Abstract
The neuropeptide substance P (SP) is a known inflammatory mediator released from cutaneous peripheral nerve terminals. SP effects on cellular composition in the cutaneous response to injury remain unclear. Based on our previous observations about SP effects on wound repair, we hypothesized that topical SP increases inflammatory cell density infiltration early after injury. A full thickness 1.5×1.5 cm-square wound was created on the dorsum of 8–9 wk old C57BL/6J-m+Leprdb mice (db/db). Wounds were treated daily with 300μl of either normal saline (0.9% NaCl) or 10−9M SP for seven days. Three wounds from each group were harvested at 2,3,7,14, and 28 days. Samples underwent enzymatic digestion and were incubated with fluorescent-labeled antibodies. Using flow cytometry, cellular content and density for each sample was derived. Masson Trichrome stained histology specimens were prepared to confirm results. Cell density in the SP-treated wounds (11.3×107 cells/gram tissue, SD +/−1.5×107) was greater than in NaCl-treated wounds (7×107 cells/gram tissue, SD +/−2.3×107, p<.05) at day 7 post-wounding. Substance P significantly increased the density of leukocytes (2.1×107, SD +/−3.6×106 vs. 1.8×107, SD+/−4.9×105, p<.02) 3 days after wounding and the density of macrophages (2.9 ×107, SD+/−7.5×106 vs. 1.3×107, SD+/−1.4×106, p<.05) 7 days after wounding. There were no significant differences in endothelial cell, leukocyte or macrophage density at later time points. Topical SP treatment increases early inflammatory density in the healing wounds of db/db mice. These data support a role for nerve-mediated inflammation in cutaneous wound repair.
Keywords: Wound, Cell, Substance P, Macrophage, Leukocyte
Introduction
Extensive data suggest that neuropeptides may be involved in normal wound repair responses. SP is released by unmyelinated cutaneous sensory nerves after noxious stimuli(1). We have previously localized nerve fibers containing SP in human skin and burn wounds, particularly near blood vessels and sweat glands(2). Efferent nerve stimulation and SP release produces local vasodilatation with plasma extravasation in peripheral skin(3, 4). SP induces vascular permeability via nitric oxide production(5) and induces endothelial cell production of cytokines(6). SP stimulates angiogenesis(7), mast cell release of histamine(8, 9), mitogenesis and migration of keratinocytes, macrophages, T cells, smooth muscle cells and fibroblasts(10–12). Further, it recruits macrophages and neutrophils in vitro(13–15). However, the definitive role of SP in cellular response to injury in the healing wound remains undefined.
Diabetic wounds remain a profound clinical problem, likely affected by complex factors, including vascular, immune, neural and biochemical pathways(16). Recently peripheral neurosensory pathways responsible for wound healing dysfunction have been suggested (6). Wound healing in diabetic tissues may be negatively affected by diminished proinflammatory neuropeptides such as substance P (SP). Diabetic skin has been noted to have decreased nerve numbers (17) and elevated neutral endopeptidase (NEP) activity, which is known to degrade SP(18). The diabetic murine excisional wound model has been validated by several investigators as a useful model for studying perturbation of the wound environment(19, 20). We have shown that like human skin, diabetic murine skin has fewer epidermal nerves than that of non-diabetic littermates, and have correspondingly longer wound healing times in diabetic mice versus non-diabetic littermates(21). We have also reported that topical SP(21) and NEP inhibition(22) improve wound repair kinetics in diabetic mice. However, the specific effects of SP on the cellular composition of healing wounds are not clear. The role of inflammation in healing diabetic wounds has been controversial. Whereas some studies report that early inflammation is delayed in murine diabetic wounds, others report abnormally increased inflammatory responses. Based on our previous data that inflammation at 72 hours is reduced in diabetic murine wounds(22), we hypothesized that topical SP increases inflammatory cell infiltration early after injury.
