SUMMARY
Wnt signaling is required for development of mesoderm-derived lineages and expression of transcription factors associated with the primitive streak. In a functional screen, we examined the mesoderm-inducing capacity of transcription factors whose expression was Wnt-dependent in differentiating ESCs. In contrast to many inactive factors, we found that mesoderm posterior 1 (Mesp1) promoted mesoderm development independently of Wnt signaling. Transient Mesp1 expression in ESCs promotes changes associated with epithelial-mesenchymal transition (EMT) and induction of Snai1, consistent with a role in gastrulation. Mesp1 expression also restricted the potential fates derived from ESCs, generating mesoderm progenitors with cardiovascular, but not hematopoietic, potential. Thus, in addition to its effects on EMT, Mesp1 may be capable of generating the recently identified multipotent cardiovascular progenitor from ESCs in vitro.
INTRODUCTION
Differentiating ESCs approximate the gastrulating epiblast (Keller, 2005; Nishikawa et al., 2007; Wobus et al., 1991; Sakurai et al., 2006; Yamashita et al., 2000). Canonical Wnt/β-catenin signaling is required for gastrulation and mesoderm formation in vivo (Liu et al., 1999; Huelsken et al., 2000) and for ESC differentiation toward mesoderm-derived lineages (Lindsley et al., 2006). In the current study, we performed a functional screen in ESCs to identify Wnt-dependent transcription factors that could induce Wnt-independent development of mesoderm-derived lineages.
Mesp1 is a basic helix-loop-helix transcription factor that is transiently expressed by the earliest progenitors of the cardiovascular system from E6.5 to E7.5 (Saga et al., 2000). Lineage tracing studies show that virtually all cells of the heart and cells of the vascular system derive from Mesp1-expressing precursors (Saga et al., 2000). During gastrulation, progenitors of cardiogenic mesoderm arise at E6.5 in the posterior lateral epiblast and migrate to form the cardiac crescent at E7.5, when regionalized cell fates are first delineated (Meilhac et al., 2004; Lawson et al., 1991; Tam et al., 1997). Lineage tracing and heterotopic transplantation studies suggest that precursors in the earliest heart field possess potential to generate myocardium, endocardium, and epicardium, but subsequently become restricted as lineage-specific regulatory programs are activated (Kitajima et al., 2000; Tam et al., 1997). The mechanisms by which inductive signals in the primitive streak influence development of the cardiac progenitor field are incompletely understood (Kelly, 2005).
In this study, we report that Mesp1 induces features of EMT in differentiating ESCs and promotes development of mesoderm precursors of the cardiovascular lineage. The induction of EMT is likely a result of the ability of Mesp1 to induce Snai1, which we show can independently decrease E-Cadherin. Further, we show that transient expression of Mesp1 is sufficient to allow development of cardiovascular mesoderm, including endothelial cells, smooth muscle cells, and cardiomyocytes, while inhibiting development of other mesodermal lineages, correlating with changes in global gene expression induced by Mesp1. Finally, Mesp1 induces maximal cardiac Troponin T (cTnT) expression with inhibition of Wnt signaling, corresponding with the inhibition of cardiac development by Wnt signaling at later stages in vivo (Schneider and Mercola, 2001; Ueno et al., 2007). These results suggest that Mesp1 is sufficient for triggering EMT and programming nascent mesoderm toward a restricted set of cardiovascular fates.
RESULTS
Mesp1 Induces Wnt-Independent Expression of Mesodermal Markers
We previously identified transcription factors whose expression during early ESC differentiation required Wnt signaling, many being associated with the primitive streak or mesoderm (Lindsley et al., 2006). Among these were Brachyury (T), Mixl1, Evx1, Eomes, Mesp1, Cdx2, and Hand1. We hypothesized that some of these factors might induce mesoderm development independently of Wnt activity. To test this, we generated ESC lines with doxycycline (dox)-inducible expression of each candidate. We analyzed PDGFRα and Flk1 expression (Sakurai et al., 2006; Tada et al., 2005) for each candidate line after ESC differentiation in the presence of DKK1, with dox treatment from day 2 to day 4 (Figure 1A). Of the candidates tested, Mesp1 most markedly increased the frequency of PDGFRα+ and Flk1+ cells. After 2 days of Mesp1 expression, only 4% of cells failed to express Flk1 or PDGFRα, whereas the majority of cells were PDGFRα− Flk1− for all other candidates. Thus, Mesp1 robustly induces early mesoderm markers independently of Wnt signaling.
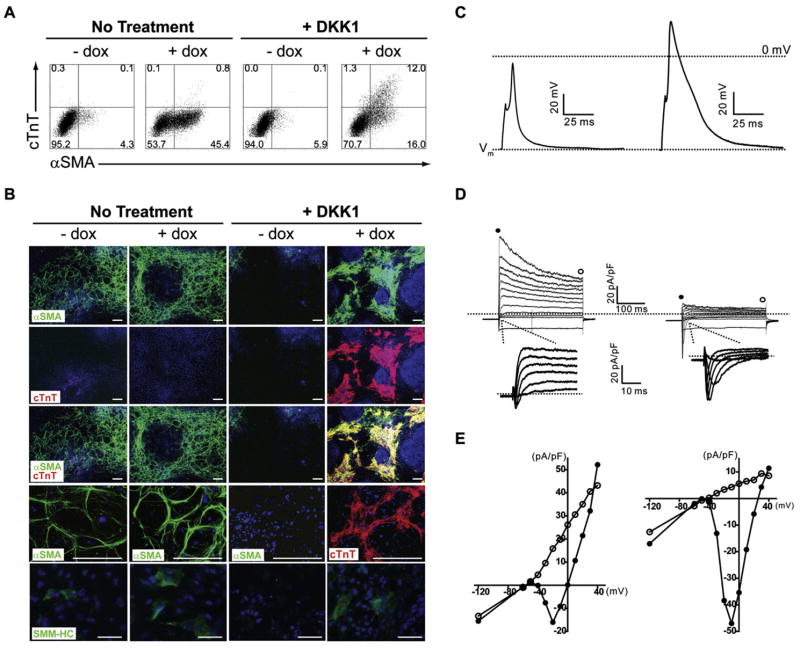
Figure 1. Mesp1 Induces Wnt-Independent Development of Flk1+ Mesoderm.
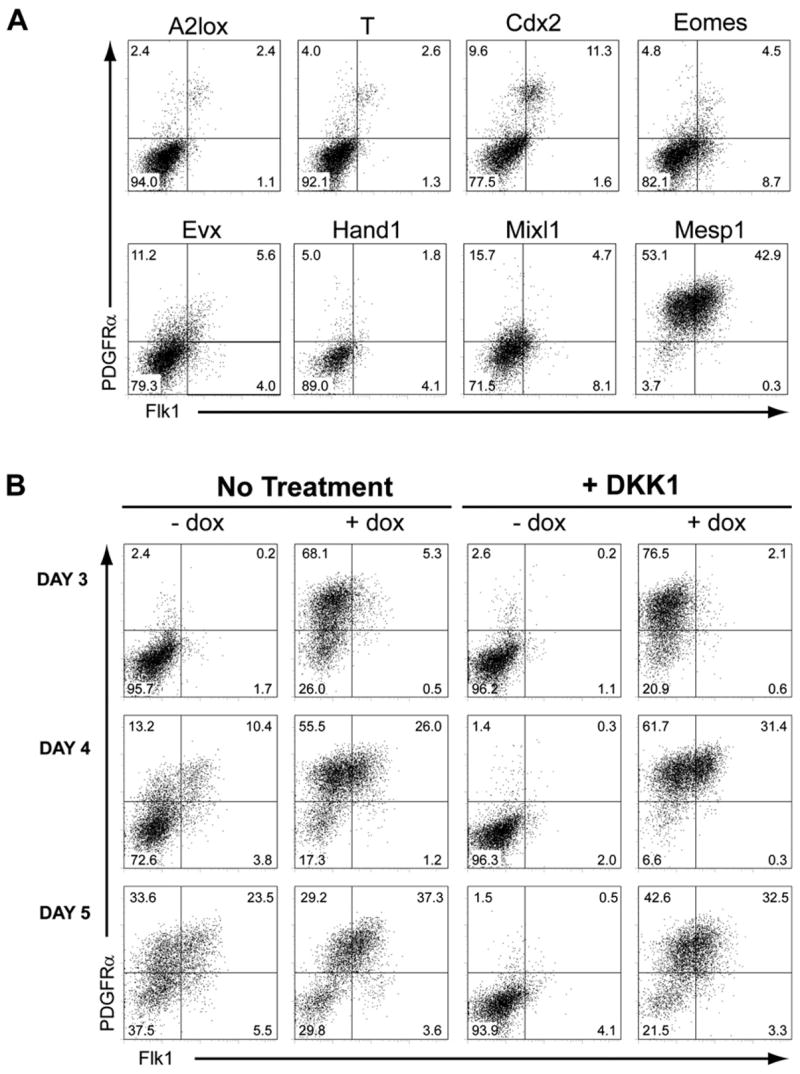
(A) A2lox ESCs, or A2lox cells targeted with the indicated cDNAs, were treated with both DKK1 and dox between days 2 and 4 of differentiation. On day 4, cells were analyzed by flow cytometry for Flk1 and PDGFRα expression. Numbers indicate the percentage of live-gated cells within each quadrant.
(B) A2lox.Mesp1 ESCs were differentiated in the absence (No Treatment) or presence (+ DKK1) of recombinant DKK1 from days 2–4 of differentiation, either with (+ dox) or without (− dox) doxycycline from days 2–4 of differentiation. Cells were analyzed at the indicated times as in (A).
During ESC differentiation, endogenous Mesp1 mRNA was detected on day 2.5, was maximal on days 3 to 3.5, and was nearly extinguished by day 4.5 (Figure S1A). We generated an A2lox ESC line with inducible V5-epitope-tagged Mesp1 (Figure S1C). Upon differentiation, V5-Mesp1 protein was not detected unless cells were treated with dox. After dox addition on day 2 and medium replacement on day 4, Mesp1 protein was detectable only on days 3 and 4, accurately reflecting the expression of endogenous Mesp1. In A2lox cells expressing either a Mesp1-GFP fusion protein or a bicistronic Mesp1-ires-GFP message (Figure S1E, upper panels), doxycycline induced uniform GFP expression in 88.2% and 95.6% of cells, respectively, indicating expression in all cells and at similar levels. Thus, we used these same conditions and timing of Mesp1 induction (i.e., adding dox from day 2 to 4) (Figure S1B) for the following studies.
