over time.
Stabilizing rates of new infections suggest that HIV prevention may be most achievable through a combination of behavioral and biomedical interventions1 that integrate antiretroviral therapy (ART) and prevention, reducing viral load and decreasing the potential for transmission.2,3 However, women will continue to be at greater risk of infection due to early sexual debut, partner violence, migration, biologic vulnerability, and lack of preventative measures such as vaginal microbicides.4
Despite voluntary counseling and testing (VCT) programs, most HIV-positive women will be infected by their primary male partners and the majority of HIV infection in sub-Saharan Africa occurs in marital and cohabiting relationships.5,6 VCT has proven successful in reducing transmission among serodiscordant couples7,8; however, a seropositive diagnosis and counseling may increase condom use only temporarily,9,10 and many couples do not adequately protect the uninfected partner.11
Effective risk reduction interventions require a complex set of changes in a variety of behaviors. These changes include assessing and accepting sexual barrier products (i.e., male and female condoms, vaginal chemical contraceptives), adhering to their use, and both sexual partners making significant changes in their sexual practices. Our previous research in both the United States and Zambia found multisession group and individual sexual behavior interventions to increase acceptability and use of sexual barrier products including vaginal lubricants as surrogates for microbicides, among HIV seropositive women12 and men.13
Globally, the total number of persons living with HIV was estimated to be 38.6 million in late 2005, of whom over 24.5 million reside in sub-Saharan Africa.14 Zambia, a sub-Saharan country of 10.2 million persons, had prevalence rates of 17% among adults and as high as 30% among women in antenatal clinics in Lusaka,15 the capital city (1.1 million estimated infections nationwide).14
Prevention programs in Zambia rely heavily on male condom promotion. Although male condoms have high efficacy (93%–95%)16 in reducing transmission of HIV and other STDs, their impact on disease prevention has been minimal, due in large part to low acceptability and to culture-specific sexual preferences (e.g., rough sex or dry sex), lack of knowledge,17 and inconsistent use.11
Men are the primary sexual decision makers7 and are in a position of dominance. Women are expected to ask their male partners for money to feed themselves and their children, for money or gifts in exchange for sex, and for permission to work and keep the money they earn.15 Women are often limited in their ability to negotiate use of male and female condoms,18,19 and rates of female condom use are low among HIV+ women.20
Limited condom use emphasizes the need for vaginal chemical barriers such as microbicides1 that offer alternative methods of protection from disease transmission for women. Small-scale and hypothetical acceptability studies have been conducted to assess preferences regarding specific characteristics of microbicidal barrier products.11,21–25
This study examines the acceptability of sexual barrier products and lubricants as surrogates for microbicides among seronegative women in Lusaka, Zambia, and the effects of a culturally tailored sexual risk reduction intervention designed to enhance the acceptability of sexual barrier products and lubricants. Finally, we explore the impact of cultural preferences and practices on acceptability and use of sexual barrier products.
MATERIALS AND METHODS
Study Design
The NOW Project was a randomized study that recruited 549 HIV seropositive (n = 248) and high-risk seronegative (n = 301) Zambian women 18 years of age or older between January 2001 and May 2004. Details regarding study outcomes with the seropositive women in the study have been provided in previous literature.26 The following analyses were conducted using data only from the high-risk seronegative participants (n = 301).
Participant Intervention and Examination Protocol
Before participant recruitment, Institutional Review Board and Ethics Committee approvals were obtained in accordance with the provisions of the US Department of Health and Human Services regarding the conduct of research. Study candidates were recruited from local hospitals, community health centers, and nongovernmental organizations. All women had been self-referred for HIV testing because of self-identified risk within the previous 2 weeks.
All participants were screened for eligibility, provided informed consent before enrollment, and completed a baseline assessment. Participants were recruited by serostatus (participants 1–248 positive, participants 249–301 negative); seropositive participants were recruited, randomized, and participated initially, followed by seronegative participants. Participants were randomized by cohort (n = 30 per cohort) to 1 of 3 conditions, group or individual intervention or enhanced usual care, using numbers generated randomly that had previously been allocated to condition assignment (group, individual, usual care). Assessors were blind to condition assignment. All participants completed 3 monthly sessions (Fig. 1). The primary reason for ineligibility was no current sexual partner or no sexual activity within the last month.
Fig. 1.