Methods and Materials
All animal work was in accordance with IACUC guidelines and approved by the Animal Care Committee of the University of Washington, Seattle, WA. Genetically diabetic male C57BL/KsJ-m+/+ Leprdb mice were purchased (Jackson Laboratories; Bar Harbor, ME) and acclimated to vivarium conditions for two weeks. Wounding was performed as previously described(21, 23). Mice (8–9wk old) were anesthetized with intraperitoneal injection of ketamine (150 mg/kg, Phoenix Pharmaceuticals, Inc., St. Joseph, MO) and xylazine (10mg/kg; Phoenix Pharmaceuticals, Inc., St. Joseph, MO). Dorsal hair was shaved and a depilatory agent (Nair, Carter Wallace, Inc., NY) was applied for 30 seconds. The skin was cleaned using 70% alcohol. A full-thickness, dorsal, 1.5×1.5 cm square dorsal full thickness excisional wound was created using a scalpel through the panniculous carnosus muscle. Wounds were tattooed 2 mm from each of four corners to mark the original wound margins and covered with a semi-occlusive dressing (Tegaderm, 3M, MN), adhered to the skin with use of a liquid adhesive spray (Mastisol, Ferndale Laboratories, MI). Mice were randomly assigned to treatment with 300 microliters of either 0.9% normal saline (NaCl) or 10−9M SP daily for seven days (day 0–6). Treatment was performed using a 30 gauge needle injection through the dressing, so as to prevent dressing changes. Following euthanasia, wounds from three mice in each group were harvested at 2,3,7,14 and 28 days. Wounds were harvested sharply with a 5mm margin around the wound bed dissecting down to the deep fascia.
In order to quantify cellular response to injury at the wound site, we used tissue dispersion followed by flow cytometry, which has been validated as a reliable cell quantification technique by comparison with immunohistochemical results (23). Briefly, tissue samples were weighed and minced to 2mm2 sections at 4°C. Following overnight incubation at 4°C in dispase solution [5mL/g tissue; HBSS containing 1mg/mL dispase I (Roche Molecular Biochemicals, Indianapolis, IN), 3% heat-inactivated fetal calf serum, 1:100 penicillin/streptomycin solution (Sigma, St. Louis, MO)], the tissue was further minced to smaller sections and placed in hyaluronidase solution [20mL/g tissue; RPMI containing hyaluronidase I (1mg/mL, Sigma, St. Louis, MO), collagenase D (1mg/mL, Roche Molecular Biochemicals, Indianapolis, IN), DNAase (150μ/mL, New England Biolabs, Beverly, MA), 1:100 penicillin/streptomycin solution (Sigma)] in a shaking water bath at 37°C for 2 hours. The dispersed tissue was agitated vigorously with a pipette and passed through a 70-μm nylon cell strainer (Sigma, St. Louis, MO) along with the dispase solutions. Samples were centrifuged at 900xg for 10 minutes at 4°C. Cells were re-suspended in 2mL buffer (PBS with 2% heat-inactivated fetal calf serum, 0.1% sodium azide) and a manual cell count, using trypan blue, was performed on each sample to exclude nonviable cells. Cells (106 cells/μL) were incubated with rat anti-mouse CD16 antibody (BD Biosciences, San Jose, CA) at 4°C for 30 minutes to minimize nonspecific staining of leukocyte Fc receptors.
Aliquots of 106 cells were incubated with 2bl FITC-labeled antibodies (F4/80 for macrophages, CD45 for leukocytes; CD31 for endothelial cells and rabbit IgG for controls; 1 μg/106 cells; BD Biosciences) for one hour at 4°C. The cells were washed with 1 mL PBS, fixed with 1% paraformaldehyde, and stored in the dark at 4°C until flow cytometry analysis. FACScan (BD Biosciences) and WinMDI software version 2.8 were used for analysis; excitation was at 488nm and fluorescence was detected at 530nm. Percent cellular content and density for each sample was derived after subtraction of Ig control staining.
The data were standardized to tissue sample weight and reported as cells per gram of tissue. Each sample was assayed in triplicate. The results were analyzed by one-way ANOVA and probability values less than 0.05 were accepted as statistically significant. The standard deviation was calculated for each comparison and indicated in the figures using error bars.
