Abstract
Wolbachiae are bacterial endosymbionts of insects and many filarial nematodes whose products trigger inflammation in filarial infections. The dependence of the parasites on their endosymbionts has also led to the use of antibiotics directed against the Wolbachiae, therapy that has been demonstrated to have a profound salutary effect on filarial infections. The identification of Wolbachiae in Mansonella species has been conclusively shown for Mansonella ozzardi (Mo), but not for Mansonella perstans (Mp) Using primers known to amplify the 16S ribosomal DNA of other filarial Wolbachiae, an identical 1393 bp band was found in all samples tested. Sequence analysis of these samples demonstrated a single consensus sequence for Mp Wolbachia 16S rDNA that was most similar to Wolbachia sequences from other filarial nematodes. When aligned with the only other Mansonella Wolbachia sequence (Mo) there were only 8 nucleotide differences in the 1369 bp overlapping sequence. Phylogenetic dendrograms, showing the relationship of the Mp Wolbachia to other Wolbachia 16S rDNA, tracked almost identically to the 5S rRNA of their parasite host. Wolbachia surface protein (WSP) was also demonstrated in protein extracted from Mp-containing whole blood. In advance of a treatment trial of Mp, a method for the quantitation of Mp Wolbachia was developed and used to demonstrate not only a relationship between microfilarial numbers and Wolbachia copy numbers, but also to demonstrate the effect of antibiotic on ridding Mp of Wolbachia.
Keywords: Wolbachia, endosymbiont, Mansonella, ribosomal
1. Introduction
Mansonella perstans is a filarial nematode endemic in tropical portions of Africa and South America. Despite very high prevalences in some regions, very little pathology has been directly attributable to this parasite, although transient angioedema, abdominal pain, and pericarditis each has been associated with infection with Mansonella perstans [1-3].
Wolbachiae are bacterial endosymbionts of insects and many filarial nematodes. It has been postulated that these bacteria and their products trigger inflammatory responses that result in filarial-induced lymphedema and elephantiasis [4, 5] and onchocercal ocular disease [6] as well as reactions seen following drug treatment of onchocerciasis [7] and lymphatic filariasis [8, 9]. The dependence of the parasites on their endosymbionts has also led to the use of antibiotics directed against the Wolbachiae, antibiotics that have been demonstrated to have a profound salutary effect on filarial infections [10-12].
The identification of Wolbachiae in Mansonella species has been conclusively shown for Mansonella ozzardi [13]. For Mp, however, one study using microfilariae of Mp from Gabon was unable to demonstrate Wolbachiae in Mp [14] using PCR. Using microfilariae from microfilaremic patients in Uganda, Fischer et al was also unable to detect Wolbachia in Mp (Peter Fischer personal communication and [15]).
Here we report the molecular identification of a Wolbachia endosymbiont in Mansonella perstans, report on its phylogenetic relationship to other Wolbachiae, demonstrate immunologic reactivity with antibodies to the Wolbachia surface protein (WSP) and provide a method for quantitating Mp Wolbachia. Moreover, we have been able to utilize this method to demonstrate the loss of Wolbachiae in Mp microfilaremic patients treated with six weeks of doxycycline.
The presence of bacterial endosymbionts in a largely non-pathogenic filarial species may shed some light on the precise role of Wolbachia in inflammatory conditions associated with other filarial infections. It also suggests that, under the rare circumstances in which Mp is suspected of causing pathology, antibiotics directed against the Wolbachiae (i.e., doxycycline) may be able to modify the disease process and parasite levels.
2. Materials and methods
2.1 Mansonella perstans
During a clinical study of post-treatment reactions in Wuchereria bancrofti in Mali [16], a number of volunteers were identified who were microfilaremic for Mansonella perstans but uninfected with W. bancrofti (as determined by circulating filarial antigen testing and midnight blood smears). Individual, frozen, EDTA containing blood samples from 12 of these individuals were the initial source of Mansonella perstans DNA. These individuals had microfilarial counts that ranged from 17 to 3,300 Mp mf per ml. Identification of the Mansonella perstans was made based on morphologic criteria of diurnal thick smears (unsheathed blood-borne microfilariae, nuclear column extending into the tip of a thick blunt tail).