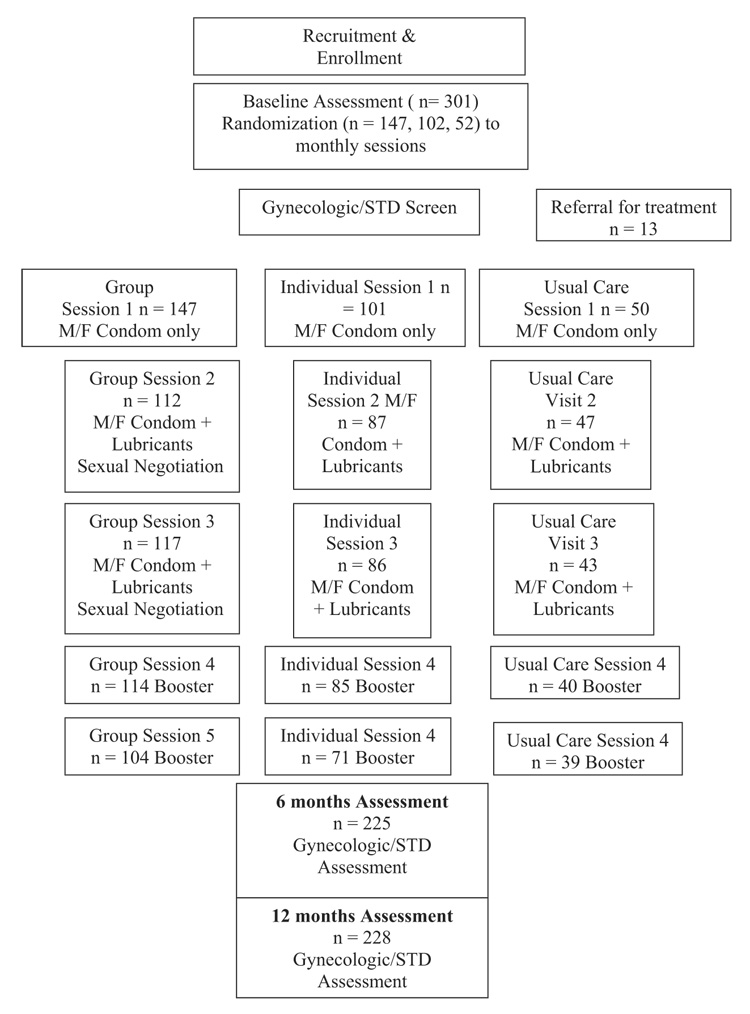
Enrollment, randomization, and assessment.
Recruiters, assessors, and interventionists were multilingual and translated into participant dialect/language (e.g., Bemba, Nyanja) any information that required clarification. Assessments were, at the request of participants, primarily conducted in English. Interventions were conducted using a combination of Bemba, Nyanja, and English, because of the mixture of audience language (73 local and 3 primary regional languages in Zambia). All participants were followed over a 12-month period (baseline assessment, sessions 1, 2, 3, brief assessments at months 4 and 5, and a 6- and 12-month postbaseline assessment). Participants were screened for STDs and vaginal infections, notified of their STD results, and provided with appropriate treatment before receiving study products. All participants received monetary compensation for their travel expenses. Assessors were blind to condition, and participants were asked not to reveal their study arm assignment.
Intervention Group Condition
The intervention group condition was developed and manualized from feedback from pilot studies with multicultural women, and has been described in earlier literature.13 The group condition emphasized participation and experimentation with sexual barrier products and lubricants and provided an opportunity for practice, feedback, and reinforcement of sexual risk reduction strategies. The group structured behavioral change intervention was limited to 10 participants. Each of the 3 monthly 2-hour sessions emphasized group cohesion and skill building in a supportive environment using communication techniques, negotiation skills, and experiential/interactive skill training to expand and reframe perceptions of sexual barrier product use, and to increase self-efficacy and skill mastery. Material was presented using the conceptual model of the theory of reasoned action and planned behavior.27 Facilitators were multilingual female registered nurses, licensed practical nurses, and health care staff trained in the administration of each condition.
The intervention and accompanying videos were developed in English and translated into Zambian local languages (Nyanja and Bemba). The correct methods of sexual barrier and lubricant use, commonly asked questions, and sexual negotiation scenarios were presented and discussed. After each session, participants were provided with a 1-month supply of male and female condoms (sessions 1–3; visits 4–5) and vaginal lubricants (sessions 2–3; visits 4–5). Participants were strongly encouraged to use condoms with the vaginal lubricants during each act of sexual intercourse.