For confirmation, wounds harvested from db/db mice after treatment with SP or NaCl according to the protocol described above were also prepared for histology to examine inflammatory cell infiltrate. Wounds were fixed in neutral buffered formalin, embedded in paraffin, and processed for conventional histology using Masson’s Trichrome stain. Given the increased inflammatory cell density by flow cytometry at day 3, we selected wounds at day 3 for histologic comparison.
Results
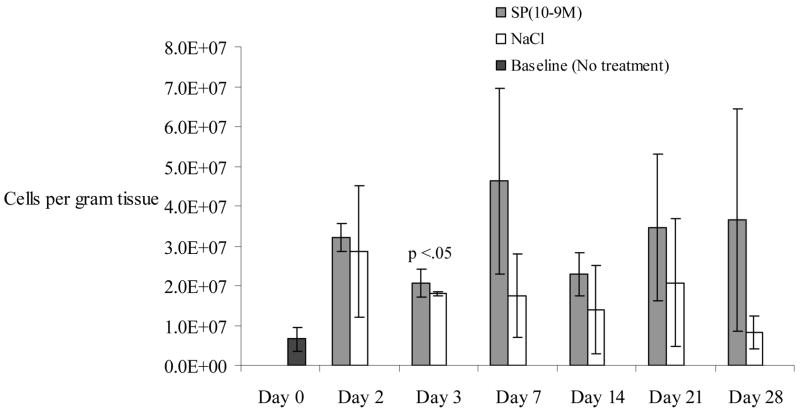
None of the 1.5 × 1.5 cm dorsal wounds had completely healed at day 28. Cell density in SP-treated wounds (5.2 × 107 cells/gram tissue) was increased on day 3 compared to NaCl-treated wounds (2.7 × 107 cells/gram tissue), but data only approached significance (p=.06). By post wounding day 7, cell density in all wounds increased (Figure 1), but more so in SP-treated wounds (11.3 × 107 cells/gram tissue, SD +/−1.5×107) than in NaCl-treated wounds (7 × 107 cells/gram of tissue, SD +/−2.3×107, p<.05). This trend in cell density decreased after day 7.
Figure 1.
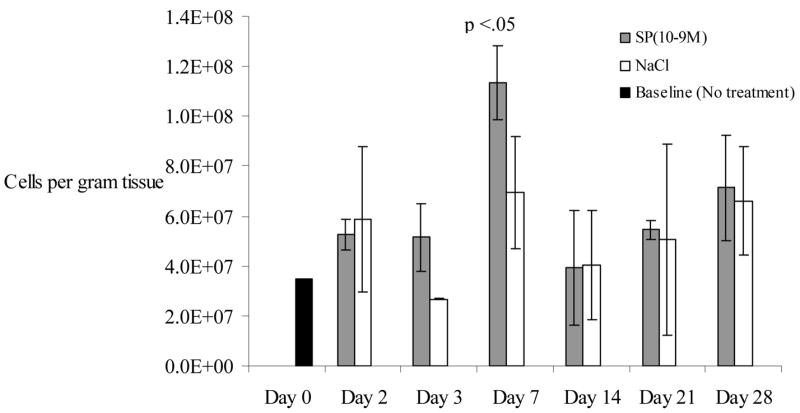
Overall cell density (# cells/gram of wound tissue) is described in the y axis of the graph below. This represents the number of cells quantified per gram of tissue harvested. Cell density is compared in cutaneous wounds following treatment with substance P (10−9M) or NaCl. Cell density is transiently increased on days 3 and 7 following injury compared to normal saline treatment, with day 7 reaching statistical significance (p<0.05). “Day 0” represents the skin harvested during the wounding, with neither SP nor NaCl treatment. N=36 for this experiment. Error bars represent standard deviation.