2.2 DNA extraction and PCR
DNA was extracted using a protocol adapted from Williams et al [17]. Briefly, to 200μL of centrifuged whole blood was added a single zinc BB in a microfuge tube. This tube was vortexed in two dimensions over a 20-minute period. DNA was then extracted using the QiaAmp DNAEasy Blood and Tissue Kit (Qiagen) according to the manufacturer’s instructions. The DNA was subsequently stored at 4° until used.
PCR for Wolbachia was performed with primers for the 16S rDNA gene as published previously [18], using the following sequence: 16S forward: GAG AGT TTG ATC CTG GCT CAG; 16S reverse: CTA CGG CTA CCT TGT TAC GA.
Mansonella perstans was confirmed by PCR using primers for the internal transcribed spacer (ITS) in the 5S rRNA gene, according to previously published methods [19] by using primers with the following sequence: S2: GTT AAG CAA CGT TGG GCC TGG; S16: TTG ACA GAT CGG ACG AGA TG.
Master mix was made for 25μL reactions to have 2.5μL of 10x master mix (0.75μL of 50mM MgCl2, 0.5μL of 2.5mM dNTP’s, 0.7μL of 10μM primers per reaction, 1μl of Amplitaq™ [ABI]) with 5μL of extracted DNA as the template. Thermal cycling was done with 94° for 1 min followed by 35 cycles at 94° for 30 sec, annealing at 52° for 1min, extension at 72° for 3 min with a final 72° extension for 9 min.
2.3 Cloning and sequencing
PCR products were cloned in the vector pCR®2.1-TOPO (Invitrogen) according to the kit manufacturer’s instructions. Purified plasmid was obtained from overnight cultures of transformed Top10 E. coli using the QiaPrep Spin MiniPrep Kit (Qiagen) according to manufacturer’s instructions. Sequencing of multiple plasmids was done in both directions using standard automated sequencing techniques. The consensus sequence has been deposited in Genbank’s non-redundant nucleotide database (accession number AY278355)
2.4 Quantitative PCR
A method for quantitating copy numbers of Mp and the Wolbachia from Mp using the strategy illustrated in Fig. 4A. Because of co-endemnicity of Wuchereria bancrofti in Mali, speciation of both Wb and Mp and their respective Wolbachia was also developed. Using primers (as illustrated) and FAM-labeled probes, along with standard curves generated by plasmid DNA containing the respective ribosomal genes, quantitation in an ABI 7900HT could be made using standard conditions recommended by the manufacturer.
The cloned 16S plasmid of the Wolbachia endosymbiont was purified, and the plasmid DNA was quantified using a spectrophotometer. This quantified plasmid was aliquoted for use in generating a standard curve for the quantitative PCR reactions. For the reactions, 5μL of extracted DNA or serial dilutions of the standards were combined with 20μL master mix in 96-well MicroAmp™ optical plates (ABI). Thermal cycling and data analysis were done on the ABI7900HT.
2.5 Immunologic reactivity using anti-WSP antibody
Because only fixed whole blood was available for protein staining, 1 ml of formalin-fixed blood from a patient (W. bancrofti negative) with M. perstans containing ~2108 microfilariae was pelleted at 14000 rpm. Protein was extracted from the pellet using the Qproteome FFPE kit (Qiagen) exactly as described by the manufacturer. Ninety-six well plates (Immulon4, Dynatech) were coated with this extracted protein at a concentration of 1ug/ml overnight at 4°C . The plates were blocked with 5% BSA in PBS-0.05%Tween20 for 1 hour and washed 6 times. High titer, individual rabbit antibodies raised against 2 separate KLH-coupled WSP-specific immunogenic peptides (CAQKDSKTNDLY and CSEEVNKGTSEDK) were added in multiple dilutions (in PBS/Tween/0.1%BSA) as were rabbit sera obtained prior to immunization (pre-bleeds). The rabbit antisera was incubated for 2 hours at RT and washed. Alkaline phosphatase-conjugated goat anti-rabbit IgG (Fc-specific) (Jackson ImmunoResearch) was added for 1 hour. The plate was washed and substrate (para nitrophenyl phosphate) was added and read in a multiwell reader (Molecular Dynamics).