We analyzed Flk1 and PDGFRα expression in differentiating A2lox.Mesp1 cells at various times (Figure 1B; Table S4). In unmanipulated cultures, differentiating ESCs developed into Flk1−PDGFRα+ (13%), Flk1+PDGFRα+ (10%), and Flk1+PDGFRα− (4%) populations on day 4 of differentiation, which increased to 34%, 24% and 6% on day 5, respectively (Figure 1B, Table S4). None of these populations developed in the presence of DKK1 alone, consistent with aWnt requirement by early mesoderm (Lindsley et al., 2006; Kelly et al., 2004). In the presence of DKK1, Mesp1 induced development of Flk1−PDGFRα+ cells to 77% after one day (Figure 1B, day 3), followed by an increase in Flk1 expression on days 4 and 5 (Figure 1B). Bromodeoxyuridine (BrdU) incorporation was similar between untreated and dox-treated cultures for both the Flk1−PDGFRα+ and Flk1+PDGFRα+ populations (Figure S1D) and both the Flk1−PDGFRα+ and Flk1+PDGFRα+ populations expressed similar levels of Mesp1 (Figure S1E, lower panels). This suggests that these two populations do not result from variable proliferative responses to dox, but instead are induced sequentially after Mesp1 expression.
Mesp1 Induces Wnt-Independent Genetic Changes Associated with Epithelial-Mesenchymal Transition
Since Mesp1−/− embryos showed altered cell migration during gastrulation (Saga et al., 1999), we tested if Mesp1 might influence aspects of EMT, such as the switch from E-cadherin to N-cadherin expression (Peinado et al., 2004; Shook and Keller, 2003; Moustakas and Heldin, 2007) (Figure 2A). On day 4, untreated differentiating ESCs show a typical heterogeneous pattern of nonoverlapping regions of E-cadherin or N-cadherin expression (Figure 2A). DKK1 treatment causes uniform maintenance of E-cadherin expression and loss of N-cadherin induction (Figure 2A). Mesp1 completely inhibited E-cadherin and uniformly induced N-cadherin expression, either with or without DKK1 treatment (Figure 2A). We next determined the changes in global gene expression induced by Mesp1 (Figure 2B). A2lox.Mesp1 ESCs were differentiated for 48 hr, were induced with dox both in the absence and presence of DKK1, and were analyzed 6, 12, 24, 48, 96, and 120 hr after dox addition (Table S1). In the presence of DKK1, Mesp1 inhibited expression of several epithelial genes, including claudin 3 (Cldn3), claudin 7 (Cldn7), occludin (Ocln), and E-cadherin (Cdh1) within 24 hr of induction (day 3), with further inhibition on day 4 (Figure 2B). At the same time, Mesp1 induced expression of many mesenchymal genes, including matrix metallopeptidase 2 (Mmp2), vimentin (Vim), N-cadherin (Cdh2), and fibronectin (Fn1). These results suggest Mesp1 induces a global transition from epithelial to mesenchymal gene expression.
Figure 2. Mesp1 Expression Is Sufficient to Induce Wnt-Independent Epithelial to Mesenchymal Transition.
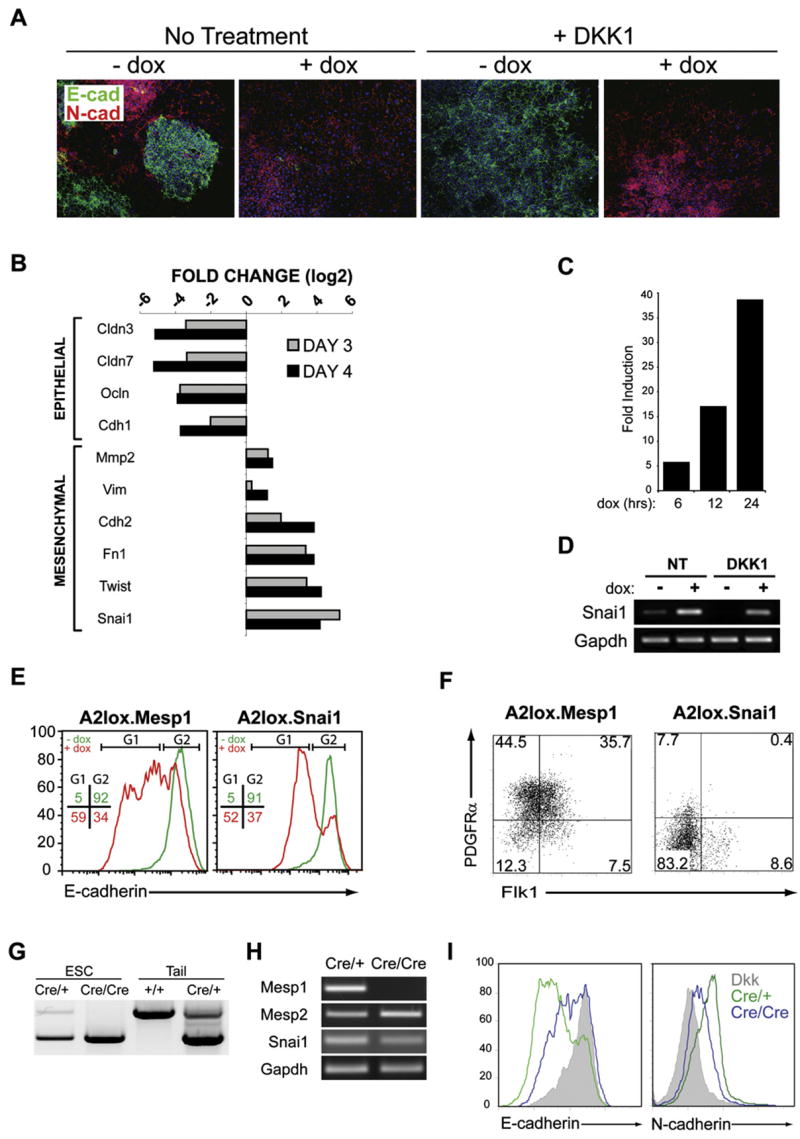
(A) A2lox.Mesp1 cells differentiated as described in Figure S1B were plated on day 2 onto Collagen I-coated slides and stained with antibodies to E-cadherin (green) and N-cadherin (red) on day 4. Nuclei were stained using Hoechst 33342 (blue).
(B) A2lox.Mesp1 cells were differentiated in the presence of DKK1, either with or without dox addition at day 2. On day 3 or day 4, gene expression was analyzed using Mouse Genome 430 2.0 arrays (Affymetrix). Shown are log2 fold change of expression of the indicated genes on day 3 (gray bar) and day 4 (black bar). Genes are arranged according to their association with either epithelial or mesenchymal phenotypes.
(C) Snai1 gene expression analysis at 6, 12, and 24 hr after Mesp1 induction. A2lox.Mesp1 cells were differentiated as in (B) and were harvested 6, 12, or 24 hr after dox addition, and gene expression was analyzed as in (B). Shown is the fold increase of Snai1 expression in cells treated with DKK1 and dox compared to timematched controls treated with DKK1 only.
(D) Snai1 and Gapdh expression were measured on day 3 of differentiation by RT-PCR. A2lox.Mesp1 ESCs were differentiated in the absence (NT) or presence of DKK1 (DKK1) beginning at day 2, either without (−) or with (+) addition of dox at day 2.
(E) A2lox.Mesp1 and A2lox.Snai1 ESCs differentiated as in (D) were analyzed on day 4 for E-cadherin levels by flow cytometry. Shown are histogram overlays of E-cadherin staining for each cell line treated with DKK1 alone (green) and DKK1 plus dox (red). Numbers represent the frequency of live-gated cells found within the indicated gates (G1 or G2) for DKK-treated (green) or DKK and dox-treated (red) cells.
(F) A2lox.Mesp1 and A2lox.Snai1 ESCs were treated with both DKK1 and dox. Cells were harvested on day 4 and analyzed by flow cytometry as in Figure 1.
(G) Mesp1 heterozygous or Mesp1-deficient ESCs were derived from blastocysts of crosses between Mesp1cre/+ heterozygous knockin mice. Heterozygous (Cre/+) or Mesp1-deficient (Cre/Cre) ESCs and wild-type (+/+) or heterozygous (Cre/+) mice were genotyped by PCR.
(H) Mesp1, Mesp2, Snai1, and Gapdh expression in heterozygous (Cre/+) or Mesp1-deficient (Cre/Cre) cells were measured by RT-PCR on day 5 of differentiation.
(I) Mesp1 heterozygous (green) or Mesp1-deficient (blue) ESCs, differentiated as embryoid bodies, were harvested on day 5. E-cadherin and N-cadherin levels, determined by flow cytometry, were compared to levels on DKK-treated cultures (shaded).
Next we examined transcription factors that regulate EMT, such as snail (Snai1) and twist (Twist) (Carver et al., 2001; Thiery and Sleeman, 2006). In the presence of DKK1, Mesp1 induced Snai1 and Twist expression within 24 hr (Figure 2B). Snai1 expression increased progressively from 5-fold induction at 6 hr to 39-fold induction after 24 hr of dox treatment (Figures 2C and 2D). To test if Snai1 could mediate Mesp1’s actions, we generated a dox-inducible A2lox.Snai1 ESC line and examined E-cadherin repression by Snai1 or Mesp1 (Figure 2E). Both Mesp1 and Snai1 decreased E-cadherin expression, by 59% and 52% respectively. However, unlike Mesp1, Snai1 failed to induce Flk1+ or PDGFRα+ expression in DKK1-treated cultures (Figure 2F), suggesting that Snai1 can mediate some, but not all, of Mesp1’s actions.
To ask if Mesp1 is necessary for EMT or for Snai1 expression in ESCs, we used mice with Cre recombinase targeted to the Mesp1 locus (Mesp1Cre/+) (Saga et al., 1999) to generate ESCs that were Mesp1 heterozygous (Cre/+) or Mesp1 deficient (Cre/Cre) (Figure 2G). After 5 days of differentiation in unmanipulated cultures, Mesp1Cre/Cre cells retained endogenous Snai1 expression (Figure 2H), had downregulated E-cadherin, and upregulated N-cadherin, indicating an EMT similar to control Mesp1Cre/+ cells (Figure 2I). Interestingly, the Mesp1Cre/Cre cells expressed a related family member Mesp2 at a slightly increased level compared to Mesp1Cre/+ cells (Figure 2H). Since Mesp2 can compensate for migratory defects in Mesp1-deficient embryos (Kitajima et al., 2000), we asked if Mesp2 can induce mesoderm and EMT in ESCs (Figure S2). Dox treatment of A2lox.Mesp2 cells induced expression of Flk1 and PDGFRα (Figure S2A), downregulated E-cadherin (Figure S2A), and induced Snai1 expression in DKK treated cultures (Figure S2B). Thus, the ability of differentiating Mesp1-deficient ESCs to express Snai1 and undergo EMT may be due to compensation by Mesp2.