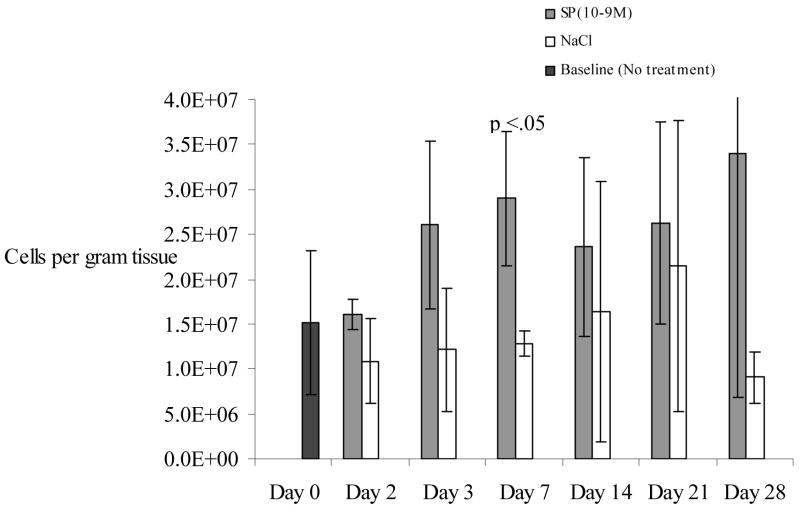
Substance P increased the density of leukocytes at all time points post-wounding (Figure 2) compared to NaCl. The effect was greatest on post wounding day 3 when the leukocyte count in SP-treated wounds (2.1 × 107 cells/gram tissue, SD +/−3.6×106) was statistically greater than NaCl treated wounds (1.8×107, SD+/−4.9×105, p<.02). In the SP-treated wounds, leukocyte number peaked at day 7 but remained elevated until day 28, when the study ended.
Figure 2.
Leukocyte density was increased at all time points in cutaneous wounds treated with substance P (10−9M) compared to NaCl. A statistically significant difference was observed at day 3 (p<0.05). N=36 for this experiment. Error bars represent standard deviation.
The density of macrophages was also increased in SP-treated wounds at all time points (Figure 3). This difference was statistically significant at day 7 in SP-treated wounds (2.9 × 107 cells/gram tissue, SD+/−7.5×106) compared to NaCl treated wounds (1.3×107 cells/gram tissue, SD+/−1.4×106, p<.05).
Figure 3.
Macrophage density was higher in cutaneous wounds following treatment with substance P (10−9M) compared to normal saline treated wounds at all time points. A statistically significant difference was observed at day 7 (p<0.05). N=36 for this experiment. Error bars represent standard deviation.
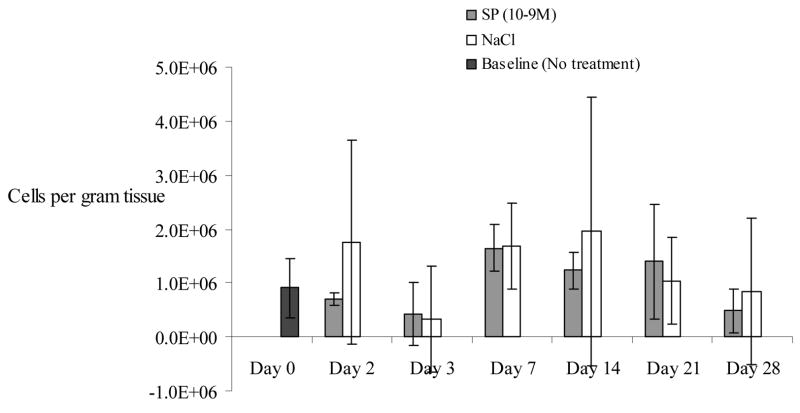
Endothelial cell density increased to the highest levels in both groups at day 7 and 14, but there was a lack of any significant difference between SP and NaCl-treated wounds (Figure 4) suggesting that endothelial density was not affected by topical SP therapy.
Figure 4.
Endothelial cell density increased on day 7 and 14 in both substance P-treated and saline-treated wounds. No statistically significant differences were identified at any time points. N=36 for this experiment. Error bars represent standard deviation.