2.6 Assessment of Wolbachia levels before and after doxycycline treatment
Blood from a small number of individuals (n = 23) with Mp microfilaremia treated with six weeks of doxycycline was examined for the presence of Mp wolbachia before and at one year following treatment using quantitative real-time PCR.
2.7 Phylogenetic analyses
Using the 16S rDNA sequence obtained from M. perstans as the query sequence we performed a BLAST search against the nonredundant nucleotide databases to identify homologues in the main Wolbachia lineages [20]. These 16S rDNA sequences were then aligned using the global alignment program ClustalW [21] with the default parameters. The 5S rDNA spacer sequences for the corresponding filarial nematodes [13, 19] were aligned using the local alignment program, Muscle [22]. All multiple sequence alignments were visually inspected and manually improved before further analysis.
Phylogenetic analysis was performed with PAUP* 4.0b10 [23] using maximum likelihood distances with Neighbor-joining clustering and 2000 bootstrap replicates. Phylogenetic trees were drawn using Interactive Tree of Life (iTOL [24]).
2.8 Statistical analyses
Spearman rank correlation was used to determine correlations among variables. The analyses were performed using GraphPad (v4.0).
3. Results
Identification of the species as Mansonella perstans was made on morphologic criteria from thick smears of peripheral blood (Fig. 1). The absence of concomitant Wuchereria bancrofti was confirmed by midnight blood filtration and negative tests (ICT™ and TropBio™) for circulating filarial antigen.
Fig. 1.

Individual microfilaria of Mansonella perstans (both panels) used for amplification of Wolbachial DNA. Note the lack of sheath, blunt tail with nuclei extending to the tail.
Molecular speciation of the M. perstans was confirmed by sequencing the internal spacer region of the 5S rRNA gene (100% identity with published sequence, accession number U31640.1; GI:975833).
When primers known to amplify the 16S ribosomal DNA of other Wolbachia were used to amplify DNA obtained from blood of multiple, individual, whole blood samples of known Mp-infected individuals, an identical 1393 bp band was found in all samples tested. These products were cloned and sequenced in both directions. Results of sequence similarity searches using BLAST demonstrated that the 16S rDNA gave the best scores with the Wolbachia sequences from other filarial nematodes and indicated that the endosymbiont of M. perstans belonged to the genus Wolbachia. When aligned with the only other Mansonella wolbachia sequence (that from Mansonella ozzardi (AJ2790434) there were only eight nucleotide differences over the 1369 bp overlapping sequence (data not shown).
A fine-scale phylogenetic analysis of 16S rDNA sequences was performed on an alignment including most of the sequences available for filarial Wolbachia, some of the available sequences for the arthropods capable of transmitting filarial parasites (i.e. Culex spp) and representatives of most of the known Wolbachiae supergroups [20,25,26]. Phylogenetic dendrograms showing the relationship of the Mp Wolbachia 16S rDNA in relation to multiple other Wolbachiae (labeled according to host species) is shown in Fig. 2A. The similar, though not identical, dendrogram based on parasite 5S internal transcribed spacer sequences is shown in Fig. 2B. As can be seen, both the M. perstans 5S rRNA sequence and the Mp Wolbachia 16S rRNA sequences track with the only other Mansonella species for which both sequences were available. Moreover, based on the known phylogenetic relationships among the Wolbachiae, the Wolbachia from Mp groups with the F supergroup, the only supergroup that contains Wolbachia from both arthropods (i.e., termites) and filarial nematodes (i.e. Mansonella ozzardi). These analyses help to corroborate the notion that an independent horizontal transfer between the Wolbachia bacteria and the Mp parasites occurred ~ 100 million years ago [20].