Vaginal lubricants used were high-viscosity gels (Astroglide Silken Secret, tube twist top applicator, single dose) and low-viscosity gels (KY Jelly, fillable tube applicator with multiple dose gel) and suppositories (Lubrin single dose vaginal insert suppository). Each lubricant was provided in 3 doses per session (9 doses in total per month).
Intervention Individual Condition
The individual condition provided participants with time-matched information in a traditional health education individual format with a health educator. Sessions included information on HIV/STD transmission, hierarchical counseling, and skill training to facilitate product use, videos, written materials on instructions for use, and supplies of male and female condoms and vaginal lubricants.
Enhanced Usual Care Condition
The enhanced usual care condition was a time-matched health education format with a health educator on health issues with instructional videos. Participants were provided with sexual barrier product supplies.
In all conditions, after the 3 sessions, participants reported their use of sexual barrier products and lubricants at months 4 and 5, at which time they received additional supplies.
Assessments
All measures were administered at baseline, 6- and 12-months postbaseline.
Demographic Questionnaire
This questionnaire included data collection on age, religion, nationality, ethnicity, educational level, employment status, residential status, HIV serostatus [date of HIV infection (if known), mode of infection with HIV], marital status/current partner status, living situation, number of children, and serostatus.
Sexual Activities Questionnaire
This 55-item scale was adapted from the Sexual Risk Behavior Assessment Schedule.28 Responses indicated the frequency of heterosexual sexual intercourse (vaginal, oral, and anal) with both primary partners (most frequent sexual relations) and nonprimary partners (any other male partners). The questionnaire also assessed sexual barrier and lubricant use, HIV status of the partner(s), known sexual practices of the partner, and alcohol or drug use before the initiation of sexual activity.
Acceptability Measures
These 25-item scales were developed using feedback from participants during pilot testing in the United States and Zambia and assessed most preferred product, perceived ease of use, comfort, fun, sexual pleasure, control, communication, confidence, and secrecy. The scales include rating and comparison between products using combined Likert-like scales of 1 to 7 (“definitely yes” to “definitely no”), personal and partner acceptability, personal and partner reactions to products, and ranking of attributes as most to least important.
Barrier Questionnaire
This measure was adapted from the University of California at San Francisco Center for AIDS Prevention Studies Barrier Questionnaire, and measures current and previous use of and willingness to use sexual barriers using a Likert-type scale based on specific characteristics (current and previous use: never used, like very much = 5, like somewhat = 4, neutral = 3, dislike somewhat = 2, strongly dislike = 1; willingness to use: not at all willing to use = 1, slightly willing = 2, moderately willing = 3, very willing = 4). Stem prompts were adapted to state willingness “to use” products. Subscale scores on barrier acceptability were compiled for (a) willingness to use product type after trial use, (b) willingness to use product, (c) product characteristics, (d) timing of product use, and (e) type of protection by product.
Lubricant Dosage
Vaginal lubricants were provided in 2 formats, full dose and chosen dose. Participants were asked to use the products at their full dosage (5 mL) initially, and to select their most preferred product and the amount of their most preferred dose at follow-up. 3–5 Reports were made using a Likert scale of 1 to 14.
Statistical Analyses
This study used a repeated measures design with experimental arm (group, individual, usual care) as the between-subjects factor and time (baseline and postintervention) as the within-subjects factor and controlled for partner serostatus. Correlations are reported as Pearson r statistics; repeated measures between arms are reported as F statistics; and effects of time on the sample are reported as t tests, and all comparisons used an α (2-tailed) of 0.05. Data were analyzed using the Statistical Package for Social Sciences (SPSS).
RESULTS
Participants
Participants (n = 301) were 18 years of age or older, HIV seronegative, sexually active, and living in urban Lusaka. The mean age of our participants was 29; 80% were unemployed, 10% worked part time. Ethnic groups included Bemba (25%), Nsenga/Ngoni/Tumbuka (31%), Tonga (12%), Lozi (13%), Mambwe/Namwanga (7%), and other ethnic groups (12%). The average level of educational attainment was completion of the 6th grade, ranging from primary school education (67%) to secondary education (33%). Most were married (94%), with children (96%; mean number of children = 3). Thirty-two percent of participants planned to have more children. There were no significant demographic differences between group, individual, and usual care arm participants. There were no significant differences in retention between condition at 6 month (78%) and 12 month (77%) follow-up. The primary reasons for loss to follow-up were death, illness, change of residence, and employment.