Mesp1 Induces a Restricted Subset of Mesodermal Gene Expression
At 96 hr after induction of Mesp1, global microarray expression data identified four gene expression patterns roughly correlating with distinct cell lineages (Figure 3A; Table S1). One set of genes was inhibited by Mesp1 but was also inhibited by Wnt signaling (Figure 3A, NEURO). These genes were only expressed in the presence of DKK1, but then were inhibited by Mesp1 in this condition, and contained genes such as Sox2 and Pax6, which are associated with neuroectoderm, and Pax7, which is associated with neuroectoderm and paraxial mesoderm. This expression pattern observed by microarray was also confirmed by RT-PCR (Figure 3B). A second set of genes was inhibited by Mesp1 but required Wnt signaling for expression (Figure 3A, HEME). These genes were only expressed in the absence of DKK1, but then were inhibited by Mesp1 in this condition, and contained many genes that were associated with the hematopoietic lineage (D’Souza et al., 2005), such as Tal1, Gata1, and Hbb-b1 (Figure 3C). A third set of genes was induced by Mesp1, but only during inhibition of Wnt signaling (Figure 3A, CARDIOMYOCYTE). These genes were only expressed in the presence of both DKK1 and Mesp1 and contained many genes that were cardiomyocyte specific, such as Myh6, Myh7, Myl2, Myl7, Tnnt2, and Nppa (Figure 3D) (Yuasa et al., 2005). A fourth set of genes was induced by Mesp1, but their endogenous expression required Wnt signaling (Figure 3A, ENDOTHELIAL, SMC). These genes were inhibited by DKK1, but were induced by Mesp1 in this condition, and contained many genes associated with endothelial and smooth muscle cells (Lindskog et al., 2006), such as Tie1, Tek and Cdh5 expressed by endothelial cells, and Acta2, Rgs5 and Pdgfrb expressed by smooth muscle cells (Figures 3E and 3F). These results are summarized in Figure 3G and are consistent with Mesp1 lineage tracing of cardiovascular cells in vivo (Saga et al., 2000). Mesp1 is expressed in lateral mesoderm, the origin of cardiovascular lineages, and in paraxial (presomitic) mesoderm (Saga et al., 1999; Takahashi et al., 2005, 2007). We asked if Mesp1 regulated paraxial mesoderm gene expression (Figure S3). Mesp1 induced the paraxial mesoderm genes Pax3 and Tcf15 on day 4, but this was not sustained at day 6 (Figures S3A and S3B). Mesp1 failed to induce other paraxial mesoderm genes, such as Mesp2, Tbx6, or Pax1 (Sakurai et al., 2006) (Figure S3C), or skeletal myogenic transcription factors Myod, Myogenin, or Myf5 (Figures S3A and S3B) (Rohwedel et al., 1995). However, this could either be due to the inability of Mesp1 to induce paraxial genes or to a bias against paraxial gene expression in the culture conditions.
Figure 3. Mesp1 Induces Expression of a Restricted Subset of Mesoderm-Associated Genes.
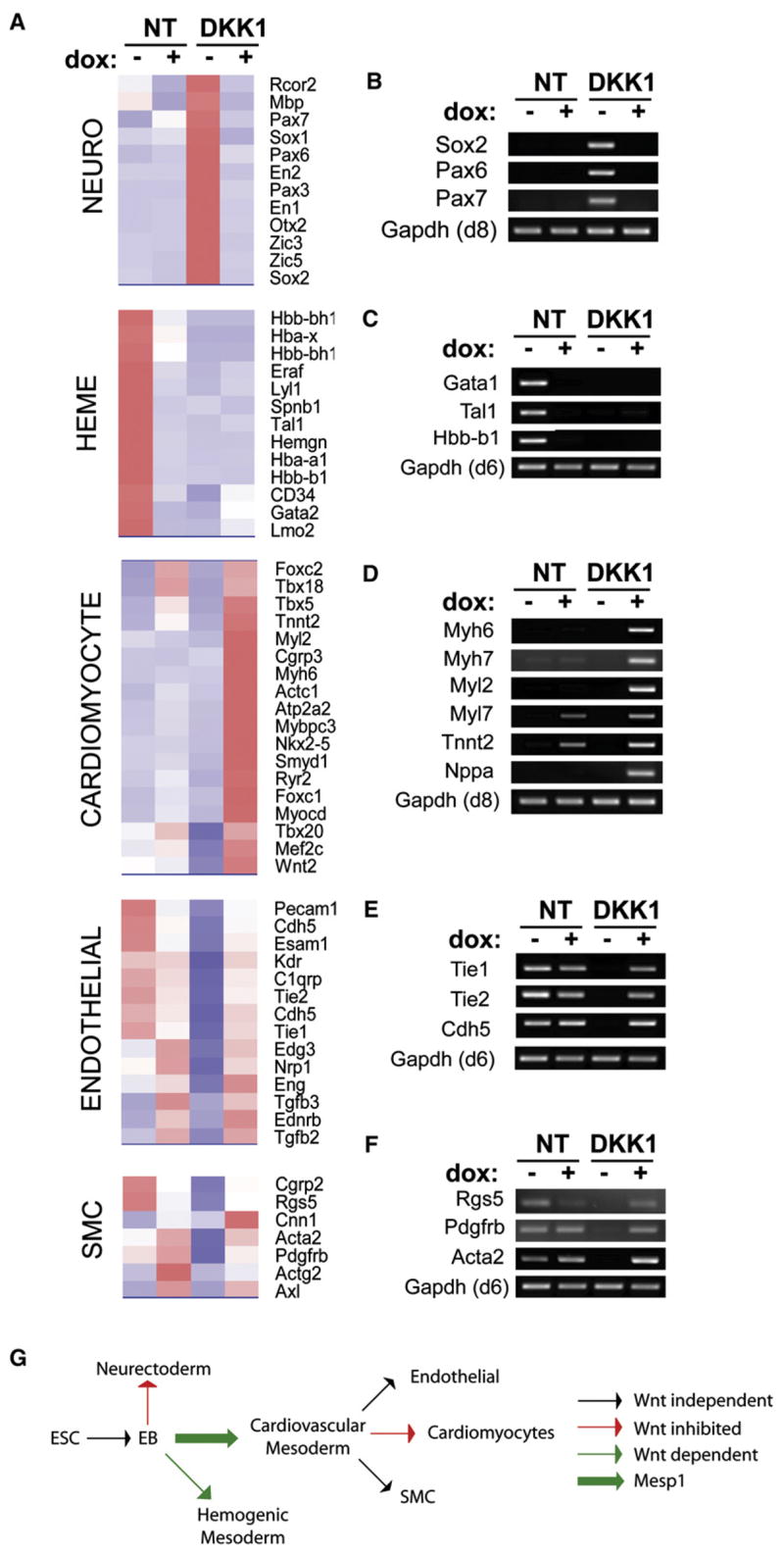
(A) A2lox.Mesp1 ESCs were differentiated in the absence (NT) or presence (DKK1) of DKK1, either without (−) or with (+) addition of dox from days 2 to 4. On day 6, gene expression was analyzed by microarray analysis. Normalized sample data were evaluated for 5-fold changes following Mesp1 induction: (NT+ dox versus NT−) or (DKK1+ dox versus DKK1−). Representative clusters of the indicated lineage-specific genes are shown as heat maps with red shading indicating increased expression and blue shading indicating decreased expression.
(B–F) Cells differentiated as in (A) were harvested at day 6 (C, E, and F) or day 8 (B and D), and expression of the indicated genes was analyzed by RT-PCR.
(G) Results described in ([A]–[F], and Figures 2A–2D) are shown schematically. ESC, embryonic stem cell; EB, embryoid bodies; EMT, epithelial mesenchymal transition.
Mesp1 Induces Differentiation of Either Smooth Muscle Cells or Cardiomyocytes, Contingent on Wnt Activity
We used FACS and immunohistochemistry for α-smooth muscle actin (αSMA, Acta2) and cardiac troponin T (cTnT, Tnnt2) to quantify commitment to cardiovascular lineages in response to Mesp1. αSMA is expressed by mesenchymal cells (Ball et al., 2007), smooth muscle cells (Kattman et al., 2006), and embryonic cardiomyocytes (Ruzicka and Schwartz, 1988), and cTnT is expressed in both embryonic and adult cardiomyocytes (Jin et al., 1996; Cooper and Ordahl, 1985). On day 6 of differentiation, untreated A2lox.Mesp1 ESCs contained a 4%population of cells expressing αSMA (Figure 4A, Table S5). Without DKK1 treatment, Mesp1 increased αSMA expression to 45% of cells (Figure 4A Table S5). cTnT was not expressed by untreated cultures, consistent with a report that embryoid bodies in suspension cultures do not efficiently differentiate into cardiomyocytes spontaneously (Ueno et al., 2007). Mesp1 did not induce cTnT without DKK1 treatment. However, with DKK1, Mesp1 induced a population of αSMA+ cTnT+ cells, and in this condition, the majority of cell clusters were spontaneously contractile (Figure S5).
Figure 4. Mesp1-Induced Expression of Cardiac Troponin T Requires Blockade of Wnt Signaling.
(A) A2.Mesp1 ESCs were differentiated with (+ dox) or without (− dox) dox and left without DKK1 treatment (No Treatment) or treated with the DKK1 from day 2–6. On day 6, cells were fixed, permeabilized, and stained with FITC-anti-αSMA, mouse-anti-cTnT, and APC-anti-mouse Ig. The numbers shown indicate the percent of live cells present within the indicated region.
(B) A2.Mesp1 ESCs were differentiated as in (A) and plated on Collagen I-coated slides on day 4 or day 7 (SMM-HC). On day 8, cells were stained with antibodies to αSMA (green), cTnT (red), and SM-MHC (green, bottom panel). Nuclei were stained using Hoechst 33342 (blue). Representative images are shown, where the scale bar is 200 μm (αSMA/cTnT) or 50 μm (SM-MHC).