Fig. 2.

Phylogenetic relationships of Mansonella perstans and its Wolbachia sp. Panel A: Phylogenetic relationship of the endosymbiont of Mansonella perstans among the Wolbachia of nematodes and arthropods. The tree, based on the 16S rDNA sequences, was obtained using a distance algorithm in PAUP. Bootstrap confidence values after 2000 replicates are shown. Panel B: Phylogeny of filarial nematodes based on the 5S internal spacer. A distance algorithm-based tree was obtained. Bootstrap confidence values after 2000 replicates are shown. Mansonella spp are in red.
To determine if Wolbachia protein could be detected in Mp, protein was extracted from formalin-fixed whole blood containing 2108 Mp mf/ml obtained from an Mp-infected individual. Using Wolbachia surface protein (WSP)-specific polyclonal antisera, it can be seen (Fig 3) that WSP could be specifically recognized in protein extracts of the mf-containing blood.
Fig. 3.
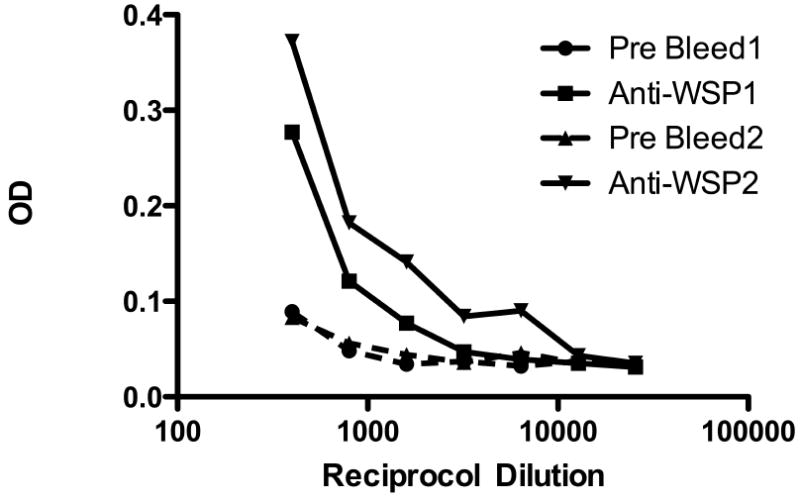
Rabbit anti-WSP recognizes Mp protein extracted from fixed whole blood. Optical densities (ODs) shown for two separate WSP-specific rabbit polyclonal antisera (solid lines) and the corresponding ODs using control (pre-bleed) rabbit sera (dashed lines).
In advance of a treatment trial of Mansonella perstans, a method was developed for the quantitation of Mp Wolbachia and to differentiate it from the Wolbachia from Wuchereria bancrofti, a filarial species often found co-infecting patients with Mp, (Fig. 4). As can be seen, one can easily quantitate the Mp Wolbachia, specifically. Moreover, there was a correlation between the numbers of microfilariae in a given human blood sample and the relative copy number of the 16S Mp Wolbachia sequence (p <0.001).
Fig. 4.