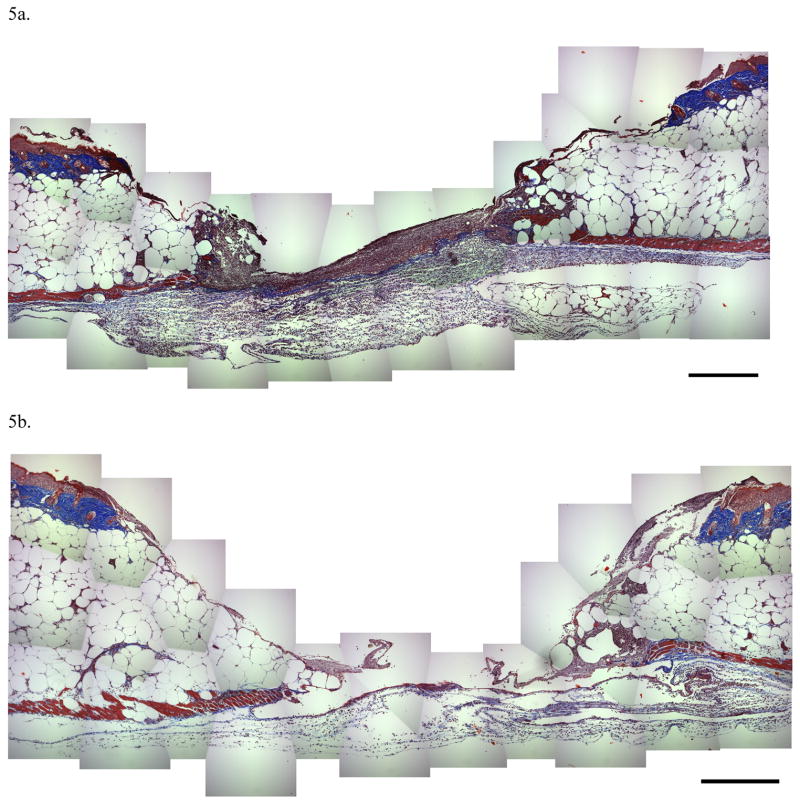
Histology specimens taken at post wounding day 3 showed a marked increased in inflammatory cell infiltrate in SP treated wounds compared with NaCl treated wounds (Figure 5a and 5b). These histology results support our flow cytometry observations.
Figure 5.
Figure 5a and 5b. Histology at day 3 in SP-treated wounds (5a) revealed a marked inflammatory cell infiltrate when compared with NaCl treated wounds (5b), confirming the flow cytometry results. Note that the magnification bar in the lower right is equal to 100 microns.
Discussion
Genetically mutant diabetic (C57BL/KsJ-db/db) mice exhibit typical characteristics of type 2 diabetes, including obesity, insulin resistance, hyperglycemia, and peripheral neuropathy(24). They have been reported to have a delayed inflammatory response to injury compared to non-diabetic littermates(20). Sensory nerve and neuropeptide depletion in diabetes mellitus is well documented(25, 26). We have previously reported that topical SP restores wound repair kinetics in diabetic C57BL/KsJ-db/db murine wounds(21). We now demonstrate that topical substance P increases overall cell density in the diabetic healing wound early after cutaneous injury. Temporally, this proinflammatory effect corresponds with known sequences of inflammatory cell migration in healing wounds, with leukocytes marginating into tissue first followed by macrophages. Since SP-induced migration of in vitro inflammatory cells has been documented(13–15) and SP has been shown to up-regulate IL-8 in several cell types(6), our data suggest that SP effects on wound healing kinetics may relate to its ability to increase early inflammation. It is not clear whether SP-induced inflammatory cell recruitment is due to its ability to increase capillary permeability(4) and up-regulation of endothelial cell adhesion molecules(27) or to direct chemoattractant effect on the inflammatory cells themselves. It is also possible that SP-induced effects are a result of macrophage activation. Macrophage activation has been studied for decades as a known mediator of inflammatory process(28, 29). Further mechanistic evaluation of the role of substance P in inflammatory cellular responses to injury is warranted.