(C–E) Action potentials and ionic currents were recorded from spontaneously beating clusters in DKK+dox cultures on day 10. Whole-cell action potentials (C) and macroscopic currents (D) were recorded with K+- (left panels) or Cs+-containing (right panels) pipette solution (D) In voltage-clamp mode, currents were evoked in response to a series of 300 ms voltage steps (−120 to +40 mV) from the holding potential of −70 mV; the dotted lines indicate the zero current level. Insets show inward currents evoked by depolarizing steps from −40 to +10 mV at a higher gain. (E) Peak (filled circles) and plateau (empty circles) current densities are plotted as a function of the test potential.
We confirmed these results by fluorescence microscopy for αSMA, smooth muscle myosin heavy chain (SM-MHC), and cTnT expression (Figure 4B). On day 8, αSMA-expressing cells were present in untreated cultures, increased by Mesp1, and decreased by DKK1 treatment (Figure 4B). αSMA-expressing cells were also induced by Mesp1 in the presence of DKK1. Cultures with αSMA-expressing cells also contained a small number of SM-MHC-positive cells (Figure 4B). RT-PCR analysis confirmed the expression of SM-MHC (Myh11) and the smooth muscle marker SM22, which had similar expression patterns to that of SMA (acta2) (Figure S2D). cTnT was induced by Mesp1 in the presence of DKK1 and, again, largely colocalized with αSMA (Figure 4B).
We examined the maturity of Mesp1-induced cells by measuring calcium fluxes and by voltage-clamp recording. The A7R5 vascular smooth muscle cells fluxed calcium in response to vasopressin and ionomycin, but not angiotensin, as described (Filipeanu et al., 2001). Mesp1-induced ESCs fluxed calcium in response to ionomycin, but not vasopressin or angiotensin (Figure S4), suggesting that Mesp1-induced α-SMA+ cells represent immature smooth muscle cells at this time. We next compared whole-cell current and voltage-clamp recordings from cultures induced by Mesp1 in the presence of DKK1 to those from cultures treated with DKK1 only (Table S3). The mean resting membrane potential (Vm) measured in Mesp1-induced clusters was significantly more hyperpolarized than the mean Vm of cells treated with DKK1 alone (Table S3). Action potentials could be evoked in some beating clusters obtained by Mesp1 induction in the presence of DKK1 (Figure 4C) and were more readily evoked, larger in amplitude, and broader when outward K+ currents were blocked (Figure 4C, right panel) but were never observed in cells treated with DKK1 alone. Voltage-gated outward K+ currents (IK) and inwardly rectifying K+ currents (IK1) were recorded in all (n = 7) spontaneously beating clusters (Figure 4D). Although K+ currents were also detected in cells treated with DKK1 alone, mean IK and IK1 densities were significantly larger in clusters obtained by Mesp1 induction in the presence of DKK1 (Table S3). Voltage-gated inward currents were also evident in some recordings (n = 2) (Figures 4D and 4E, left panels), and inward Na+ (INa) and Ca2+ (ICa) currents (Figures 4D and 4E, right panels) could be measured only in Mesp1-induced cells (Table S3). These results show that spontaneously beating clusters induced by Mesp1 in the presence of DKK1 are electrically differentiated compared to cultures treated with DKK1 alone and are consistent with an early stage of differentiation into cardiomyocytes (Nerbonne and Kass, 2005). The selective cTnT expression induced by Mesp1 with DKK1 is consistent with known effects of Wnt signaling on cardiac development, in which Wnt signaling is required for early steps but is inhibitory at later stages of cardiac development (Ueno et al., 2007; Naito et al., 2006).
Because αSMA and cTnT expression did not account for all cells responding to Mesp1, we asked if endothelial markers were induced by Mesp1 (Figures 5A and 5B). Untreated cultures had abundant Pecam1+ cells (Figure 5A), consistent with a previous report (Yamashita et al., 2000), but these were lost upon DKK1 treatment. Mesp1 restored Pecam1+ expression in DKK1-treated cultures (Figure 5A). Pecam1+ cells also expressed the endothelial marker VE-cadherin (Figure 5B). Thus, Mesp1 may induce a progenitor of cardiomyocytes, smooth muscle, and endothelial cells, with the fate selected by this progenitor depending on other signals, such as Wnt, as suggested by the promotion of cardiomyocyte development by DKK1.
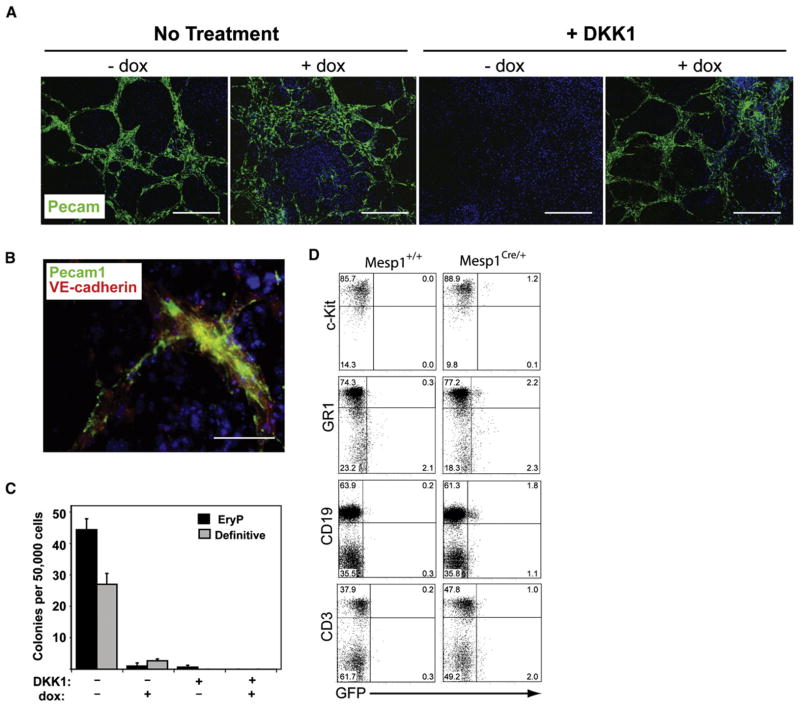
Figure 5. Mesp1 Expression Induces Endothelial Differentiation and Inhibits Hematopoietic Potential of Differentiating ESCs.
(A and B) A2lox.Mesp1 ESCs were differentiated as in Figure S1B and were plated on Collagen I-coated slides on day 4 of differentiation. On day 12, cells were stained with antibodies to Pecam1 (green) and VE-cadherin (red) as indicated. Nuclei were stained using Hoechst 33342 (blue). (A) Representative images are shown where the scale bar is 200 μm. (B) Higher power image (scale bar, 50 μm) of culture treated with both DKK1 (day 2–6) and dox (day 2–4). (C) A2lox.Mesp1 ESCs were differentiated as in Figure S1B. On day 6, cells were assessed for hematopoietic precursor potential using methycellulose colony forming assays. Primitive erythroid (black) and definitive hematopoietic (gray) colonies were counted at days 12 and 16, respectively. (D) Cells were obtained from adult Rosa R26R-eGFP+/−mice that were either Mesp1+/+ or Mesp1Cre/+. Bone marrow cells were analyzed by flow cytometry for GFP expression in cKithi lineage− live cells or Gr1+ cells contained in a granulocyte live-cell gate. Splenocytes were analyzed for GFP expression in either CD19+ or CD3+ cells contained in a lymphocyte live gate. Numbers shown are the percentage of live-gated cells in each quadrant.
Mesp1 Inhibits Hematopoietic Potential of ESCs In Vitro and Is Expressed by Only a Small Percentage of Hematopoietic Progenitors In Vivo
We asked if Mesp1 functionally inhibited hematopoietic competence of differentiating ESCs (Figure 5C). Development of primitive and definitive hematopoietic colonies (Kyba et al., 2003) was inhibited by DKK1 (Figure 5C) as described (Lindsley et al., 2006). Mesp1 failed to restore hematopoietic competence in DKK1-treated differentiating ESCs and eliminated primitive and definitive hematopoietic colonies in the absence of DKK1 (Figure 5C). However, these results do not distinguish active inhibition of hematopoietic development from skewing ES cultures toward cardiovascular development.
Successive populations of Flk1+ cells arise during ESC differentiation that contain either hematopoietic or cardiovascular progenitors (Kattman et al., 2006). To test if Mesp1-expressing Flk1+ cells could contribute to the hematopoietic system in vivo, we extended Mesp1 lineage tracing to the hematopoietic system (Figure 5D). We analyzed GFP expression in hematopoietic progenitor cells and mature blood cells isolated from adult Mesp1-Cre (Mesp1Cre/+) mice (Saga et al., 1999) crossed to the Cre-reporter mouse strain R26R-eGFP (Rosa26R26R–eGFP/+) (Figure 5D). In Mesp1+/+ R26R-eGFP mice, no GFP+ blood cells were observed, as expected. In Mesp1+/Cre R26R-eGFP mice, the majority (>96%) of hematopoietic cells were negative for GFP, but a small population of GFP+ cells was reproducibly present in hematopoietic cells of thesemice. GFP+ cells comprised 1.3%of lineageneg cKithi bonemarrow progenitor cells (1.2%of the total gate), 2.8% of GR1+ bone marrow granulocytes (2.2% of the total gate), 2.8% of CD19+ splenic B cells (1.8% of the total gate), and 2%of CD3+ splenic T cells (1%of the total gate) (Figure 5D). Thus, during embryogenesis, Mesp1-expressing cells are largely excluded from the hematopoietic mesoderm, consistent with our in vitro results (Figure 5C).
Mesp1 Induces Stable Expression of Cardiovascular Transcription Factors
Mesp1 could induce cardiovascular mesoderm either by restoring Wnt-dependent primitive streak gene expression or by directly specifying a cardiovascular progenitor. Thus, we examined expression of primitive streak-associated genes and core cardiac transcription factors (Srivastava, 2006) after Mesp1 induction (Figure 6A). T (brachyury), MixL1, and endogenous Mesp1 were expressed in control cultures on day 3 and day 4 of differentiation, markedly inhibited by Mesp1 induction, not expressed in DKK1-treated cultures and not restored by Mesp1 (Figure 6A). Thus, Mesp1 does not appear to restore primitive streak-associated gene expression. In contrast, Gata4, Foxc1, Foxc2, and Isl1, transcription factors which act in the first and second heart fields (Srivastava, 2006), were induced by Mesp1 with or without DKK1 (Figures 6B and 6C). Tbx1, a factor regulated by forkhead proteins in the second heart field (Maeda et al., 2006), was detected only in Mesp1-induced cultures (Figure 6B). Mesp1 also induced Zfhx1a, implicated in smooth muscle differentiation (Nishimura et al., 2006) and myocardin (Myocd), important for smooth muscle and cardiomyocyte differentiation (Li et al., 2003) (Figure 6B; Table S1). Moreover, the transcription factors Gata4, Gata6, Hand1, Hand2, Mef2c, Smyd1 (Bop), Tbx5, and Tbx20 were all induced by Mesp1 and were maintained for at least 8 days after dox removal (Figure 6C). Endogenous expression of these genes was inhibited by DKK1, and their expression was induced by Mesp1 in the presence of DKK1.