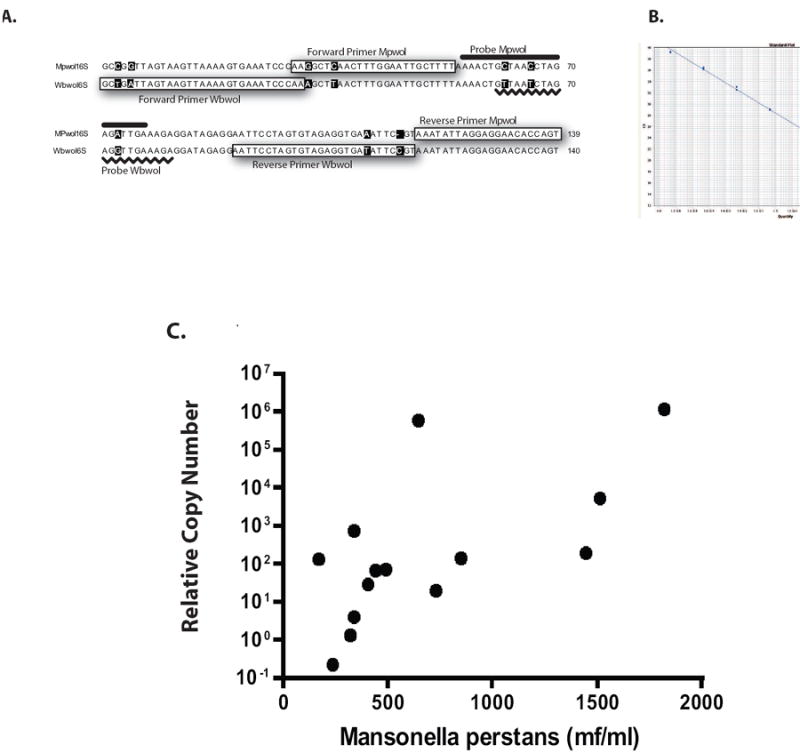
Quantitative and specific amplification of the endosymbiont of Mansonella perstans. Panel A: Sequence based strategy to detect the Wolbachia endosymbiont Mansonella perstans (Mp) and Wuchereria bancrofti (Wb). Panel B: Standard curve used to quantitate the Mp Wolbachia using dilutions of known quantities of Mp Wolbachia DNA plotted in terms of CT. Note: the higher the CT value, the lower the amount of input DNA. Panel C: Relationship between microfilarial numbers per ml of whole blood and the relative copy number of the Wolbachia of Mp.
Finally, this real-time PCR method was used to demonstrate that antibiotics directed against the endosymbiont in Mp was useful in clearing the Wolbachia (Fig. 5). As seen, in 23 individuals followed longitudinally before and one year after a six-week course of doxycycline, all but one was able to completely clear their Wolbachia endosymbiont.
Fig. 5.
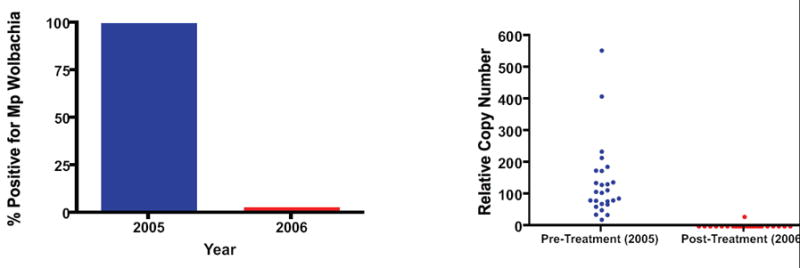
Effect of doxycycline on Mansonella perstans Wolbachia. Left panel: shows relative copy number of Mp Wolbachia DNA before (2005) and one year following (2006) the administration of 6 weeks of doxycycline. Each dot represents an individual patient. Right panel: The percentage of individuals with demonstrable Mp wolbachia DNA before (2005) and 1 year (2006) following the administration of six weeks of doxycycline.
4. Discussion
We present molecular evidence that Mansonella perstans contains a bacterial endosymbiont with high sequence homology to Wolbachiae of other filarial nematodes, most notably Mansonella ozzardi.
Although Wolbachiae have been implicated as a source of pro-inflammatory products among those filarial infections of humans, the presence of a similar endosymbiont in a largely non-pathogenic filarial species suggests that the mere presence of bacterial endosymbionts is not sufficient to trigger pathology. This same conclusion could be made based on the fact that most filarial infections, even those known to induce pathology (W. bancrofti, B. malayi, B. timori, O. volvulus), cause generally asymptomatic (or subclinical) infection.