Cell dispersion with flow cytometric analysis provides an opportunity to quantify specific cells in wounds rather than counting cells in stained tissue sections. Wilson and colleagues reported a cell loss rate of 9–11% with this technique raising the potential that cell loss may be differential with some cell types dissociating more or less readily from the surrounding matrix(23). Whereas, this could be a concern for quantifying absolute counts, the rate of cell loss should be comparable between wounds from different treatment groups. However, we agree that flow cytometry counts are best interpreted as relative rather than absolute, and that further optimization of the protocol could be tailored to each individual cell type for more accurate absolute cell counts. Furthermore, in their study the investigators correlated the flow cytometry results with cell counts in immunostained tissue sections suggesting that the flow cytometry results are reliable. Our results correspond well with known temporal dynamics of leukocyte and macrophage migration(30, 31). In order to verify our results, we used histology to confirm the increase in inflammatory cells in SP and NaCl-treated diabetic wounds on post-wounding day 3. As with the flow cytometry results, histology specimens demonstrate increased inflammatory cell density in SP-treated wounds compared with NaCl treated wounds at day 3. These results correlate with prior studies in which we found that inhibition of the metallopeptidase neutral endopeptidase, a SP-degrading enzyme, had a maximal effect on inflammation on post wounding day 3(22). Therefore, we propose that tissue dispersion with flow cytometry provides excellent methodology for quantifying cellular responses to cutaneous injury.
In designing studies of topically applied mediators to wound surfaces, a realistic aim should be to restore physiologic concentrations to the site of injury. Our group has previously reported that uninjured human skin has 1698 picograms (pg) of SP per gram of tissue, and that burned skin contains 958 pg SP per gram of tissue(32). Since the average weight of our diabetic murine wounds was 0.3 grams we extrapolate that normal human skin of this size would contain 509 pg of SP. Our group has previously demonstrated that topical administration of 10−9 M SP improves wound healing kinetics in diabetic mice(21). In this study we delivered approximately 405 pg of SP (300μl of 10−9 M SP) per day to each wound. Thus, we have applied a low-physiologic daily concentration of SP to the wound environs. Whether or not supra-physiologic concentrations of substance P would have more dramatic effects on cell recruitment into the diabetic wound site has not been demonstrated. As with all mediators, increasing SP concentration alone may not be sufficient to modulate inflammatory effects. Our studies demonstrating increased cellular NEP activity in endothelial cells with elevated glucose and fatty acid levels seen in type 2 diabetes(33) and improved wound healing kinetics with NEP inhibition(22) suggest that neutral endopeptidase inhibition may enhance exogenous SP modulation of in vivo cellular responses to cutaneous injury.
Conclusion
Topical SP treatment increases early leukocyte and macrophage density in healing wounds of db/db mice. These data support a role for neuroinflammatory responses to injury in cutaneous wound repair. Manipulation of the neurogenic inflammatory axis to increase the local cutaneous substance P concentration may have potential therapeutic benefits for patients with diabetic neuropathic wounds.
Acknowledgments
We would like to acknowledge Joanne Anthony and Lara Muffley for technical assistance. This work was supported by the NIH grants R01 DK58007, R01 GM056483 and T32 GM007037.