Figure 6. Mesp1 Activates Stable Expression of the Core Regulatory Network of Cardiomyogenic Transcription Factors.
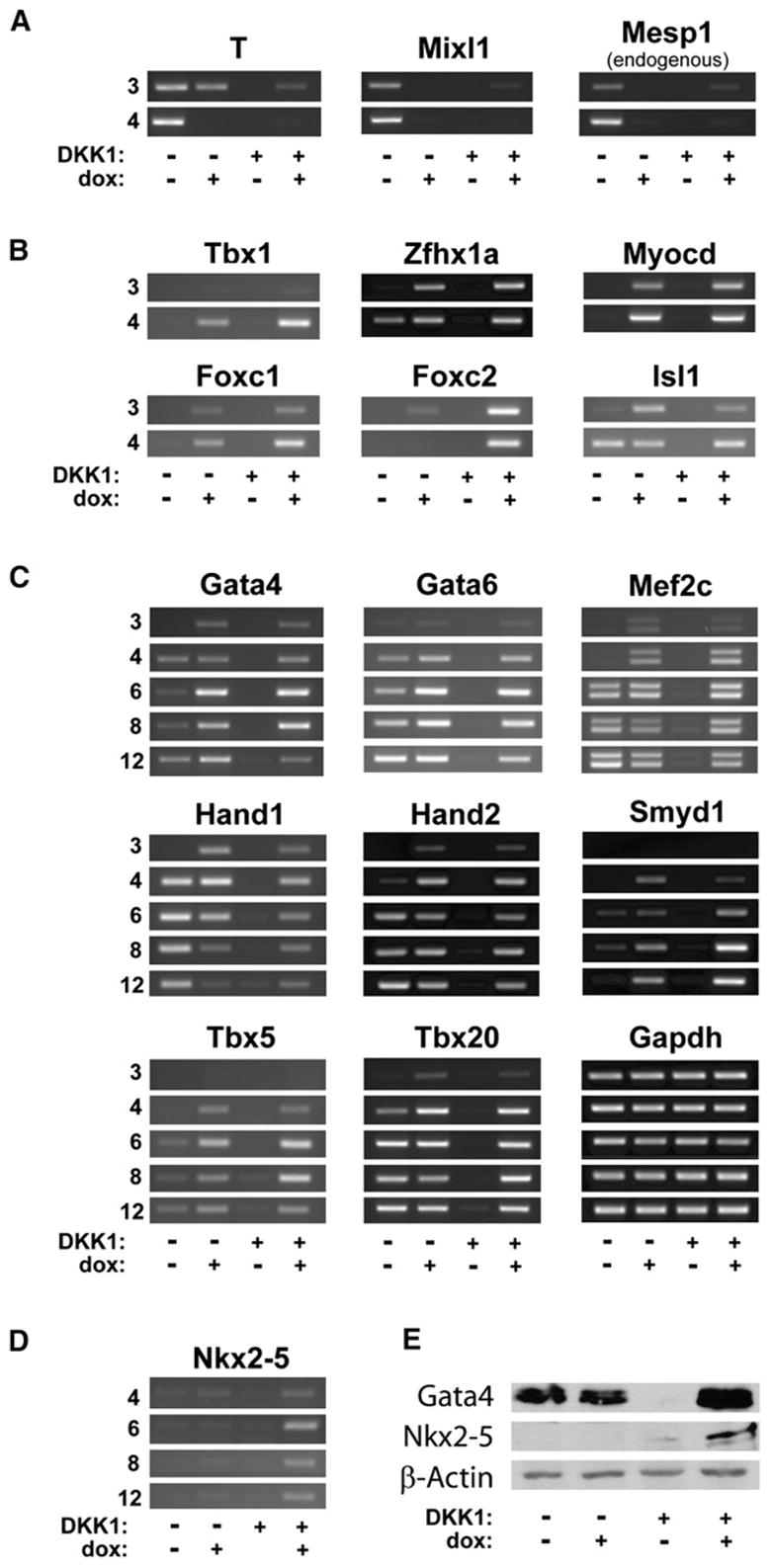
(A–D) A2lox.Mesp1 ESCs were differentiated as in Figure S1B. Cells were harvested on days 3, 4, 6, 8, and 12, and expression of the indicated genes was analyzed by RT-PCR.
(E) A2lox.Mesp1 ESCs, differentiated as indicated in Figure S1B, were harvested on day 6. Expression of the indicated proteins was analyzed by western blotting (see the Supplemental Experimental Procedures).
Since Mesp1 induced cTnT only with DKK1 treatment (Figures 4A and 4B), we asked if any transcription factors were induced preferentially with DKK1. Nkx2-5 was more strongly induced by Mesp1 in the presence of DKK1 (Figure 6D), consistent with microarray data (Figure 3A), which was confirmed at the level of protein by western analysis (Figure 6E). Although Gata4 was expressed in untreated cultures and dox-treated cultures, was inhibited by DKK1, and was induced by Mesp1 in the presence of DKK1, Nkx2.5 was much more strongly induced by Mesp1 in cultures treated with DKK1 compared to non-DKK1-treated cultures (Figure 6E), consistent with RT-PCR results (Figure 6D). In summary, the induction by Mesp1 of multiple cardiogenic transcription factors, but not primitive streak-, hematopoietic-, or paraxial mesoderm-associated genes, suggests that Mesp1 might selectively program cardiovascular development.
Identification of Early Transcriptional Targets of Mesp1 Activity
We used reporter analysis to ask if Nkx2.5 or Myocd were direct targets of Mesp1 (Figure S6A). Activation of the EphA4 enhancer required both Mesp1 and E47, as expected (Nakajima et al., 2006). However, cardiac-specific enhancers for Nkx2.5 (Searcy et al., 1998) and Myocd (Creemers et al., 2006) were not activated by cotransfection of Mesp1 alone or with E47, indicating they may not be direct targets of Mesp1. However, other enhancer regions of Nkx2.5 (Lien et al., 1999) or Myocd might be responsive to Mesp1, or other factors not present in 293T cells may be required in order for Mesp1 to act. Since direct Mesp1 targets should be induced earlier than indirect targets, we characterized Mesp1-induced gene expression after 6, 12, and 24 hr of dox treatment on day 2 of differentiation in DKK1-treated cultures (Figure 7A; Table S1). After 6 hr, 41 genes were induced >3-fold; after 12 hr, 152 genes were induced; and after 24 hr, >500 genes were induced (Figure 7A). Nearly 1/3 of genes induced by Mesp1 after 12 hr were regulators of transcription or signaling and included the transcription factors Foxc1, Foxc2, Cited1, and Zfhx1b, which were induced within 6 hr (Table S1).
Figure 7. Mesp1 Rapidly Induces Expression of Many Genes, Including Myocardin, which Is Required for Differentiation of Cardiomyocyte and SMC Lineages.
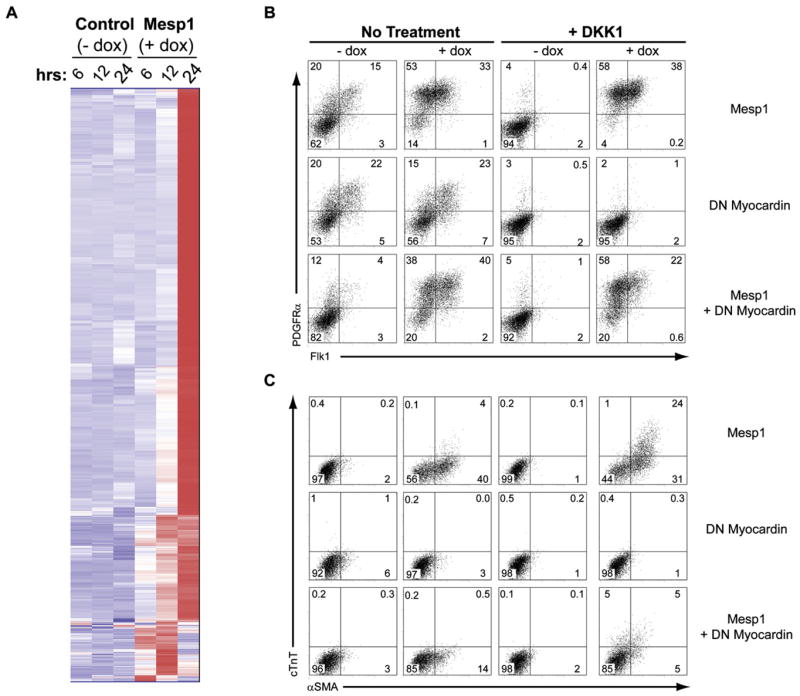
(A) A2lox.Mesp1 ESCs were differentiated in the presence of DKK1 without dox (Control) or with addition of dox (Mesp1) at day 2. Gene expression was assessed by microarray analysis at the indicated times (hrs) after doxcycyline addition. Normalized expression values were compared for 3-fold changes between time-matched samples that were either untreated or treated with dox. 592 genes satisfying the comparison criteria are shown using hierarchical clustering analysis. (B and C) A2lox.Mesp1 ESCs containing inducible Mesp1 (Mesp1), A2lox.Myocd DN ESCs containing inducible DN myocardin (Δ585) lacking the transactivation domain (DN Myocardin) (Wang et al., 2001), and A2lox.Mesp1-Myocd DN ESCs containing simultaneously inducible Mesp1 and DN myocardin (Mesp1 + DN Myocardin) were differentiated as in Figure S1B, and cells were harvested on day 4 (B) and day 6 (C). (B) Expression of PDGFRα and Flk1 was assessed by flow cytometry as in Figure 1B. (C) Expression of αSMA and cTnT was assessed by flow cytometry as in Figure 4B. The numbers shown indicate the percent of live cells present within each quadrant.