Other investigators, using methods similar to ours, have concluded that Mansonella perstans does not possess an endosymbiotic Wolbachia [14, 15]. This may simply reflect technical or methodological differences in that we used a bead-based DNA extraction method shown to break open filarial parasites more efficiently than other methods [17].
The possibility that some geographically distinct strains of Mansonella perstans possess an endosymbiont while others do not remains a possibility. However, the notion that these Wolbachia and Mansonella species share a very long and co-dependent relationship [13, 26],as suggested by the similarity in phylogenetic relationships (Fig. 2) makes this a little less likely. Nevertheless, there is clearly data to suggest that some very closely related filarial species (e.g. Onchocerca volvulus and Onchocerca flexuosa) have evolved in such a manner that one has and the other does not have the Wolbachia endosymbiont [27]. Moreover, the very recent data that lateral transfer can occur of an entire Wolbachia genome to its host, suggests that there is the possibility at least, that this process might have begun to occur in some geographic isolates of Mansonella perstans [28].
To date, there has been no effective therapy for clearing infection with Mansonella perstans [29, 30]. With the demonstration that M. perstans possesses a Wolbachia endosymbiont, studies using doxycycline therapies are being investigated for their ability to clear Mp microfilariae. As a proof of concept, we have been able to demonstrate the loss of measurable Wolbachia DNA up to one year following a six-week course of doxycycline (see Fig. 5). These data suggest that Wolbachia can be targeted to rid humans of Mp when necessary, and also suggests there may be effective therapeutic approaches to those rare patients whose symptoms or persistent eosinophilia have been attributed to this filarial species.
Acknowledgments
This work was supported by the Division of Intramural Research, NIAID, NIH. The authors wish to thank Dr. Kurt Wollenberg for his help with the phylogenetic analyses and Ms. Nancy Shulman for editorial help in the formatting of this manuscript.
Abbreviations
- Mp
Mansonella perstans
- Mo
Mansonella ozzardi
- Mf
Microfilaria
- WSP
Wolbachia surface protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adolph PE, Kagan IG, McQuay RM. Diagnosis and treatment of Acanthocheilonema perstans filariasis. Am J Trop Med Hyg. 1962;11:76. doi: 10.4269/ajtmh.1962.11.76. [DOI] [PubMed] [Google Scholar]
- 2.Bourgeade A, Nosny Y, Olivier-Paufique M, Faugere B. 32 cases of recurrent localized edema on return from the Tropics. Bull Soc Pathol Exot Filiales. 1989;82:21–28. [PubMed] [Google Scholar]
- 3.Scott G. Pathogenicity of Acanthocheilonema perstans. J Trop Med Hyg. 1962;65:230–232. [PubMed] [Google Scholar]
- 4.Punkosdy GA, Dennis VA, Lasater BL, Tzertzinis G, Foster JM, Lammie PJ. Detection of serum IgG antibodies specific for Wolbachia surface protein in rhesus monkeys infected with Brugia malayi. J Infect Dis. 2001;184:385–389. doi: 10.1086/322023. [DOI] [PubMed] [Google Scholar]
- 5.Lammie PJ, Cuenco KT, Punkosdy GA. The pathogenesis of filarial lymphedema: is it the worm or is it the host? Ann N Y Acad Sci. 2002;979:131–142. doi: 10.1111/j.1749-6632.2002.tb04874.x. discussion 188-196. [DOI] [PubMed] [Google Scholar]