References
- 1.Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24(3):739–68. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- 2.Dunnick CA, Gibran NS, Heimbach DM. Substance P has a role in neurogenic mediation of human burn wound healing. J Burn Care Rehabil. 1996;17(5):390–6. doi: 10.1097/00004630-199609000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Iwamoto I, Ueki IF, Borson DB, Nadel JA. Neutral endopeptidase modulates tachykinin-induced increase in vascular permeability in guinea pig skin. Int Arch Allergy Appl Immunol. 1989;88(3):288–93. doi: 10.1159/000234808. [DOI] [PubMed] [Google Scholar]
- 4.Holzer P. Neurogenic vasodilatation and plasma leakage in the skin. Gen Pharmacol. 1998;30(1):5–11. doi: 10.1016/s0306-3623(97)00078-5. [DOI] [PubMed] [Google Scholar]
- 5.Hughes S, Williams T, Brain S. Evidence that endogenous nitric oxide modulates oedema formation induced by substance P. Eur J Pharmacol. 1990;191:481–4. doi: 10.1016/0014-2999(90)94184-y. [DOI] [PubMed] [Google Scholar]
- 6.Ansel JC, Armstrong CA, Song I, Quinlan KL, Olerud JE, Caughman SW, Bunnett NW. Interactions of the skin and nervous system. J Investig Dermatol Symp Proc. 1997;2(1):23–6. doi: 10.1038/jidsymp.1997.6. [DOI] [PubMed] [Google Scholar]
- 7.Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, Geppetti P, Ledda F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994;94(5):2036–44. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weidner C, Klede M, Rukwied R, Lischetzki G, Neisius U, Skov PS, Petersen LJ, Schmelz M. Acute effects of substance P and calcitonin gene-related peptide in human skin--a microdialysis study. J Invest Dermatol. 2000;115(6):1015–20. doi: 10.1046/j.1523-1747.2000.00142.x. [DOI] [PubMed] [Google Scholar]
- 9.Johnson AR, Erdos EG. Release of histamine from mast cells by vasoactive peptides. Proc Soc Exp Biol Med. 1973;142(4):1252–6. doi: 10.3181/00379727-142-37219. [DOI] [PubMed] [Google Scholar]
- 10.Ziche M, Morbidelli L, Pacini M, Geppetti P, Alessandri G, Maggi CA. Substance P stimulates neovascularization in vivo and proliferation of cultured endothelial cells. Microvasc-Res. 1990;40(2):264–78. doi: 10.1016/0026-2862(90)90024-l. [DOI] [PubMed] [Google Scholar]
- 11.Kahler CM, Herold M, Reinisch N, Wiedermann CJ. Interaction of substance P with epidermal growth factor and fibroblast growth factor in cyclooxygenase-dependent proliferation of human skin fibroblasts. J-Cell-Physiol. 1996;166(3):601–8. doi: 10.1002/(SICI)1097-4652(199603)166:3<601::AID-JCP15>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Kahler CM, Herold M, Wiedermann CJ. Substance P: a competence factor for human fibroblast proliferation that induces the release of growth-regulatory arachidonic acid metabolites. J Cell Physiol. 1993;156(3):579–87. doi: 10.1002/jcp.1041560318. [DOI] [PubMed] [Google Scholar]
- 13.Helme RD, Eglezos A, Hosking CS. Substance P induces chemotaxis of neutrophils in normal and capsaicin-treated rats. Immunol Cell Biol. 1987;65:267–9. doi: 10.1038/icb.1987.30. [DOI] [PubMed] [Google Scholar]
- 14.Roch-Arveiller M, Regoli D, Chanaud B, Lenoir M, Muntaner O, Stralzko S, Giroud JP. Tachykinins: effects on motility and metabolism of rat polymorphonuclear leucocytes. Pharmacology. 1986;33(5):266–73. doi: 10.1159/000138225. [DOI] [PubMed] [Google Scholar]
- 15.Carolan EJ, Casale TB. Effects of neuropeptides on neutrophil migration through noncellular and endothelial barriers. J Allergy Clin Immunol. 1993;92(4):589–98. doi: 10.1016/0091-6749(93)90083-r. [DOI] [PubMed] [Google Scholar]
- 16.Greenhalgh D. Wound healing and diabetes mellitus. Clin Plast Surg. 2003;30(1):37–45. doi: 10.1016/s0094-1298(02)00066-4. [DOI] [PubMed] [Google Scholar]
- 17.Levy D, Karanth S, Springall D, Polak J. Depletion of cutaneous nerves and neuropeptides in diabetes mellitus: an immunocytochemical study. Diabetologia. 1989;32(7):427–33. doi: 10.1007/BF00271262. [DOI] [PubMed] [Google Scholar]