Figures S6B and S6C show the fold changes in expression of transcription factors and signaling molecules induced by Mesp1 in the presence of DKK1 after 12 hr. Mesp1 induced many genes relevant to EMT and development of cardiovascular lineages in vivo. Snai1 and Lhx1, related to EMT (Carver et al., 2001; Hukriede et al., 2003), were induced 6 hr after dox addition. Gata2, which promotes endothelial cell development and inhibits hematopoietic development from multipotent progenitors (Persons et al., 1999; Minegishi et al., 2003; Lugus et al., 2007), was induced 5-fold by 6 hr and 14-fold by 12 hr after dox treatment. A number of Wnt-related factors were induced by Mesp1 (Table S1). Wnt5a, which acts in the noncanonical Wnt pathway important in several models of cardiogenesis (Eisenberg and Eisenberg, 2006; Fraidenraich et al., 2004), was induced 55-fold and 93-fold at 6 and 12 hr after dox addition, respectively. DKK1, recently proposed as a direct target of Mesp1 (David et al., 2008), was induced 50-fold by Mesp1 in the presence of DKK1, but not until 24 hr after dox addition (Table S1). Notably, Myocd, a coactivator which regulates serum response factor (SRF) and Mef2c activity during embryogenesis and which activates smooth muscle and cardiac gene expression programs in vitro and in vivo (Pipes et al., 2006; Du et al., 2003), was induced 74-fold by Mesp1 in DKK1-treated cultures after 24 hr.
Myocardin Activity Is Required for Mesp1 Induction of cTnT+aSMA+ Cardiomyocytes and cTnT−aSMA+ Smooth Muscle Cells
A dominant-negative form of Myocardin (Myocd) lacking a transactivation domain inhibits cardiogenesis in Xenopus embryos (Wang et al., 2001). Myocd−/− murine embryos show disruption of vascular smooth muscle, but not cardiac development (Li et al., 2003), suggesting other members of the myocardin-related transcription factor (MRTF) family besides Myocd might function in murine cardiac development. To test if Myocd is important for Mesp1-induced cardiovascular differentiation, we developed an inducible dual-expression system to allow simultaneous induction of Mesp1 and dominant-negative Myocd (Figure 7B). We first confirmed that this system can inducibly coexpress two different cDNAs (Figure S7). In A2lox.GFP-RFP cells, GFP and RFP were both induced upon dox treatment, but not in the absence of dox, indicating this system allows for coinduction by dox of two cDNAs. We examined the effect of dominant-negative (DN) Myocd on Mesp1 induction of the mesoderm markers PDGFRα and Flk1. Mesp1 increased the frequency of PDGFRα+ and Flk1+ cells, both in the absence and presence of DKK1 (Figure 7B, top row). Expression of DN-Myocd alone, without Mesp1, did not change the frequency of PDGFRα+ or Flk1+ cells in the absence of DKK1 and failed to induce these populations in the presence of DKK1 (Figure 7B, middle row). Coexpression of DN-Myocd, along with Mesp1, did not alter the frequency of PDGFRα+ or Flk1+ cells induced by Mesp1 either in the absence or presence of DKK1 (Figure 7B, bottom row). Thus, induction by Mesp1 of the mesoderm markers PDGFRα and Flk1 is independent of Myocd.
We next examined the effect of DN-Myocd on Mesp1 induction of αSMA and cTnT (Figure 7C). Mesp1 alone induced αSMA+cTnT− cells and αSMA+cTnT+ cells in the absence and presence of DKK1, respectively (Figure 7C, top row). DN-Myocd alone slightly inhibited αSMA expression (Figure 7C, middle row). When coexpressed with Mesp1, DN-Myocd strongly inhibited αSMA+cTnT− cells, with DKK1 (30.8% to 5.3%) or without DKK1 (40.2% to 13.9%) (Figure 7C, bottom row). Also, DN-Myocd inhibited Mesp1-induced development of αSMA+cTnT+ cells in the presence of DKK1 (24.2% to 4.5%). These results are consistent with a role for Myocd in Mesp1-induced smooth muscle cell and cardiomyocyte differentiation.
DISCUSSION
Identification of Mesp1 as a Transcription Factor Sufficient for Wnt-Independent Mesoderm Induction
Mesp1 was identified in our screen as driving Wnt-independent expression of mesoderm markers, but we found that it also induces features of epithelial-mesenchymal transition (EMT) and restricts potential fates of differentiating ESCs to cardiovascular lineages. Mesp1 initiates large-scale changes in global gene expression that evolved over time in contrast to limited changes induced by other candidates (data not shown). Our approach could fail to identify mesoderm-inducing factors that are expressed at low levels during ESC differentiation (Lindsley et al., 2006) or that require Wnt signaling to induce mesoderm. A recent study claimed that Mesp1 promotes cardiovascular differentiation in ESCs by induction of DKK1 (David et al., 2008) but did not examine Mesp1 in regulating Snai1 or EMT. Our study has shown that the mechanism by which Mesp1 restricts the fate of differentiating ESCs is not exclusively through induction of DKK1, but likely involves more direct actions on induction or suppression of many transcription factors as discussed below.
Correlation of In Vivo and In Vitro Actions of Mesp1 and Mesp2 Activity
Mesp1 was cloned by subtractive hybridization from the posterior primitive streak of 7.5 day embryos (Saga et al., 1996). Mesp2, a closely related homolog located near the Mesp1 gene, was cloned by homology to Mesp1 (Saga et al., 1997). Mesp1 is required for normal heart development, and Mesp1−/− embryos display early lethality (Saga et al., 1999). In Mesp1−/− mice in which βGal is targeted to the Mesp1 locus, βGal-expressing cells accumulate in the primitive streak (Saga et al., 1999). However, Mesp1−/− embryos do generate cardiac mesoderm, although their heart tubes fail to fuse, leading to cardia bifida and embryonic death (Saga et al., 1999). Mesp1−/− embryos also show increased and prolonged expression of Mesp2 in the primitive streak, suggesting that Mesp2 may compensate for migratory defects, but not for heart development seen in Mesp1−/− embryos (Kitajima et al., 2000). In Mesp1−/−Mesp2−/− double-deficient embryos, there is an accumulation of nonmigrating cells in the primitive streak and complete failure to form cardiac mesoderm (Kitajima et al., 2000). Thus, Mesp1 andMesp2may share potential transcriptional targets, consistent with finding common induction of Snai1, but private functions may arise from expression at distinct sites and times in the embryo, and verifying targets of Mesp1 in vivo may require examination of Mesp1−/−Mesp2−/− embryos.
Mesp1 and Generation of Cardiovascular Progenitors
Recent studies identified a multipotent Isl1+ cardiovascular progenitor cell (MICP) in vivo that gave rise to endothelial, cardiac, and smooth muscle cells (Moretti et al., 2006; Wu et al., 2006). Cardiovascular colony-forming cells (CV-CFC) identified in differentiating ESCs may represent this divergence between cardiovascular and hemogenic lineages (Moretti et al., 2006; Kattman et al., 2006). Our study suggests that Mesp1 may represent the transcriptional basis for development of the MICP and CV-CFC and is consistent with a study suggesting that the orthologous Ciona intestinalis gene Mesp specifies the cardiomyocyte lineage independently of cell migration (Satou et al., 2004; Davidson et al., 2005). Early transcriptional targets of Mesp1 include Snai1 and Myocd, representing plausible links to EMT and cardiovascular commitment, although we have not determined whether these are direct Mesp1 targets.
Recent studies have found both positive and negative effects of Wnt signaling in the proliferation (Kwon et al., 2007; Cohen et al., 2007; Ai et al., 2007) and the specification and differentiation of cardiovascular progenitors (Lin et al., 2007; Ueno et al., 2007; Naito et al., 2006; Kwon et al., 2007; Eisenberg and Eisenberg, 2006; Tzahor, 2007; Qyang et al., 2007). Our previous study showed that development of Flk1+ mesoderm precursors, including cardiovascular mesoderm, required Wnt signaling (Lindsley et al., 2006). Our current findings suggest that the early requirement for Wnt signaling on cardiovascular development may stem from the Wnt-dependence of Mesp1 expression. The inhibition by Wnt signaling of later cardiovascular differentiation (Eisenberg and Eisenberg, 2006) would represent actions of Wnt on the specified MICP (or its progeny) that influence cardiac muscle or smooth muscle differentiation.
A recent study suggested that Mesp1 induced cardiovascular differentiation in ESCs through induction of DKK1 (David et al., 2008). That conclusion was based on a correlation of Mesp1’s actions with the induction of DKK1 by Mesp1. In this and a previous study (Lindsley et al., 2006), we have shown that treatment of differentiating ESCs with DKKI actually inhibits mesoderm lineage development. We suggest that the induction of cardiovascular fates by Mesp1 might derive instead from the rapid induction of transcription factors that are targets of Mesp1 rather than induction of DKK1. Identifying the mechanisms by which Mesp1 acts may be relevant to understanding cardiac development and eventual applications to regenerative therapy.
EXPERIMENTAL PROCEDURES
ESC Generation and Differentiation
MC50 (Dr. Robert Schreiber, Washington University School of Medicine) and modified A2lox ESCs (Kyba et al., 2002) were maintained as described (Lindsley et al., 2006). Individual cDNAs were amplified from embryoid body RNA using gene-specific primers (Table S2) and were cloned into the p2lox targeting vector (Kyba et al., 2003). Site specific recombination into A2lox ESCs using cotransfected Cre recombinase was as described (Lindsley et al., 2006; Kyba et al., 2003). An inducible, dual-expression vector was prepared by cloning a cDNA (either GFP or DN Myocd [aa 1–585] [Wang et al., 2001]) into the vector pTet-CMVmin pA-zeocin (Dr. Jonathan Green, Washington University School of Medicine), transferring this Tet-CMVmin promoter-cDNA-pA cassette into the p2lox targeting vector downstream of the original p2lox pA site, and followed by inserting a second cDNA (either RFP or Mesp1) into the resulting plasmid, allowing simultaneous dox induction of both cDNAs in transfected A2lox ESCs.
For differentiation, ESCs were plated in suspension in Petri dishes at 1.5 × 104 cells/ml in Iscove’s modified Dulbecco’s medium with 10% FCS, NEAA, L-glutamine, NaPyruvate, Pen/Strep, and 2-mercaptoethanol and supplemented where indicated with DKK1-his as described (Lindsley et al., 2006). Gene expression was induced by addition of dox (250 ng/ml). Hematopoietic colony assays were as described (Lindsley et al., 2006).