- 6.Saint Andre A, Blackwell NM, Hall LR, Hoerauf A, Brattig NW, Volkmann L, Taylor MJ, Ford L, Hise AG, Lass JH, Diaconu E, Pearlman E. The role of endosymbiotic Wolbachia bacteria in the pathogenesis of river blindness. Science. 2002;295:1892–1895. doi: 10.1126/science.1068732. [DOI] [PubMed] [Google Scholar]
- 7.Keiser PB, Reynolds SM, Awadzi K, Ottesen EA, Taylor MJ, Nutman TB. Bacterial endosymbionts of Onchocerca volvulus in the pathogenesis of posttreatment reactions. J Infect Dis. 2002;185:805–811. doi: 10.1086/339344. [DOI] [PubMed] [Google Scholar]
- 8.Haarbrink M, Abadi GK, Buurman WA, Dentener MA, Terhell AJ, Yazdanbakhsh M. Strong association of interleukin-6 and lipopolysaccharide-binding protein with severity of adverse reactions after diethylcarbamazine treatment of microfilaremic patients. J Infect Dis. 2000;182:564–569. doi: 10.1086/315735. [DOI] [PubMed] [Google Scholar]
- 9.Cross HF, Haarbrink M, Egerton G, Yazdanbakhsh M, Taylor MJ. Severe reactions to filarial chemotherapy and release of Wolbachia endosymbionts into blood. Lancet. 2001;358:1873–1875. doi: 10.1016/S0140-6736(01)06899-4. [DOI] [PubMed] [Google Scholar]
- 10.Hoerauf A, Mand S, Adjei O, Fleischer B, Buttner DW. Depletion of wolbachia endobacteria in Onchocerca volvulus by doxycycline and microfilaridermia after ivermectin treatment. Lancet. 2001;357:1415–1416. doi: 10.1016/S0140-6736(00)04581-5. [DOI] [PubMed] [Google Scholar]
- 11.Taylor MJ, Makunde WH, McGarry HF, Turner JD, Mand S, Hoerauf A. Macrofilaricidal activity after doxycycline treatment of Wuchereria bancrofti: a double-blind, randomised placebo-controlled trial. Lancet. 2005;365:2116–2121. doi: 10.1016/S0140-6736(05)66591-9. [DOI] [PubMed] [Google Scholar]
- 12.Turner JD, Mand S, Debrah AY, Muehlfeld J, Pfarr K, McGarry HF, Adjei O, Taylor MJ, Hoerauf A. A randomized, double-blind clinical trial of a 3-week course of doxycycline plus albendazole and ivermectin for the treatment of Wuchereria bancrofti infection. Clin Infect Dis. 2006;42:1081–1089. doi: 10.1086/501351. [DOI] [PubMed] [Google Scholar]
- 13.Casiraghi M, Favia G, Cancrini G, Bartoloni A, Bandi C. Molecular identification of Wolbachia from the filarial nematode Mansonella ozzardi. Parasitol Res. 2001;87:417–420. doi: 10.1007/s004360000368. [DOI] [PubMed] [Google Scholar]
- 14.Grobusch MP, Kombila M, Autenrieth I, Mehlhorn H, Kremsner PG. No evidence of Wolbachia endosymbiosis with Loa loa and Mansonella perstans. Parasitol Res. 2003;90:405–408. doi: 10.1007/s00436-003-0872-z. [DOI] [PubMed] [Google Scholar]
- 15.Buttner DW, Wanji S, Bazzocchi C, Bain O, Fischer P. Obligatory symbiotic Wolbachia endobacteria are absent from Loa loa. Filaria J. 2003;2:10. doi: 10.1186/1475-2883-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keiser PB, Coulibaly YI, Keita F, Traore D, Diallo A, Diallo DA, Semnani RT, Doumbo OK, Traore SF, Klion AD, Nutman TB. Clinical characteristics of posttreatment reactions to ivermectin/albendazole for Wuchereria bancrofti in a region co-endemic for Mansonella perstans. Am J Trop Med Hyg. 2003;69:331–335. [PubMed] [Google Scholar]