- 18.Antezana M, Sullivan S, Usui M, Gibran N, Spenny M, Larsen J, Ansel J, Bunnett N, Olerud J. Neutral endopeptidase activity is increased in the skin of subjects with diabetic ulcers. J Invest Dermatol. 2002;119(6):1400–4. doi: 10.1046/j.1523-1747.2002.19618.x. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan S, Underwood R, Gibran N, Sigle R, Usui M, Carter W, Olerud JE. Validation of a model for the study of multiple wounds in the diabetic mouse (db/db) Plast Reconstr Surg. 2004;113(3):953–60. doi: 10.1097/01.prs.0000105044.03230.f4. [DOI] [PubMed] [Google Scholar]
- 20.Greenhalgh DG, Sprugel KH, Murray MJ, Ross R. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol. 1990;136(6):1235–46. [PMC free article] [PubMed] [Google Scholar]
- 21.Gibran NS, Jang YC, Isik FF, Greenhalgh DG, Muffley LA, Underwood RA, Usui ML. Diminished neuropeptide levels contribute to the impaired cutaneous healing response associated with diabetes mellitus. J Surg Res. 2002;108(1):122–8. doi: 10.1006/jsre.2002.6525. [DOI] [PubMed] [Google Scholar]
- 22.Spenny ML, Muangman P, Sullivan SR, Bunnett NW, Ansel JC, Olerud JE, Gibran NS. Neutral endopeptidase inhibition in diabetic wound repair. Wound Repair Regen. 2002;10(5):295–301. doi: 10.1046/j.1524-475x.2002.10504.x. [DOI] [PubMed] [Google Scholar]
- 23.Wilson L, Fathke C, Isik F. Tissue dispersion and flow cytometry for the cellular analysis of wound healing. Biotechniques. 2002;32(3):548–51. doi: 10.2144/02323st07. [DOI] [PubMed] [Google Scholar]
- 24.Sima AA, Robertson DM. Peripheral neuropathy in mutant diabetic mouse [C57BL/Ks (db/db)] Acta Neuropathol (Berl) 1978;41(2):85–9. doi: 10.1007/BF00689757. [DOI] [PubMed] [Google Scholar]
- 25.Levy DM, Terenghi G, Gu XH, Abraham RR, Springall DR, Polak JM. Immunohistochemical measurements of nerves and neuropeptides in diabetic skin: relationship to tests of neurological function. Diabetologia. 1992;35(9):889–97. doi: 10.1007/BF00399938. [DOI] [PubMed] [Google Scholar]
- 26.Lindberger M, Schroder HD, Schultzberg M, Kristensson K, Persson A, Ostman J, Link H. Nerve fibre studies in skin biopsies in peripheral neuropathies. I. Immunohistochemical analysis of neuropeptides in diabetes mellitus. J Neurol Sci. 1989;93(2–3):289–96. doi: 10.1016/0022-510x(89)90198-6. [DOI] [PubMed] [Google Scholar]
- 27.Lindsey K, Caughman S, Olerud J, Bunnett N, Armstrong C, Ansel J. Neural regulation of endothelial cell-mediated inflammation. J Investig Dermatol Symp Proc. 2000;5(1):74–8. doi: 10.1046/j.1087-0024.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- 28.Leibovich S, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975;78(1):71–100. [PMC free article] [PubMed] [Google Scholar]
- 29.Leibovich S, Ross R. A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol. 1976;84(3):501–14. [PMC free article] [PubMed] [Google Scholar]
- 30.Kovacs E, DiPietro L. Fibrogenic cytokines and connective tissue production. FASEB J. 1994;8(11):854–61. doi: 10.1096/fasebj.8.11.7520879. [DOI] [PubMed] [Google Scholar]
- 31.Ross R, Benditt E. Wound healing and collagen formation. II. Fine structure in experimental scurvy. J Cell Biol. 1962;12:533–51. doi: 10.1083/jcb.12.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott JR, Muangman PR, Tamura RN, Zhu KQ, Liang Z, Anthony J, Engrav LH, Gibran NS. Substance P levels and neutral endopeptidase activity in acute burn wounds and hypertrophic scar. Plast Reconstr Surg. 2005;115(4):1095–102. doi: 10.1097/01.prs.0000156151.54042.da. [DOI] [PubMed] [Google Scholar]
- 33.Muangman P, Spenny ML, Tamura RN, Gibran NS. Fatty acids and glucose increase neutral endopeptidase activity in human microvascular endothelial cells. Shock. 2003;19(6):508–12. doi: 10.1097/01.shk.0000055815.40894.16. [DOI] [PubMed] [Google Scholar]