Immunofluorescence
Embryoid bodies (EB) were plated at the indicated time onto Collagen I-coated slides (BD Biosciences), fixed in2%formaldehyde in PBS, and blocked with1% BSA/0.5% saponin in PBS. Primary antibodies: biotinylated E-cadherin (2.5 μg/ml, R and D Systems), N-cadherin (2.5 μg/ml, BD Transduction Laboratories), cTnT (2 μg/ml, 1C11, Abcam), αSMA (13.3 μg/ml, Sigma), SM-MHC(1:100, Biomedical Technologies), Pecam1 (5 μg/ml, 390, eBioscience), VE-cadherin (2.5 μg/ml, R and D Systems). Secondary detection: FITC F(ab′)2 α-rat IgG, Cy3 α-mouse IgG, Cy3 α-mouse IgG1, and FITC α-rabbit IgG (3 μg/ml, Jackson Immunoresearch Laboratories) and streptavidin-Alexa488 (Molecular Probes). Nuclei were stained with Hoechst 33342 (1 μg/ml, Molecular Probes).
Flow Cytometry
EBs were dissociated with trypsin and were stained directly or fixed with 2% formaldahyde and permeabilized with 0.5% saponin before staining. Blood cells were obtained from mouse bone marrow or spleen. Primary antibodies: biotin α-mE-cadherin (R&D Systems, 1.25ug/ml), PE α-Flk1 (1 μg/ml, Avas12a1), PE α-Pecam1 (1 μg/ml, 390), PE α-Tie2 (1 μg/ml, Tek4), and APC α-PDGFRα (1 μg/ml, APA5) (eBioscience), FITC α-αSMA (230 ng/ml, 1A4, Sigma), biotin α-VE-cadherin (250 ng/ml, R and D Systems), and α-cTnT (2 μg/ml, 1C11, Abcam), APC α-CD19 (BD-PharMingen, 1 μg/ml), APC α-CD3 (BD-PharMingen, 1 μg/ml), APC α-Gr1 (BD-PharMingen, 1 μg/ml), APC α-cKit (eBioscience, 1 μg/ml), and biotinylated Lineage cocktail (10 μl/ml, Miltenyi). Secondary detection reagents: SA/APC (BD PharMingen, 0.5 μg/ml), SA/PE-Cy7 (BD Biosciences), and PE or APC α-mouse IgG1 (3 μg/ml, Jackson Immunoresearch Laboratories). Data were acquired on a FACS Calibur (Becton Dickinson) and analyzed using FloJo (Tree Star).
Gene Expression Analysis
Large-scale gene expression analysis was performed using MOE430_2.0 arrays (Affymetrix) as described (Lindsley et al., 2006). Data were normalized, and expression values were modeled using DNA-Chip Analyzer (dChip) (Li and Hung Wong, 2001). Raw data are available at the Gene Expression Omnibus (GEO) Repository, accession number GSE5976. RT-PCR analysis was as described (Lindsley et al., 2006) using primers in Table S2. Cycle numbers varied according to expression level and were between 25 and 36 cycles.
Mice
Mesp1-Cre mice were obtained from the Riken BioResource Center as cryo-preserved embryos (Saga et al., 2000). Heterozygous Mesp1Cre/+ mice were bred to homozygous R26R-GFP+/+ mice (The Jackson Laboratory).
Electrophysiological Studies
A2lox.Mesp1 ESCs were differentiated in the presence of DKK1 alone (days 2–10), or in the presence of DKK1 (days 2–10) and doxycyline (days 2–4) to produce spontaneously beating clusters. On day 10, cells were lightly dissociated using trypsin and small clusters were plated on laminin-coated glass cover-slips. Whole cell recordings were obtained as described in the Supplemental Experimental Procedures.
Supplementary Material
SUPPLEMENTAL DATA
The Supplemental Data include seven figures, five tables, and Supplemental Experimental Procedures and can be found with this article online at http://www.cellstemcell.com/cgi/content/full/3/1/55/DC1/.
Acknowledgments
We thank Drs. Ken Blumer and Peirong Hu for the A7r5 cells, Dr. Shreeram Akilesh for help with immunofluorescence microscopy, Dr. Scott Weber for help with calcium imaging, and Michael White for help with Mesp1Cre/Cre ESCs. Part of this work was supported by grant number R01-HL-34161 (J.M.N.) and an American Heart Association Postdoctoral Fellowship (W.W.).
References
- Ai D, Fu X, Wang J, Lu MF, Chen L, Baldini A, Klein WH, Martin JF. Canonical Wnt signaling functions in second heart field to promote right ventricular growth. Proc Natl Acad Sci USA. 2007;104:9319–9324. doi: 10.1073/pnas.0701212104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball SG, Shuttleworth CA, Kielty CM. Platelet-derived growth factor receptor-alpha is a key determinant of smooth muscle alpha-actin filaments in bone marrow-derived mesenchymal stem cells. Int J Biochem Cell Biol. 2007;39:379–391. doi: 10.1016/j.biocel.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Carver EA, Jiang R, Lan Y, Oram KF, Gridley T. The Mouse Snail Gene Encodes a Key Regulator of the Epithelial-Mesenchymal Transition. Mol Cell Biol. 2001;21:8184–8188. doi: 10.1128/MCB.21.23.8184-8188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ED, Wang Z, Lepore JJ, Lu MM, Taketo MM, Epstein DJ, Morrisey EE. Wnt/beta-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling. J Clin Invest. 2007;117:1794–1804. doi: 10.1172/JCI31731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper TA, Ordahl CP. A single cardiac troponin T gene generates embryonic and adult isoforms via developmentally regulated alternate splicing. J Biol Chem. 1985;260:11140–11148. [PubMed] [Google Scholar]
- Creemers EE, Sutherland LB, McAnally J, Richardson JA, Olson EN. Myocardin is a direct transcriptional target of Mef2, Tead and Foxo proteins during cardiovascular development. Development. 2006;133:4245–4256. doi: 10.1242/dev.02610. [DOI] [PubMed] [Google Scholar]
- D’Souza SL, Elefanty AG, Keller G. SCL/Tal-1 is essential for hematopoietic commitment of the hemangioblast but not for its development. Blood. 2005;105:3862–3870. doi: 10.1182/blood-2004-09-3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David R, Brenner C, Stieber J, Schwarz F, Brunner S, Vollmer M, Mentele E, Muller-Hocker J, Kitajima S, Lickert H, et al. MesP1 drives vertebrate cardiovascular differentiation through Dkk-1-mediated blockade of Wnt-signalling. Nat Cell Biol. 2008;10:338–345. doi: 10.1038/ncb1696. [DOI] [PubMed] [Google Scholar]
- Davidson B, Shi W, Levine M. Uncoupling heart cell specification and migration in the simple chordate Ciona intestinalis. Development. 2005;132:4811–4818. doi: 10.1242/dev.02051. [DOI] [PubMed] [Google Scholar]
- Du KL, Ip HS, Li J, Chen M, Dandre F, Yu W, Lu MM, Owens GK, Parmacek MS. Myocardin is a critical serum response factor cofactor in the transcriptional program regulating smooth muscle cell differentiation. Mol Cell Biol. 2003;23:2425–2437. doi: 10.1128/MCB.23.7.2425-2437.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg LM, Eisenberg CA. Wnt signal transduction and the formation of the myocardium. Dev Biol. 2006;293:305–315. doi: 10.1016/j.ydbio.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Filipeanu CM, Brailoiu E, Kok JW, Henning RH, De Zeeuw D, Nelemans SA. Intracellular angiotensin II elicits Ca2+ increases in A7r5 vascular smooth muscle cells. Eur J Pharmacol. 2001;420:9–18. doi: 10.1016/s0014-2999(01)01004-4. [DOI] [PubMed] [Google Scholar]
- Fraidenraich D, Stillwell E, Romero E, Wilkes D, Manova K, Basson CT, Benezra R. Rescue of cardiac defects in id knockout embryos by injection of embryonic stem cells. Science. 2004;306:247–252. doi: 10.1126/science.1102612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W. Requirement for beta-catenin in anterior-posterior axis formation in mice. J Cell Biol. 2000;148:567–578. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukriede NA, Tsang TE, Habas R, Khoo PL, Steiner K, Weeks DL, Tam PP, Dawid IB. Conserved requirement of Lim1 function for cell movements during gastrulation. Dev Cell. 2003;4:83–94. doi: 10.1016/s1534-5807(02)00398-2. [DOI] [PubMed] [Google Scholar]
- Jin JP, Wang J, Zhang J. Expression of cDNAs encoding mouse cardiac troponin T isoforms: characterization of a large sample of independent clones. Gene. 1996;168:217–221. doi: 10.1016/0378-1119(95)00803-9. [DOI] [PubMed] [Google Scholar]
- Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11:723–732. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- Kelly OG, Pinson KI, Skarnes WC. The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development. 2004;131:2803–2815. doi: 10.1242/dev.01137. [DOI] [PubMed] [Google Scholar]
- Kelly RG. Molecular inroads into the anterior heart field. Trends Cardiovasc Med. 2005;15:51–56. doi: 10.1016/j.tcm.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Kitajima S, Takagi A, Inoue T, Saga Y. MesP1 and MesP2 are essential for the development of cardiac mesoderm. Development. 2000;127:3215–3226. doi: 10.1242/dev.127.15.3215. [DOI] [PubMed] [Google Scholar]
- Kwon C, Arnold J, Hsiao EC, Taketo MM, Conklin BR, Srivastava D. CanonicalWnt signaling is a positive regulator ofmammalian cardiac progenitors. Proc Natl Acad Sci USA. 2007;104:10894–10899. doi: 10.1073/pnas.0704044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- Kyba M, Perlingeiro RC, Hoover RR, Lu CW, Pierce J, Daley GQ. Enhanced hematopoietic differentiation of embryonic stem cells conditionally expressing Stat5. Proc Natl Acad Sci USA. 2003;(Suppl 1):11904–11910. doi: 10.1073/pnas.1734140100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson KA, Meneses JJ, Pedersen RA. Clonal analysis of epiblast fate during germ layer formation in the mouse embryo. Development. 1991;113:891–911. doi: 10.1242/dev.113.3.891. [DOI] [PubMed] [Google Scholar]
- Li C, Hung Wong W. Model-based analysis of oligonucleotide arrays: model validation, design issues and standard error application. Genome Biol. 2001;2:RESEARCH0032. doi: 10.1186/gb-2001-2-8-research0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Wang DZ, Wang Z, Richardson JA, Olson EN. The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc Natl Acad Sci USA. 2003;100:9366–9370. doi: 10.1073/pnas.1233635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien CL, Wu C, Mercer B, Webb R, Richardson JA, Olson EN. Control of early cardiac-specific transcription of Nkx2–5 by a GATA-dependent enhancer. Development. 1999;126:75–84. doi: 10.1242/dev.126.1.75. [DOI] [PubMed] [Google Scholar]