Baseline Sexual Behavior
The majority of women had been sexually active in the last month (86%). Women with seropositive partners were significantly more likely to use sexual barriers and lubricants (F = 4.99, P = 0.008); 8% of the sample had seropositive partners. In contrast, there was no difference in the use of sexual barriers or lubricants between those who knew their partner’s status and those who did not (F = 2.99, P = 0.05); 42% knew their partner’s serostatus. Contrary to expectations of higher levels of the practice, only 7% of participants reported using products to make sex drier. Twelve percent of women thought their partners had additional sexual partners outside their primary relationship, though there was no difference in sexual barrier and lubricant use between those women whose partners potentially had additional sexual partners and those who did not (F = 0.16, P = 85). Consistent male condom use was low at baseline (Table 1). A linear regression analysis was conducted to assess the relative weight of the barrier and lubricant product types and characteristics on sexual barrier and lubricant use at baseline. Because of lack of previous exposure to most products, only timing was associated with product use (r = 0.203, P <0.001) but accounted for only 0.4% of the variance. At baseline, 69% of participants had tried male condoms, 15% had tried female condoms, and 1% had tried gels or suppositories. There were no differences in sexual behavior between arms at baseline.
TABLE 1.
Sexual Behavior: One-Month Survey at Baseline, 6 and 12 Month
| Domain | I Base-line | G Base-line | U/C Base-line | I: 6 mo | G: 6 mo | U/C: 6 mo | I: 12 mo | G: 12 mo | U/C: 12 mo |
|---|---|---|---|---|---|---|---|---|---|
| Consistent use of | |||||||||
| Any type of protection used consistently | 16 | 16 | 22 | 43 | 29 | 24 | 30 | 24 | 21 |
| What type of protection chosen when used | |||||||||
| Male condom use | 47 | 41 | 56 | 36 | 18 | 9 | 27 | 24 | 21 |
| Female condom use | 0 | 2 | 0 | 1 | 1 | 0 | 0 | 3 | 3 |
| Lubricants | 0 | 0 | 0 | 1 | 0 | 3 | 0 | 0 | 0 |
| Lubricants + condoms | 0 | 0 | 0 | 15 | 9 | 6 | 4 | 3 | 10 |
All values are given in percentages.
Acceptability and Willingness to Use Products: 6 and 12 Months
At 6 months, virtually all participants in all 3 conditions had tried (trial use) all sexual barrier and lubricant products, male (99%) and female (99%) condoms, gels (99%), and suppositories (98%). At 12 months, participants expressing “willingness to use” all sexual barrier and lubricant products in all 3 conditions comprised more than 90% of the sample. Willingness to use sexual barriers and lubricants increased in all conditions across the duration of the intervention (Table 1). A linear regression analysis was conducted to assess the relative weight of the barrier and lubricant product types and characteristics on sexual barrier and lubricant use at both follow-up time points. At time 2, after 6 months of product exposure, all characteristics were associated with product use (r = 0.39–0.42); the type of barrier and lubricant participants were willing to use and timing accounted for 18% and 19% of the variance, respectively, R2 change = 0.02, P = 0.037.
At time 3, after 12 months of exposure, willingness to use, characteristics, and protection were moderately associated with product use (r = 0.26–0.44), and timing was no longer associated (r = 0.003, P = 0.49). Only product characteristics and the protection offered accounted for a significant proportion of the variance, 19% and 24% of the variance, respectively, R2 change = 0.04, P =0.001.
Product acceptability of all products was significantly higher among the group condition for each of the 3 products at each time point using an analysis of variance (ANOVA; see Table 3). However, a repeated measures analysis did not reveal significant differences between conditions over time. At time 3, a linear regression analysis was conducted to assess the relative weight of acceptability on product use; variables entered included participant liking and acceptability and partner liking and acceptability. All variables were correlated with product type used, but only partner acceptability accounted for a significant proportion of the variance (19%; R2 = 0.17, R2 change P <0.001). In addition, when product willingness to use, characteristics, timing, protection, and partner acceptability were entered in a linear regression, all variables, excluding timing, were moderately associated with product use (r = 0.18–0.45). Product characteristics (R2 = 0.19, R2 change = 0.19, P <0.001), partner acceptability (R2 = 0.31; R2 change = 0.11, P <0.001), willingness to use (R2 = 0.33, R2 change = 0.02, P = 0.006), and protection (R2 = 0.35; R2 change = 0.02, P = 0.01) accounted for a significant proportion of the variance in product use. Finally, the relative weight of partner acceptability in combination with all barrier and lubricant product subscales was analyzed; partner acceptability accounted for 75% of the variance (R2 change = 0.75, P <0.001).