- 17.Williams SA, Laney SJ, Bierwert LA, Saunders LJ, Boakye DA, Fischer P, Goodman D, Helmy H, Hoti SL, Vasuki V, Lammie PJ, Plichart C, Ramzy RM, Ottesen EA. Development and standardization of a rapid, PCR-based method for the detection of Wuchereria bancrofti in mosquitoes, for xenomonitoring the human prevalence of bancroftian filariasis. Ann Trop Med Parasitol. 2002;96(Suppl 2):S41–46. doi: 10.1179/000349802125002356. [DOI] [PubMed] [Google Scholar]
- 18.McGarry HF, Pfarr K, Egerton G, Hoerauf A, Akue JP, Enyong P, Wanji S, Klager SL, Bianco AE, Beeching NJ, Taylor MJ. Evidence against Wolbachia symbiosis in Loa loa. Filaria J. 2003:2–9. doi: 10.1186/1475-2883-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie H, Bain O, Williams SA. Molecular phylogenetic studies on filarial parasites based on 5S ribosomal spacer sequences. 1994;1:141–151. doi: 10.1051/parasite/1994012141. [DOI] [PubMed] [Google Scholar]
- 20.Casiraghi M, Bordenstein SR, Baldo L, Lo N, Beninati T, Wernegreen JJ, Werren JH, Bandi C. Phylogeny of Wolbachia pipientis based on gltA, groEL and ftsZ gene sequences: clustering of arthropod and nematode symbionts in the F supergroup, and evidence for further diversity in the Wolbachia tree. Microbiology. 2005;151:4015–4022. doi: 10.1099/mic.0.28313-0. [DOI] [PubMed] [Google Scholar]
- 21.Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003;31:3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swofford DL. PAUP * Phylogenetic Analysis Using Parsimony (and Other Methods) 4.0. Sunderland, MA: Sinauer Associates, Inc.; 2003. [Google Scholar]
- 24.Letunic I, Bork P. Interactive Tree Of Life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics. 2007;23:127–128. doi: 10.1093/bioinformatics/btl529. [DOI] [PubMed] [Google Scholar]
- 25.Lo N, Casiraghi M, Salati E, Bazzocchi C, Bandi C. How many wolbachia supergroups exist? Mol Biol Evol. 2002;19:341–346. doi: 10.1093/oxfordjournals.molbev.a004087. [DOI] [PubMed] [Google Scholar]
- 26.Covacin C, Barker SC, Supergroup F. Wolbachia bacteria parasitise lice (Insecta: Phthiraptera) Parasitol Res. 2007;100:479–485. doi: 10.1007/s00436-006-0309-6. [DOI] [PubMed] [Google Scholar]
- 27.Brattig NW, Buttner DW, Hoerauf A. Neutrophil accumulation around Onchocerca worms and chemotaxis of neutrophils are dependent on Wolbachia endobacteria. Microbes Infect. 2001;3:439–446. doi: 10.1016/s1286-4579(01)01399-5. [DOI] [PubMed] [Google Scholar]
- 28.Hotopp JC, Clark ME, Oliveira DC, Foster JM, Fischer P, Torres MC, Giebel JD, Kumar N, Ishmael N, Wang S, Ingram J, Nene RV, Shepard J, Tomkins J, Richards S, Spiro DJ, Ghedin E, Slatko BE, Tettelin H, Werren JH. Widespread lateral gene transfer from intracellular bacteria to multicellular eukaryotes. Science. 2007;317:1753–1756. doi: 10.1126/science.1142490. [DOI] [PubMed] [Google Scholar]
- 29.Luder CG, Soboslay PT, Prince AM, Greene BM, Lucius R, Schulz-Key H. Experimental onchocerciasis in chimpanzees: cellular responses and antigen recognition after immunization and challenge with Onchocerca volvulus infective third-stage larvae. Parasitology. 1993;107(Pt 1):87–97. doi: 10.1017/s0031182000079440. [DOI] [PubMed] [Google Scholar]
- 30.Van den Enden E, Van Gompel A, Vervoort T, Van der Stuyft P, Van den Ende J. Mansonella perstans filariasis: failure of albendazole treatment. Ann Soc Belg Med Trop. 1992;72:215–218. [PubMed] [Google Scholar]