- Lin L, Cui L, Zhou W, Dufort D, Zhang X, Cai CL, Bu L, Yang L, Martin J, Kemler R, et al. Beta-catenin directly regulates Islet1 expression in cardiovascular progenitors and is required for multiple aspects of cardiogenesis. Proc Natl Acad Sci USA. 2007;104:9313–9318. doi: 10.1073/pnas.0700923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindskog H, Athley E, Larsson E, Lundin S, Hellstrom M, Lindahl P. New Insights to Vascular Smooth Muscle Cell and Pericyte Differentiation of Mouse Embryonic Stem Cells In Vitro. Arterioscler Thromb Vasc Biol. 2006;26:1457–1464. doi: 10.1161/01.ATV.0000222925.49817.17. [DOI] [PubMed] [Google Scholar]
- Lindsley RC, Gill JG, Kyba M, Murphy TL, Murphy KM. Canonical Wnt signaling is required for development of embryonic stem cellderived mesoderm. Development. 2006;133:3787–3796. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Lugus JJ, Chung YS, Mills JC, Kim SI, Grass J, Kyba M, Doherty JM, Bresnick EH, Choi K. GATA2 functions at multiple steps in hemangioblast development and differentiation. Development. 2007;134:393–405. doi: 10.1242/dev.02731. [DOI] [PubMed] [Google Scholar]
- Maeda J, Yamagishi H, McAnally J, Yamagishi C, Srivastava D. Tbx1 is regulated by forkhead proteins in the secondary heart field. Dev Dyn. 2006;235:701–710. doi: 10.1002/dvdy.20686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meilhac SM, Esner M, Kelly RG, Nicolas JF, Buckingham ME. The clonal origin of myocardial cells in different regions of the embryonic mouse heart. Dev Cell. 2004;6:685–698. doi: 10.1016/s1534-5807(04)00133-9. [DOI] [PubMed] [Google Scholar]
- Minegishi N, Suzuki N, Yokomizo T, Pan X, Fujimoto T, Takahashi S, Hara T, Miyajima A, Nishikawa S, Yamamoto M. Expression and domain-specific function of GATA-2 during differentiation of the hematopoietic precursor cells in midgestation mouse embryos. Blood. 2003;102:896–905. doi: 10.1182/blood-2002-12-3809. [DOI] [PubMed] [Google Scholar]
- Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007;98:1512–1520. doi: 10.1111/j.1349-7006.2007.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, Komuro I. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci USA. 2006;103:19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Morimoto M, Takahashi Y, Koseki H, Saga Y. Identification of Epha4 enhancer required for segmental expression and the regulation by Mesp2. Development. 2006;133:2517–2525. doi: 10.1242/dev.02422. [DOI] [PubMed] [Google Scholar]
- Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev. 2005;85:1205–1253. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Jakt LM, Era T. Embryonic stem-cell culture as a tool for developmental cell biology. Nat Rev Mol Cell Biol. 2007;8:502–507. doi: 10.1038/nrm2189. [DOI] [PubMed] [Google Scholar]
- Nishimura G, Manabe I, Tsushima K, Fujiu K, Oishi Y, Imai Y, Maemura K, Miyagishi M, Higashi Y, Kondoh H, Nagai R. DeltaEF1 mediates TGF-beta signaling in vascular smooth muscle cell differentiation. Dev Cell. 2006;11:93–104. doi: 10.1016/j.devcel.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Peinado H, Portillo F, Cano A. Transcriptional regulation of cadherins during development and carcinogenesis. Int J Dev Biol. 2004;48:365–375. doi: 10.1387/ijdb.041794hp. [DOI] [PubMed] [Google Scholar]
- Persons DA, Allay JA, Allay ER, Ashmun RA, Orlic D, Jane SM, Cunningham JM, Nienhuis AW. Enforced expression of the GATA-2 transcription factor blocks normal hematopoiesis. Blood. 1999;93:488–499. [PubMed] [Google Scholar]
- Pipes GCT, Creemers EE, Olson EN. The myocardin family of transcriptional coactivators: versatile regulators of cell growth, migration, and myogenesis. Genes Dev. 2006;20:1545–1556. doi: 10.1101/gad.1428006. [DOI] [PubMed] [Google Scholar]
- Qyang Y, Martin-Puig S, Chiravuri M, Chen S, Xu H, Bu L, Jiang X, Lin L, Granger A, Moretti A, et al. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway. Cell Stem Cell. 2007;1:165–179. doi: 10.1016/j.stem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Rohwedel J, Horak V, Hebrok M, Fuchtbauer EM, Wobus AM. M-twist Expression Inhibits Mouse Embryonic Stem Cell-Derived Myogenic Differentiation in Vitro. Exp Cell Res. 1995;220:92–100. doi: 10.1006/excr.1995.1295. [DOI] [PubMed] [Google Scholar]
- Ruzicka DL, Schwartz RJ. Sequential activation of alpha-actin genes during avian cardiogenesis: vascular smooth muscle alpha-actin gene transcripts mark the onset of cardiomyocyte differentiation. J Cell Biol. 1988;107:2575–2586. doi: 10.1083/jcb.107.6.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga Y, Hata N, Kobayashi S, Magnuson T, Seldin MF, Taketo MM. MesP1: a novel basic helix-loop-helix protein expressed in the nascent mesodermal cells during mouse gastrulation. Development. 1996;122:2769–2778. doi: 10.1242/dev.122.9.2769. [DOI] [PubMed] [Google Scholar]
- Saga Y, Hata N, Koseki H, Taketo MM. Mesp2: a novel mouse gene expressed in the presegmented mesoderm and essential for segmentation initiation. Genes Dev. 1997;11:1827–1839. doi: 10.1101/gad.11.14.1827. [DOI] [PubMed] [Google Scholar]
- Saga Y, Miyagawa-Tomita S, Takagi A, Kitajima S, Miyazaki J, Inoue T. MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development. 1999;126:3437–3447. doi: 10.1242/dev.126.15.3437. [DOI] [PubMed] [Google Scholar]
- Saga Y, Kitajima S, Miyagawa-Tomita S. Mesp1 Expression Is the Earliest Sign of Cardiovascular Development. Trends Cardiovasc Med. 2000;10:345–352. doi: 10.1016/s1050-1738(01)00069-x. [DOI] [PubMed] [Google Scholar]
- Sakurai H, Era T, Jakt LM, Okada M, Nakai S, Nishikawa S, Nishikawa S. In vitro modeling of paraxial and lateral mesoderm differentiation reveals early reversibility. Stem Cells. 2006;24:575–586. doi: 10.1634/stemcells.2005-0256. [DOI] [PubMed] [Google Scholar]
- Satou Y, Imai KS, Satoh N. The ascidian Mesp gene specifies heart precursor cells. Development. 2004;131:2533–2541. doi: 10.1242/dev.01145. [DOI] [PubMed] [Google Scholar]
- Schneider VA, Mercola M. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev. 2001;15:304–315. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searcy RD, Vincent EB, Liberatore CM, Yutzey KE. A GATA-dependent nkx-2.5 regulatory element activates early cardiac gene expression in transgenic mice. Development. 1998;125:4461–4470. doi: 10.1242/dev.125.22.4461. [DOI] [PubMed] [Google Scholar]
- Shook D, Keller R. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech Dev. 2003;120:1351–1383. doi: 10.1016/j.mod.2003.06.005. [DOI] [PubMed] [Google Scholar]
- Srivastava D. Making or breaking the heart: from lineage determination to morphogenesis. Cell. 2006;126:1037–1048. doi: 10.1016/j.cell.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Tada S, Era T, Furusawa C, Sakurai H, Nishikawa S, Kinoshita M, Nakao K, Chiba T, Nishikawa S. Characterization of mesendoderm: a diverging point of the definitive endoderm and mesoderm in embryonic stem cell differentiation culture. Development. 2005;132:4363–4374. doi: 10.1242/dev.02005. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Kitajima S, Inoue T, Kanno J, Saga Y. Differential contributions of Mesp1 and Mesp2 to the epithelialization and rostro-caudal patterning of somites. Development. 2005;132:787–796. doi: 10.1242/dev.01597. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Yasuhiko Y, Kitajima S, Kanno J, Saga Y. Appropriate suppression of Notch signaling by Mesp factors is essential for stripe pattern formation leading to segment boundary formation. Dev Biol. 2007;304:593–603. doi: 10.1016/j.ydbio.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Tam PPL, Parameswaran M, Kinder SJ, Weinberger RP. The allocation of epiblast cells to the embryonic heart and other mesodermal lineages: The role of ingression and tissue movement during gastrulation. Development. 1997;124:1631–1642. doi: 10.1242/dev.124.9.1631. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- Tzahor E. Wnt/beta-catenin signaling and cardiogenesis: timing does matter. Dev Cell. 2007;13:10–13. doi: 10.1016/j.devcel.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, Reinecke H, Moon RT, Murry CE. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Chang PS, Wang Z, Sutherland L, Richardson JA, Small E, Krieg PA, Olson EN. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell. 2001;105:851–862. doi: 10.1016/s0092-8674(01)00404-4. [DOI] [PubMed] [Google Scholar]
- Wobus AM, Wallukat G, Hescheler J. Pluripotent mouse embryonic stem cells are able to differentiate into cardiomyocytes expressing chronotropic responses to adrenergic and cholinergic agents and Ca2+ channel blockers. Differentiation. 1991;48:173–182. doi: 10.1111/j.1432-0436.1991.tb00255.x. [DOI] [PubMed] [Google Scholar]
- Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien CL, Schultheiss TM, Orkin SH. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–1150. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K, Nishikawa S. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- Yuasa S, Itabashi Y, Koshimizu U, Tanaka T, Sugimura K, Kinoshita M, Hattori F, Fukami S, Shimazaki T, Okano H, et al. Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. Nat Biotechnol. 2005;23:607–611. doi: 10.1038/nbt1093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL DATA
The Supplemental Data include seven figures, five tables, and Supplemental Experimental Procedures and can be found with this article online at http://www.cellstemcell.com/cgi/content/full/3/1/55/DC1/.