TABLE 3.
Mean Willingness to Use Products After Trial Use: Barrier Subscale Scores Baseline, 6 and 12 Months
| Percent Willing to Use: |
|||||||
|---|---|---|---|---|---|---|---|
| Entire Sample | I: 6 mo | G: 6 mo | U/C: 6 mo | I: 12 mo | G: 12 mo | U/C: 12 mo | |
| Type of product | 21.18 | 20.38 | 20.83 | 22.28 | 22.15 | 22.14 | 21.63 |
| Characteristics | 36.00 | 37.54 | 37.21 | 37.51 | 38.48 | 28.82 | 36.71 |
| Timing | 12.98 | 12.85 | 13.02 | 12.95 | 13.53 | 12.78 | 13.37 |
| Protection | 22.70 | 22.82 | 23.01 | 32.74 | 23.13 | 23.50 | 23.52 |
The group intervention had the highest comparative acceptability scores on each product at each follow-up time point, though the within group result did not differ significantly over time; suppositories were the most preferred in the group condition. The perceived acceptability by partners regarding suppositories was significantly higher for the group condition over time (F = 3.20, P = 0.005). Preferences regarding product delivery system, characteristics, timing, and protection are presented in Table 2.
TABLE 2.
Percent Willing to Use Products After Trial Use: Baseline, 6 and 12 Months
| Percent Willing to Use: |
|||||||
|---|---|---|---|---|---|---|---|
| Entire Sample | I: 6 mo | G: 6 mo | U/C: 6 mo | I: 12 mo | G: 12 mo | U/C: 12 mo | |
| Type of product | |||||||
| Male condoms | 84.6 | 95.0 | 96.6 | 97.4 | 96.3 | 99.1 | 100 |
| Female condoms | 91.0 | 98.7 | 94.1 | 97.3 | 96.3 | 98.3 | 100 |
| Gel | 82.0 | 84.8 | 86.3 | 89.7 | 92.6 | 89.2 | 84.2 |
| Suppositories | 73.4 | 86.1 | 91.5 | 89.7 | 91.3 | 97.3 | 92.1 |
| Characteristics | |||||||
| Products with no smell | 85.0 | 92.4 | 88.9 | 84.7 | 98.8 | 94.6 | 94.7 |
| Products with no taste | 64.0 | 73.4 | 81.2 | 79.5 | 87.6 | 85.6 | 68.4 |
| Products with a range of flavors | 79.7 | 87.3 | 79.5 | 82.1 | 78.8 | 79.3 | 86.9 |
| Products with an applicator | 77.3 | 84.8 | 89.7 | 92.3 | 87.5 | 93.7 | 89.5 |
| Products requiring touching genitals | 87.4 | 81.0 | 90.6 | 97.4 | 95.0 | 99.1 | 92.1 |
| Timing | |||||||
| Products requiring insertion just before sex | 79.9 | 74.7 | 90.6 | 92.3 | 92.5 | 98.2 | 94.7 |
| Products that could be inserted 4 h before sex | 65.0 | 59.5 | 60.3 | 58.9 | 66.3 | 50.4 | 73.7 |
| Products requiring insertion with each sex act | 63.9 | 73.4 | 80.4 | 82.1 | 78.8 | 89.2 | 81.6 |
| Products requiring a 20 min wait before sex | 79.0 | 78.5 | 64.1 | 58.9 | 79.1 | 48.6 | 65.8 |
| Characteristics | |||||||
| Products that leak a small amount | 64.6 | 79.7 | 65.8 | 43.6 | 57.6 | 67.6 | 55.3 |
| Products that leak a large amount | 14.6 | 21.5 | 14.6 | 23.1 | 19.0 | 22.5 | 10.5 |
| Products that stay slippery for 20 min | 57.0 | 69.6 | 66.7 | 51.3 | 62.5 | 66.7 | 57.9 |
| Products that make sex wet | 29.5 | 38.0 | 40.2 | 43.6 | 46.3 | 54.1 | 42.1 |
| Products that make sex dry | 68.7 | 49.4 | 46.1 | 59.0 | 58.8 | 42.3 | 42.1 |
| Products that can be used without partner knowledge | 67.0 | 51.9 | 58.1 | 74.4 | 70.0 | 66.7 | 68.4 |
| Products that can be tasted by a partner | 53.0 | 72.2 | 67.5 | 59.0 | 65.0 | 72.1 | 63.2 |
| Products that could cause itching, burning, tingling | 10.0 | 7.6 | 7.7 | 12.8 | 7.5 | 4.5 | 2.6 |
| Protection | |||||||
| Products protecting against HIV | 98.3 | 100 | 98.3 | 97.4 | 100 | 100 | 100 |
| Products allowing pregnancy and preventing HIV/STDs | 75.0 | 68.4 | 76.1 | 76.9 | 77.2 | 86.4 | 89.5 |
Barrier and lubricant product subscale scores did not differ between condition over time (willingness to use product type after trial use [F = 1.49, P = 0.20], product characteristics [F = 0.51, P = 0.73], timing of product use [F = 1.55, P = 0.19], type of protection by product [F = 0.77, P = 0.55]) with the exception of willingness to use specific products (F = 3.84, P = 0.023). When the items within the subscales were extrapolated, the group condition was found to be significantly more willing over time to use products that increased wetness after trial use (F = 3.86, P = 0.004). Both intervention conditions reported greater willingness to use products in general at the 12-month follow up (F = 3.84, P = 0.023). Subscale means are presented in Table 3.
Product Characteristic Importance
After trial use, participants identified knowing that it works, increasing sexual pleasure, comfort of use, and ease of use as being the most important factors in product preference; being “fun” to use and having the potential to use a product secretly were considered “least important.”
Product Preference
Overall product ratings based on product selection and stated preference indicated that vaginal suppositories (53%) were the most preferred delivery system and product in contrast to a highviscosity gel (29%) or low-viscosity gel (18%).
Lubricant Dosage
Vaginal lubricant chosen dose ranged from partial dose (2–3 mL) to full dose (5 mL). Participants were asked to use the products at their full dosage (5 mL) initially and to select their most preferred product and the amount of their most preferred dose at follow-up (2–3–5 mL). The mean level of dose differed between products, and least viscous gels were preferred at the lowest dose, i.e., half of the full dose, suppositories at one-half of 5 mL, and highly viscous gels at the highest dose (5 mL). The modal distribution of responses for gels indicated a preference for no greater than 3 mL, and the most preferred product, suppositories, was preferred at a dose below 2.5 mL.
DISCUSSION
Although adding lubrication to sex is not a preferred practice in sub-Saharan Africa, this study suggests that interventions can influence the use and acceptability of lubricating products. In addition, it is clear that the basis for decisions regarding vaginal product acceptability change over time and after product exposure and are greatly influenced by perceptions of partner acceptability. In keeping with cultural preferences, the majority of participants preferred drier products. Results illustrate the importance of a variety of delivery systems and product characteristics to enhance product acceptability in the Zambian context.
At baseline, the majority of women reported minimal previous exposure to vaginal products, moderate levels of acceptability of sexual barrier and lubricants, and low levels of condom use. After exposure, participants reported increased willingness to use sexual barriers and lubricants and increased acceptability of all products. Product type, such as suppositories, and timing, such as immediate use, were the most acceptable and predicted decisions regarding product use.
At long-term follow-up, a suppository delivery system was the most popular product, and the lowered doses (e.g., 2–3 mL) of all lubricant products were preferred. Product characteristics, such as wetness and delayed use, were most disliked. Perceptions of product characteristics, perceptions of partner’s level of acceptability of products and protection were predictive of decisions to use products. Participant acceptability was primarily predicted by perceived partner preferences. Participants in the group condition reported comparatively greater acceptability than individual or usual care conditions, and male preference appeared to influence product selection. In contrast with previous studies assessing sexual behavior over shorter time periods, participants reported sustained increases in use of sexual barrier and lubricant products up to 12 months postbaseline.
Overall response to these adapted sexual barrier interventions was quite favorable. Our previous research with HIV seropositive men and women in Zambia found that interventions increased acceptability and barrier and lubricant use,26 but this finding does not seem equally generalizable to seronegative women. Although acceptability increased, consistent use of protection decreased over time. It must be remembered that the women participating were enrolled after an HIV seronegative test, and at baseline were highly motivated to maintain their negative status. However, sexual health behaviors diminished over time, consistent with other populations studied over time.9,10 It may also be that baseline scores for condom use reflect a social desirability bias that should be controlled for in future studies through measurement of this phenomenon or the use of computer-assisted interviewing techniques.
Although this study focused on preferences of Zambian women, the role of Zambian men as the culturally defined decision makers must also be considered in product development and future interventions to increase acceptability. Sexual behavior does not occur in a vacuum. As new strategies for HIV prevention are developed, research on acceptability and sexual barrier use must address the preferences of both men and women within the context of both their sexual and cultural relationships.
From a public health standpoint, vaginal microbicides seem to represent a potentially effective and culturally congruent HIV intervention. The challenge remains to establish microbicides as a viable prevention alternative using behavioral interventions to enhance acceptability.
Acknowledgments
This research was made possible by a grant from the National Institute of Mental Health RO1MH63630. The authors acknowledge the members of our research teams at the University of Miami Miller School of Medicine, the University Teaching Hospital in Lusaka, community sites providing referrals in Zambia and their study participants.
Footnotes
Publisher's Disclaimer: This PDF receipt will only be used as the basis for generating PubMed Central (PMC) documents. PMC documents will be made available for review after conversion (approx. 2–3 weeks time). Any corrections that need to be made will be done at that time. No materials will be released to PMC without the approval of an author. Only the PMC documents will appear on PubMed Central -- this PDF Receipt will not appear on PubMed Central.
References
- 1.Ramjee G. Microbicides and other prevention technologies; Program and abstracts of the XVI International AIDS Conference; August 13–18; Toronto, Ontario, Canada. 2006. Plenary presentation TUPL02. [Google Scholar]
- 2.Bunnell R, Wamai N, Ekwaru JP, et al. Changes in sexual behavior and risk of HIV transmission after two years of antiretroviral therapy and prevention interventions in rural Uganda; Program and abstracts of the XVI International AIDS Conference; August 13–18; Toronto, Ontario, Canada. 2006. Abstract MOAC0204. [DOI] [PubMed] [Google Scholar]
- 3.Rapatski BL, Suppe F, Yorke JA. Reconciling different infectivity estimates for HIV-1. J Acquir Immun Defic Syndr. 2006;43:253–256. doi: 10.1097/01.qai.0000243095.19405.5c. [DOI] [PubMed] [Google Scholar]
- 4.Beyrer C. HIV epidemiology update and transmission factors: Risks and risk contexts; Program and abstracts of the XVI International AIDS Conference; August 13–18; Toronto, Ontario, Canada. 2006. Plenary presentation MOPL02. [DOI] [PubMed] [Google Scholar]
- 5.Hira SK, Feldblum PJ, Kamanga J, et al. Condom and nonoxynol-9 use and the incidence of HIV infection in serodiscordant couples in Zambia. Int J STD AIDS. 1997;8:243–250. doi: 10.1258/0956462971919994. [DOI] [PubMed] [Google Scholar]
- 6.McKenna SL, Muyinda GK, Roth D, et al. Rapid HIV testing and counseling for voluntary testing centers in Africa. AIDS. 1997;11 suppl 1:S103–S110. [PubMed] [Google Scholar]
- 7.Roth DL, Stewart KE, Clay OJ, et al. Sexual practices of HIV discordant couples in Rwanda: Effects of a testing and counseling program for men. Int J STD AIDS. 2001;12:181–188. doi: 10.1258/0956462011916992. [DOI] [PubMed] [Google Scholar]
- 8.Allen SA, Karita E, N'Gandu N, et al. The evolution of voluntary testing and counseling as an HIV prevention strategy. In: Gibney L, DiClemente RJ, Vermund SH, editors. Preventing HIV in Developing Countries: Biomedical and Behavioral Approaches. New York: Plenum Press; 1999. pp. 87–108. [Google Scholar]
- 9.Greenberg J, Hennessy M, MacGowan R, et al. Modeling intervention efficacy for high-risk women. The WINGS Project. Eval Health Prof. 2000;23:123–148. doi: 10.1177/016327870002300201. [DOI] [PubMed] [Google Scholar]
- 10.Rugpao S, Koonlertkit S, Pinjaroen S, et al. Patterns of male condom use and risky sexual behaviors in Thai couples receiving ongoing HIV risk reduction counseling; XV International AIDS Conference; July 11–16, 2004; Bangkok. 2004. (abstract no. ThPeC7408) [Google Scholar]
- 11.Whitehead SJ, Chaikummao S, Uthaiworavit W, et al. HIV-discordant couples identified through screening for a clinical trial of microbicide safety: A chance to prevent HIV transmission; XV International AIDS Conference; July 11–16, 2004; Bangkok. 2004. [Google Scholar]
- 12.Jones DL, Weiss SM, Bhat GJ, et al. A sexual barrier intervention for HIV+/− Zambian women: Acceptability and use of vaginal chemical barriers. J Multicult Nurs Health. 2004;10:27–31. [PMC free article] [PubMed] [Google Scholar]
- 13.Jones D, Ross D, Weiss SM, et al. Influence of partner participation on sexual risk behavior reduction among HIV-positive Zambian women. J Urban Health. 2005;82:92–100. doi: 10.1093/jurban/jti111. [DOI] [PubMed] [Google Scholar]
- 14.UNAIDS. Executive Summary. 2006 Report on the Global AIDS Epidemic. 2006 Available from: www.unaids.org/bangkok2004/GAR2004>html/ExecSummar. Retrieved January 31, 2007.
- 15.Central Statistical Office. ANC Sentinel Surveillance of HIV/Syphilis Trends in Zambia 1994–2002. Zambia: Central Statistical Office; 2002. [Google Scholar]
- 16.Kamb ML, Fishbein M, Douglas JM, Jr, et al. Project RESPECT study group. Efficacy of risk reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases: A randomized controlled trial. JAMA. 1998;280:1161–1167. doi: 10.1001/jama.280.13.1161. [DOI] [PubMed] [Google Scholar]
- 17.Lagarede E, Carael M, Glynn JR, et al. The Study Group on the Heterogeneity of HIV Epidemics in African Cities. Educational level is associated with condom use within non-spousal partnerships in four cities of sub-Saharan Africa. AIDS. 2001;15:1399–1408. doi: 10.1097/00002030-200107270-00009. [DOI] [PubMed] [Google Scholar]
- 18.Macaluso M, Demand M, Artz LM, et al. Female condom use among women at high risk of sexually transmitted disease. Fam Plan Perspect. 2000;32:138–144. [PubMed] [Google Scholar]
- 19.Francis-chizororo M, Natshalaga NR. The female condom: Acceptability and perception among rural women in Zimbabwe. African J Reprod Health. 2003;7:101–116. [PubMed] [Google Scholar]
- 20.Kalichman SC, Cain D, Zweben A, et al. Sensation seeking, alcohol use and sexual risk behaviors among men receiving services at a clinic for sexually transmitted infections. J Stud Alcohol. 2003;64:564–569. doi: 10.15288/jsa.2003.64.564. [DOI] [PubMed] [Google Scholar]
- 21.Mantell JE, Myer L, Carballo-Dieguez A, et al. Microbicide acceptability research: Current approaches and future directions. Soc Sci Med. 2005;60:319–330. doi: 10.1016/j.socscimed.2004.05.011. Review. [DOI] [PubMed] [Google Scholar]
- 22.Holt BY, Morwitz VG, Ngo L, et al. Microbicide preference among young women in California. Women’s Health (Larchmt) 2006;15:281–294. doi: 10.1089/jwh.2006.15.281. [DOI] [PubMed] [Google Scholar]
- 23.Hammett TM, Mason T, Joanis C. Acceptability of formulations and application methods for vaginal microbicides among drug-involved women: Results of product trials in three cities. Sex Transm Dis. 2000;27:119–126. doi: 10.1097/00007435-200002000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Elias C, Coggins C. Acceptability research on female-controlled barrier methods to prevent heterosexual transmission of HIV: Where have we been? Where are we going? J Womens Health Gend Based Med. 2001;10:163–173. doi: 10.1089/152460901300039502. Review. [DOI] [PubMed] [Google Scholar]
- 25.Tolley EE, Eng E, Kohli R, et al. Examining the context of microbicide acceptability among married women and men in India. Cult Health Sex. 2006;8:351–369. doi: 10.1080/13691050600793071. [DOI] [PubMed] [Google Scholar]
- 26.Jones DL, Bhat GJ, Weiss SM, et al. Influencing sexual practices among HIV positive Zambian women. AIDS Care. 2006;18:629–634. doi: 10.1080/09540120500415371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albarracin D, Johnson BT, Fishbein M, et al. Theories of reasoned action and planned behavior as models of condom use: A meta-analysis. Psych Bull. 2001;127:142–161. doi: 10.1037/0033-2909.127.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer-Bahlberg HFL, Ehrhardt AA, Exner TM, et al. Sexual Risk Behavior Assessment Schedule: Adult (SERBAS-A-DF-4) Manual. 1990 [Google Scholar]


